Abstract
Cyclin-dependent kinase 5 (Cdk5) is an atypical but essential member of the Cdk kinase family, and its dysregulation or deletion has been implicated in inflammation-related disorders by an undefined mechanism. Here we show that Cdk5 is an indispensable activator of the GAIT (IFN-γ–activated inhibitor of translation) pathway, which suppresses expression of a posttranscriptional regulon of proinflammatory genes in myeloid cells. Through induction of its regulatory protein, Cdk5R1 (p35), IFN-γ activates Cdk5 to phosphorylate Ser886 in the linker domain of glutamyl-prolyl tRNA synthetase (EPRS), the initial event in assembly of the GAIT complex. Cdk5/p35 also induces, albeit indirectly via a distinct kinase, phosphorylation of Ser999, the second essential event in GAIT pathway activation. Diphosphorylated EPRS is released from its residence in the tRNA multisynthetase complex for immediate binding to NS1-associated protein and subsequent binding to ribosomal protein L13a and GAPDH. The mature heterotetrameric GAIT complex binds the 3′ UTR GAIT element of VEGF-A and other target mRNAs and suppresses their translation in myeloid cells. Inhibition of Cdk5/p35 inhibits both EPRS phosphorylation events, prevents EPRS release from the tRNA multisynthetase complex, and blocks translational suppression of GAIT element–bearing mRNAs, resulting in increased expression of inflammatory proteins. Our study reveals a unique role of Cdk5/p35 in activation of the major noncanonical function of EPRS, namely translational control of macrophage inflammatory gene expression.
Aminoacyl-tRNA synthetases (AARSs) are ubiquitous enzymes with evolutionarily conserved catalytic cores that perform their major function, ligation of amino acids to cognate tRNAs for protein synthesis (1, 2). AARSs also contribute to key biological processes beyond their canonical activities (3, 4). In most cases, AARS phosphorylation initiates the noncanonical function with little disruption of aminoacylation activity (5). Phosphorylation of ThrRS, SerRS, and LysRS increