Abstract
Virulence of the intracellular pathogen Listeria monocytogenes (Listeria) requires escape from the phagosome into the host cytosol, where the bacteria replicate. Phagosomal escape is a multistep process characterized by perforation, which is dependent on the pore-forming toxin listeriolysin O (LLO), followed by rupture. The contribution of host factors to Listeria phagosomal escape is incompletely defined. Here we show that the cystic fibrosis transmembrane conductance regulator (CFTR) facilitates Listeria cytosolic entry. CFTR inhibition or mutation suppressed Listeria vacuolar escape in culture, and inhibition of CFTR in wild-type mice before oral inoculation of Listeria markedly decreased systemic infection. We provide evidence that high chloride concentrations may facilitate Listeria vacuolar escape by enhancing LLO oligomerization and lytic activity. We propose that CFTR transiently increases phagosomal chloride concentration after infection, potentiating LLO pore formation and vacuole lysis. Our studies suggest that Listeria exploits mechanisms of cellular ion homeostasis to escape the phagosome and emphasize host ion-channel function as a key parameter of bacterial virulence.
Keywords: ion flux, listeriosis, membrane integrity
The bacterial pathogen, Listeria monocytogenes, enters mammalian hosts by oral infection, traversing the intestinal barrier to cause systemic disease. Upon entering a host cell, Listeria must escape from the pathogen-containing vacuole, or phagosome, into the cytosol to replicate (1). Phagosomal escape is largely mediated by the cholesterol-dependent cytolysin listeriolysin O (LLO), which is essential for Listeria virulence. Rupture of the Listeria-containing phagosome is a dynamic multistep process. After internalization, an LLO-dependent perforation of the phagosome distinct from rupture occurs, resulting in transient changes in vacuolar pH and calcium (2). Although LLO is the only bacterial factor required for vacuole perforation and escape, the contribution of host factors is less well defined.
Host regulation of the biochemical environment of the phagosome can facilitate bacterial virulence. Phagosome acidification by the vacuolar ATPase is sensed by intracellular pathogens and can increase activity or expression of bacterial virulence determinants, e.g., low pH triggers the PhoQ sensor kinase of Salmonella enterica, leading to changes in bacterial physiology and membrane permeability (3, 4). LLO is regulated at multiple levels by the bacterium, but host regulatory mechanisms also modulate LLO function during infection (5). Maximizing LLO activity in the vacuole but not in the cytosol is a critical aspect of the intracellular lifestyle of Listeria because LLO mutations with increased expression or pore-forming activity destroy the host cell and decrease virulence (6). LLO pore formation proceeds by oligomerization of cholesterol-bound monomers into a prepore complex, followed by insertion into the lipid bilayer (7). LLO oligomerization increases at low pH, suggesting optimal activity in acidifying phagosomes (8). A recent study also showed regulation of bacterial escape by γ-IFN–induced lysosomal thiol reductase, which reduces the single cysteine of LLO to permit pore formation (9). Thus, Listeria relies on host regulation of the phagosome for efficient escape into the cytosol. The phagosomal environment is dynamically modulated by many host proteins, including ion channels and transporters (10). Because ion flux occurs while Listeria is in the phagosome, we hypothesized that host ion transport could affect Listeria escape by altering activity of host or bacterial factors (2).
Results
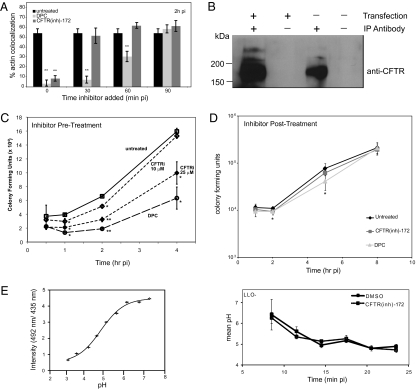
Previous studies demonstrated optimal hemolytic activity from supernatants when Listeria were grown in 428 mM KCl, and increased oligomerization of recombinant LLO (rLLO) occurs when purified in high-salt buffer, suggesting that high chloride concentrations could alter virulence properties of Listeria (8, 11). To determine whether chloride transport aids Listeria escape from the phagosome, we used host chloride channel inhibitors during infection. We treated the murine peritoneal macrophage cell line RAW264.7 (RAW) with the anion channel inhibitor diphenylamine-2-carboxylic acid (DPC) at the indicated times and infected with Listeria. Infected cells were fixed at 2 h postinfection (pi) and subjected to immunofluorescence staining to measure percentage actin colocalization with intracellular bacteria as an indicator of phagosomal escape (Fig. 1A). DPC treatment significantly decreased Listeria escape into the cytosol, even when added at 60 min pi. One DPC-sensitive chloride channel is the cystic fibrosis transmembrane conductance regulator (CFTR) (12). We asked whether CFTR contributed to Listeria phagosomal escape by infecting RAW cells in the presence of CFTR inhibitors CFTR(inh)-172 or GlyH-101 (Fig. 1A and Fig. S1A) (13, 14). Listeria escape into the cytosol was decreased in cells treated with CFTR inhibitor when added up to 30 min pi, compared with untreated cells. To confirm CFTR expression in RAW macrophages, cell lysates were analyzed for the presence of CFTR protein, which could be detected by immunoprecipitation, in agreement with previous work implicating low endogenous CFTR function in these cells (15) (Fig. 1B). CFTR was also required for efficient Listeria escape in the human intestinal epithelial cell line Caco-2 (Fig. S1B). Inhibition of CFTR by CFTR(inh)-172 did not significantly alter bacterial uptake, as indicated by similar cfu at 0.5 h pi (Fig. 1C). CFTR inhibition resulted in decreased numbers of intracellular bacteria in a dose-dependent manner compared with untreated cells, consistent with more bacteria trapped in phagosomes, but did not affect intracellular growth when added after the time window of escape (Fig. 1D). Together, these data implicate host chloride channel function in efficient Listeria phagosomal escape and suggest the involvement of distinct CFTR-dependent and -independent mechanisms.
Fig. 1.
Host chloride channels contribute to Listeria vacuolar escape. (A) RAW cells were infected with Listeria and left untreated or treated with CFTR(inh)-172 or DPC at the indicated times pi. Cells were fixed at 2 h pi, stained with rhodamine-phalloidin and anti-Listeria antibody, and analyzed by epifluorescence microscopy. Percentages represent number of bacteria per 100 colocalized with actin compared with untreated; the same untreated sample is shown with each time point of inhibitor addition (n = 3). (B) Endogenous or transfected CFTR was immunoprecipitated from RAW cells in the absence or presence of CFTR antibodies and immunoblotted with anti-CFTR antibody. (C) RAW cells were left untreated or pretreated for 1 h with the indicated concentrations of CFTR(inh)-172 or DPC. Cells were then infected with Listeria, and colony forming units (CFU) were enumerated at indicated times pi. (D) RAW cells were left untreated or treated with CFTR(inh)-172 or DPC at 1 h pi. Macrophages were infected with Listeria, and CFU were enumerated at indicated times pi. (E) Calibration curve of Oregon Green 492 nm/435 nm ratio plotted against pH (Left), and measurement of pH over time in Listeria-containing vacuoles (Right). RAW cells were pretreated with CFTR(inh)-172 or DMSO and infected with LLO− Listeria for 5 min. Cells were washed, and images were acquired at 2.5-min intervals over the 25 min after infection (≥90 vacuoles per condition). Mean pH represents AF of Listeria-containing vacuoles excited at 500 nm divided by AF of vacuoles excited at 435 nm based on a standard curve. For all graphs, *P < 0.05 and **P < 0.001, comparing untreated and treated cells. Data shown are representative of at least three independent experiments.
CFTR localizes to pathogen-containing phagosomes of alveolar macrophages and may aid in fully acidifying phagosomes by transporting chloride in as a counter ion in some cell types (16, 17). If chloride channel inhibitors prevented full phagosome acidification, LLO-dependent escape of Listeria might be altered because LLO has an acidic pH optimum (18). To determine whether CFTR was altering acidification of Listeria-containing phagosomes in RAW cells, we used live-cell microscopy to track changes in phagosomal pH over time. Macrophages were treated with CFTR(inh)-172 or DMSO and infected for 5 min with LLO-deficient Listeria along with a 10-kDa dextran conjugated to the pH-sensitive Oregon Green fluorophore (Fig. 1E). Ratiometric pH measurements of phagosomes over time revealed no significant difference between cells treated with CFTR(inh)-172 and DMSO nor was there a significant effect of CFTR(inh)-172 on lysosomal-associated membrane protein 1 (LAMP-1) acquisition, a pH-dependent process (Fig. S2). These data are consistent with previous studies in peritoneal macrophages (15–17). We conclude that CFTR facilitates Listeria phagosomal escape by a pH-independent mechanism.
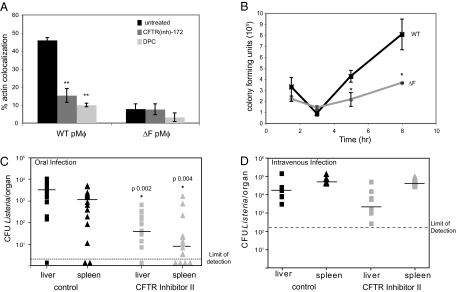
CFTR regulates ion homeostasis in respiratory and intestinal epithelium and can act as a binding determinant for some bacterial pathogens (19, 20). The most common CFTR mutation associated with human cystic fibrosis is deletion of phenylalanine 508(ΔF), which results in decreased trafficking of CFTR and associated proteins to the plasma membrane (21). To further define the contribution of CFTR to Listeria infection, we isolated primary peritoneal macrophages (pMϕ) from wild-type mice or littermates homozygous for the CFTRΔF allele (Fig. 2A). Listeria escape was suppressed in the CFTRΔF pMϕ compared with wild type, suggesting that CFTR must reach the plasma membrane, where it may be incorporated in phagosomes, to mediate Listeria vacuolar escape. We also infected pMϕ from wild-type or CFTRΔF mice and analyzed changes in CFU over time (Fig. 2B). Bacterial numbers at 1 h pi were not significantly affected by CFTR mutation, suggesting unimpaired phagocytosis, but intracellular replication in this short-term infection was delayed in CFTRΔF pMϕ, consistent with an early defect in escape from the phagosome, as observed in RAW macrophages. The growth phenotype in infected primary CFTRΔF pMϕ was more pronounced than in RAW cells with CFTR(inh)-172 likely because of increased bactericidal capacity associated with primary peritoneal macrophages. From these data, we conclude that CFTR dysfunction impedes Listeria vacuolar escape in primary peritoneal macrophages.
Fig. 2.
CFTR potentiates Listeria vacuolar escape and infection. (A) Wild type or ΔF primary pMϕ were untreated or treated for 1 h with CFTR(inh)-172 or DPC and infected with Listeria. Cells were fixed at 3 h pi, stained with rhodamine-phalloidin and anti-Listeria antibody, and analyzed by epifluorescence microscopy. Percentages represent number of bacteria per 100 colocalized with actin (n = 3; **P < 0.001 compared with untreated wild-type pMϕ). Data shown are representative of at least three independent experiments. (B) PMϕ were infected as in A, and, at the time points indicated, infected cells were lysed to enumerate CFU. (C) C57BL/6 mice were i.p. injected with Gly-H101 (6 mg/kg) or DMSO and, 30 min later, orally inoculated with 2 × 109 Listeria in 0.1 mL of PBS. At 18 h pi, spleens and livers were harvested to enumerate CFU. These data represent pooling of two independent experiments. The limit of detection (dotted line) was 2 CFU per organ (*P < 0.05). (D) C57BL/6 mice were i.v. injected with Gly-H101 (6 mg/kg) or DMSO concomitantly with 2 × 104 Listeria in 0.05 mL of PBS and analyzed as in C. The limit of detection (dashed line) was 100 CFU per organ.
CFTR expression is most abundant in epithelial cells of the lungs and gastrointestinal tract of mammals (17, 21). As a food-borne pathogen, the natural route of infection of Listeria is through the gastrointestinal tract, where Listeria could exploit CFTR to infect epithelial cells, a natural bottleneck, followed by systemic dissemination (22). CFTR-deficient mice suffer from intestinal dysfunction and hyperinflammatory responses, complicating interpretation of in vivo Listeria infection (23–25). As an alternative, we treated wild-type mice with GlyH-101, which exhibits superior pharmacokinetics in mice compared with CFTR(inh)-172 (26), and had similar effects on Listeria escape in cell culture (Fig. S1A). At 30 min after i.p. injection with one dose of GlyH-101 or DMSO control, mice were orally infected with 2 × 109 Listeria, and systemic dissemination was measured at 18 h pi (Fig. 2C). We observed ≈100-fold less Listeria in the liver and spleen of GlyH-101–treated mice compared with controls. The magnitude of the effect of the CFTR inhibitor on Listeria infection in mice compared with results in cell culture may reflect the importance of early translocation across the epithelium, which is not modeled in cell culture. In contrast, i.v. Listeria infection, which bypasses this bottleneck, revealed no difference in bacterial burden at 18 h pi between mice treated with one dose of DMSO or Gly-H101; thus, a limited window of CFTR inhibition is not sufficient to alter the course of systemic infection (Fig. 2D). These results suggest that functional CFTR is critical for Listeria to establish infection via the oral route in a murine model of listeriosis.
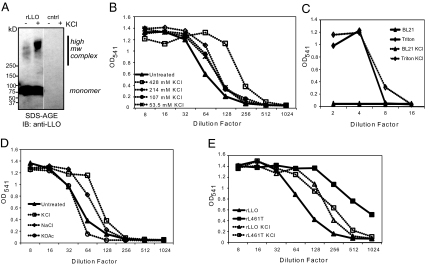
Host ion channel function could contribute to phagosomal escape of Listeria by altering function of bacterial or host factors. Results from previous studies led us to hypothesize that high NaCl or KCl concentrations could stabilize LLO oligomerization, increasing hemolytic activity (8, 11, 27). We therefore compared oligomerization and hemolytic activity of wild-type rLLO ± 428 mM KCl. Oligomers of the cholesterol-dependent cytolysin family of toxins are partially resistant to denaturation by heat and detergent, so we heated rLLO-containing lysates ± 428 mM KCl in SDS sample buffer, followed by SDS-agarose gel electrophoresis to permit visualization of high molecular weight protein complexes (Fig. 3A) (28). We observed an increase in higher molecular weight complexes of wild-type rLLO treated with 428 mM KCl, suggesting enhanced oligomerization. To determine the effect of chloride on LLO activity, we briefly treated rLLO with increasing KCl and measured hemolysis of sheep red blood cells (SRBC) at pH 5.5 (Fig. 3B). High salt enhanced hemolytic activity of rLLO but did not alter hemolytic activity of control lysate or Triton X-100 (Fig. 3C). Treatment of rLLO with 428 mM KCl or NaCl increased hemolytic activity, but 428 mM KOAc did not, indicating specificity for the chloride anion (Fig. 3D). If high KCl increased hemolytic activity by promoting LLO oligomerization, we reasoned that KCl might not enhance activity of a gain-of-function LLO mutant that oligomerizes faster than wild-type LLO because of a leucine substitution at threonine 461 (rL461T) (8). High KCl enhanced hemolytic activity of wild-type rLLO but did not increase activity of rL461T (Fig. 3E). These in vitro data suggest that a high chloride environment could potentiate oligomerization and activity of LLO.
Fig. 3.
LLO oligomerization and hemolytic activity is enhanced in vitro by high KCl concentration. (A) SDS/agarose gel electrophoresis analysis of lysates followed by immunoblotting using an anti-LLO antibody. (B and C) Hemolytic activity of rLLO lysates at pH 5.5 without (closed triangles) or with (open symbols) increasing KCl. BL21(DE3) lysate (triangles) and Triton X-100 (diamonds) represent negative and positive controls, respectively. (D) Hemolytic activity of rLLO lysates at pH 5.5 in HAB (closed triangles) or HAB with 428 mM KCl (open squares), NaCl (open diamonds), or KOAc (open circles). (E) Hemolytic activity of BL21(DE3) lysates expressing rLLO (triangles) or rL461T (squares) with (open symbols) or without (closed symbols) 428 mM KCl on SRBC at pH 5.5. Results shown in all panels represent at least three independent experiments.
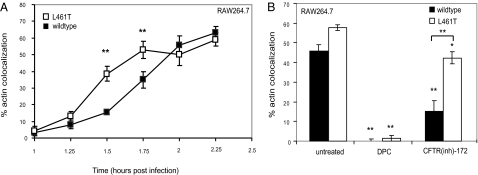
Based on our in vitro data, we hypothesized that CFTR-mediated chloride flux enhanced LLO oligomerization within the vacuole, resulting in efficient phagosome rupture. This hypothesis predicts that CFTR would be preferentially required for escape of the wild-type strain over Listeria expressing LLO L461T because this mutant protein oligomerizes faster than wild-type LLO (8). We first tested whether faster oligomerization would result in more efficient escape by infecting RAW cells with Listeria expressing wild-type LLO or L461T (Fig. 4A). Listeria expressing LLO L461T escaped more quickly than wild-type bacteria, although maximal escape was similar. We then treated RAW cells with CFTR(inh)-172 or DPC and measured Listeria escape (Fig. 4B). Escape of wild-type Listeria was markedly inhibited by CFTR(inh)-172 and DPC, whereas the strain expressing LLO L461T was only modestly affected by CFTR inhibition. Escape of the L461T mutant was still strongly inhibited by DPC, suggesting that CFTR-independent anion channels may play a distinct role in phagosome rupture. Moreover, the L461T mutant could escape more efficiently from CFTRΔF pMϕ than wild-type Listeria (Fig. S3). The L461T expressing strain escaped somewhat less from CFTRΔF pMϕ than from wild-type pMϕ + CFTR inhibitor, suggesting that loss of surface CFTR in the mutant pMϕ impacted escape even by the L461T-expressing strain, perhaps because of other proteins that require CFTR for localization or function. These data provide evidence for a model in which CFTR-mediated chloride flux contributes to efficient Listeria phagosomal escape by favoring increased LLO oligomerization and hemolytic activity in a compartmentalized manner.
Fig. 4.
CFTR function is not required for escape of Listeria expressing a gain-of-function LLO allele. (A) RAW macrophages were infected with Listeria expressing wild-type LLO or LLO L461T. Cells were fixed at the times indicated pi, stained with rhodamine-phalloidin and anti-Listeria antibody, and analyzed by fluorescence microscopy. Percentages represent number of bacteria per 100 colocalized with actin (n = 3; **P < 0.001 for comparison between strains). (B) RAW cells were untreated or treated for 1 h with CFTR(inh)-172 or DPC, infected with wild-type or the L461T mutant Listeria, fixed at 2 h pi, and analyzed as in A (n = 3; *P < 0.05 and **P < 0.001, comparing treated with untreated cells or as indicated by the bracket).
Discussion
Our studies reveal an unanticipated role for CFTR in promoting escape of Listeria from the phagosome. CFTR was previously reported to act as a binding determinant for Pseudomonas aeruginosa and Salmonella typhi (20, 29). In the case of Listeria infection, upon CFTR inhibition or mutation, we observed no reproducible differences in bacterial uptake in cultured cells that might suggest a role in mediating recognition or phagocytosis. Our favored model is that CFTR-mediated chloride movement into the phagosome enhances Listeria escape. CFTR-dependent chloride flux into phagosomes has been demonstrated in neutrophils and contributes to the substantial production of hypochlorous acid in that compartment (30, 31). Enteroinvasive bacteria can induce chloride secretion by intestinal epithelial cells; similarly, upon Listeria infection, host cells could transiently upregulate chloride flux by both CFTR-dependent and -independent mechanisms (32, 33). Transient acute increases in chloride in the lumen of the phagosome would then enhance LLO oligomerization and pore formation in the vacuolar membrane. Although we observed optimal LLO hemolytic activity at 428 mM KCl in vitro, the concentration of chloride in phagosomes is likely to be dynamic, and whether it reaches 428 mM is unclear. A study of elemental concentrations in Mycobacterium avium–containing phagosomes within BALB/c macrophages indicated that phagosomal Cl levels were markedly higher than the cytosol or free bacteria (34). It is therefore possible that, in the phagosome, chloride ions may transiently reach high concentrations in a spatially and temporally regulated manner. It is also worth noting that CFTR has many cellular functions, including glutathione flux, which may additionally alter the dynamics of intracellular infection (35). Further, CFTR inhibition or mutation can affect cholesterol homeostasis, with long-term CFTR inhibition decreasing cellular cholesterol, whereas cells with the CFTRΔF508 mutation exhibit increased cholesterol (36). Because we observe similar escape trends in cells with CFTR inhibition or mutation rather than opposite phenotypes, the effects of CFTR on cholesterol homeostasis are unlikely to be the key parameter affecting Listeria escape. CFTR may play an underappreciated role in modulating ionic strength and membrane integrity of the phagosome during host–microbe interactions. Indeed, the physiological function of CFTR in macrophage phagosomes remains poorly understood and is a matter of current debate (37). Studies characterizing the phagosome proteome have revealed the presence of multiple ion channels and transporters (10, 38). Other than the vacuolar ATPase, the contribution of these proteins to phagosome function and microbial infection is largely unknown. Further investigation of the role of ion transport in bacterial infection at the molecular and cellular level is likely to yield insights into key interactions of bacterial pathogens with their host that alter the outcome of infection.
Methods
Antibodies and Reagents.
RAW cells were treated with 0.25 mM DPC, 10–25 μM CFTR(inh)-172, or 50 μM Gly-H101 unless otherwise indicated. Antibodies and chemical reagents are fully described in SI Methods.
Cell Culture, Bacterial Strains, and Infections.
RAW cells were grown as previously described (39). The wild-type Listeria strain used was 10403S; the LLO-deficient strain (DP-L2161) and LLO L461T strain (DP-L4017) are derivatives of 10403S. rLLO was expressed as described (8). For infections in cell culture, bacteria were grown in brain–heart infusion (BHI) broth at 30 °C overnight. Host cells were infected at a multiplicity of infection (MOI) of 1 for 30 min, then washed three times with PBS, followed by addition of medium with 10 μg/mL gentamicin.
Isolation and Culture of Peritoneal Macrophages.
Cftr(ΔF/ΔF) mice have a 3-bp (CTT) deletion resulting in loss of a Phe residue corresponding to position 508 of human CFTR (40). Cftr(ΔF/WT) breeders were genotyped as described (40). Offspring from Cftr(ΔF/WT) breeders were fed a liquid elemental diet containing hydrolyzed milk protein (Peptamen; Nestle Clinical Nutrition) beginning at 10 d (41). To isolate peritoneal macrophages, Cftr(ΔF/ΔF) mice or littermates were euthanized, and peritoneal lavage collected for culture in DMEM + 20% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1% 2-mercaptoethanol, and 30% L929 conditioned medium.
SDS Gel Electrophoresis, Immunoprecipitation, and Immunoblotting.
SDS/agarose gel electrophoresis was performed as previously described (28). Samples were prepared by incubating 5 μL of reduced rLLO or rL461T Escherichia coli lysate in 428 mM KCl or PBS for 5 min at 37 °C. SRBC (0.5%) were added and incubated for 5 min at 37 °C before adding 5 μL of 5× SDS sample buffer, boiling, and running electrophoresis on a 1% SDS-agarose gel, followed by immunoblotting. Immunoprecipitation of CFTR from 2 × 106 RAW cells using 1.2 μg of M3A7 and 1.2 μg of 24-1 anti-CFTR antibodies was performed as previously described (42). Samples were run on 6% SDS-PAGE gels and immunoblotted with anti-CFTR antibody clone 24-1.
Microscopy.
An Olympus BX60 microscope with epifluorescence was used to quantitate Listeria colocalized with F-actin as described (43). To determine pH of Listeria-containing phagosomes, RAW cells were incubated with CFTR(inh)-172 or DMSO for 1 h and infected with SNARF-1–labeled DP-L2161 with 0.75 mg/mL 10-kDa Oregon Green/dextran. After 5 min, cells were rinsed and imaged in Ringer's buffer with 10 μM CFTR(inh)-172 or DMSO. Images were collected as described (2). The pH of Listeria-containing vacuoles was determined by dividing average fluorescence (AF) of the endosome excited at 500 nm by AF of the endosome excited at 435 nm. This ratio was used to calculate endosomal pH based on a standard curve of 492 nm/435 nm ratio plotted against pH.
Animal Infections.
For oral infections, food was removed 4 h before infection of female C57BL/6J mice (12–16 wk; Jackson Laboratory). At 30 min before infection, mice were fed 50 μL of 10% NaHCO3 orally and injected i.p. with 6 mg/kg GlyH-101 or DMSO in PBS. At 30 min after inhibitor treatment, mice were orally gavaged with 2 × 109 midlog Listeria in 0.1 mL of PBS. For i.v. infections, mice were injected with 2 × 104 midlog Listeria and either 6 mg/kg GlyH-101 or DMSO in a total volume of 50 μL. For both routes of infection, mice were euthanized at 18 h pi, and organs were homogenized in 0.2% Nonidet P-40 in PBS and plated on LB-agar to enumerate cfu.
Hemolytic Assay.
Hemolytic assays were performed as described (43). Lysates were used for the hemolytic assay rather than purified protein, as previously published purification procedures included high salt elution. Lysates were diluted 1:10 in hemolytic assay buffer (HAB; 35 mM sodium phosphate, 125 mM NaCl, and 0.1 mg/mL BSA, pH 5.5) and reduced for 1 h with 5 mM DTT. Lysates were serially diluted in HAB with indicated concentrations of KCl, NaCl, or KOAc in 96-well plates before adding 1% SRBC (43).
Statistical Analysis.
Data were analyzed by using Student's unpaired t test. For in vivo experiments, outlier box and quartile box plots were used to identify outliers with the definition of up-outlier > upper quartile + 1.5(interquartile range) and down-outlier < lower quartile − 1.5(interquartile range). For all experiments, error bars indicate SD.
Supplementary Material
Acknowledgments
We thank Drs. V. DiRita and K. Burkholder for critical review of the manuscript and members of M.X.O.’s laboratory for helpful discussions. We gratefully acknowledge Drs. D. Portnoy and R. Tweten for sharing reagents and Drs. C. H. Chang and Yu Qiao for sharing invaluable expertise. This work was funded by a Ellison Medical Foundation and the American Cancer Society grant (to M.X.O.) and National Institutes of Health Grants 1F32AI084431 (to K.L.A.), R01AI35950 (to J.A.S.), and R01DK073298 (to M. J. DiMagno). A.L.R., M. J. Davis, and K.L.A. were trainees of the University of Michigan Cellular Biotechnology Training Program (T32GM08353) or the Research Training in Experimental Immunology Program (T32A1007413).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013262108/-/DCSupplemental.
References
- 1.Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: The intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol. 2002;158:409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaughnessy LM, Hoppe AD, Christensen KA, Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. 2006;8:781–792. doi: 10.1111/j.1462-5822.2005.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prost LR, et al. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. Acquisition of the vacuolar ATPase proton pump and phagosome acidification are essential for escape of Francisella tularensis into the macrophage cytosol. Infect Immun. 2008;76:2671–2677. doi: 10.1128/IAI.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnupf P, Portnoy DA. Listeriolysin O: A phagosome-specific lysin. Microbes Infect. 2007;9:1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Glomski IJ, Decatur AL, Portnoy DA. Listeria monocytogenes mutants that fail to compartmentalize listerolysin O activity are cytotoxic, avirulent, and unable to evade host extracellular defenses. Infect Immun. 2003;71:6754–6765. doi: 10.1128/IAI.71.12.6754-6765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuerch DW, Wilson-Kubalek EM, Tweten RK. Molecular basis of listeriolysin O pH dependence. Proc Natl Acad Sci USA. 2005;102:12537–12542. doi: 10.1073/pnas.0500558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh R, Jamieson A, Cresswell P. GILT is a critical host factor for Listeria monocytogenes infection. Nature. 2008;455:1244–1247. doi: 10.1038/nature07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers LD, Foster LJ. The dynamic phagosomal proteome and the contribution of the endoplasmic reticulum. Proc Natl Acad Sci USA. 2007;104:18520–18525. doi: 10.1073/pnas.0705801104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers ER, Dallmier AW, Martin SE. Sodium chloride, potassium chloride, and virulence in Listeria monocytogenes. Appl Environ Microbiol. 1993;59:2082–2086. doi: 10.1128/aem.59.7.2082-2086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZR, Zeltwanger S, McCarty NA. Direct comparison of NPPB and DPC as probes of CFTR expressed in Xenopus oocytes. J Membr Biol. 2000;175:35–52. doi: 10.1007/s002320001053. [DOI] [PubMed] [Google Scholar]
- 13.Caci E, et al. Evidence for direct CFTR inhibition by CFTR(inh)-172 based on Arg347 mutagenesis. Biochem J. 2008;413:135–142. doi: 10.1042/BJ20080029. [DOI] [PubMed] [Google Scholar]
- 14.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barriere H, et al. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–3141. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem. 2007;282:31422–31428. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 17.Di A, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 18.Beauregard KE, Lee KD, Collier RJ, Swanson JA. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J Exp Med. 1997;186:1159–1163. doi: 10.1084/jem.186.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pier GB, Grout M, Zaidi TS. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pier GB, et al. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;393:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 21.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 22.Cossart P. Listeriology (1926–2007): The rise of a model pathogen. Microbes Infect. 2007;9:1143–1146. doi: 10.1016/j.micinf.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Davidson DJ, Rolfe M. Mouse models of cystic fibrosis. Trends Genet. 2001;17:S29–S37. doi: 10.1016/s0168-9525(01)02452-0. [DOI] [PubMed] [Google Scholar]
- 24.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–G1041. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 25.Canale-Zambrano JC, Poffenberger MC, Cory SM, Humes DG, Haston CK. Intestinal phenotype of variable-weight cystic fibrosis knockout mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G222–G229. doi: 10.1152/ajpgi.00405.2006. [DOI] [PubMed] [Google Scholar]
- 26.Muanprasat C, et al. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: Mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walton CM, Wu CH, Wu GY. A method for purification of listeriolysin O from a hypersecretor strain of Listeria monocytogenes. Protein Expr Purif. 1999;15:243–245. doi: 10.1006/prep.1998.1022. [DOI] [PubMed] [Google Scholar]
- 28.Shepard LA, Shatursky O, Johnson AE, Tweten RK. The mechanism of pore assembly for a cholesterol-dependent cytolysin: Formation of a large prepore complex precedes the insertion of the transmembrane β-hairpins. Biochemistry. 2000;39:10284–10293. doi: 10.1021/bi000436r. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder TH, et al. CFTR is a pattern recognition molecule that extracts Pseudomonas aeruginosa LPS from the outer membrane into epithelial cells and activates NF-κB translocation. Proc Natl Acad Sci USA. 2002;99:6907–6912. doi: 10.1073/pnas.092160899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Painter RG, Wang G. Direct measurement of free chloride concentrations in the phagolysosomes of human neutrophils. Anal Chem. 2006;78:3133–3137. doi: 10.1021/ac0521706. [DOI] [PubMed] [Google Scholar]
- 31.Painter RG, et al. CFTR-mediated halide transport in phagosomes of human neutrophils. J Leukoc Biol. 2010;87:933–942. doi: 10.1189/jlb.1009655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resta-Lenert S, Barrett KE. Enteroinvasive bacteria alter barrier and transport properties of human intestinal epithelium: Role of iNOS and COX-2. Gastroenterology. 2002;122:1070–1087. doi: 10.1053/gast.2002.32372. [DOI] [PubMed] [Google Scholar]
- 33.Epple HJ, et al. Aeromonas hydrophila β-hemolysin induces active chloride secretion in colon epithelial cells (HT-29/B6) Infect Immun. 2004;72:4848–4858. doi: 10.1128/IAI.72.8.4848-4858.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner D, et al. Elemental analysis of the Mycobacterium avium phagosome in Balb/c mouse macrophages. Biochem Biophys Res Commun. 2006;344:1346–1351. doi: 10.1016/j.bbrc.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 35.Kogan I, et al. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang D, et al. Increased plasma membrane cholesterol in cystic fibrosis cells correlates with CFTR genotype and depends on de novo cholesterol synthesis. Respir Res. 2010;11:61. doi: 10.1186/1465-9921-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haggie PM, Verkman AS. Defective organellar acidification as a cause of cystic fibrosis lung disease: Reexamination of a recurring hypothesis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L859–L867. doi: 10.1152/ajplung.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trost M, et al. The phagosomal proteome in interferon-γ-activated macrophages. Immunity. 2009;30:143–154. doi: 10.1016/j.immuni.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Radtke AL, O'Riordan MX. Homeostatic maintenance of pathogen-containing vacuoles requires TBK1-dependent regulation of aquaporin-1. Cell Microbiol. 2008;10:2197–2207. doi: 10.1111/j.1462-5822.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 40.Zeiher BG, et al. A mouse model for the ΔF508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol. 1995;269:L625–L630. doi: 10.1152/ajplung.1995.269.5.L625. [DOI] [PubMed] [Google Scholar]
- 42.Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin-dependent endocytosis and is regulated by protein phosphorylation. Biochem J. 1997;328:353–361. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol. 2002;156:1029–1038. doi: 10.1083/jcb.200201081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.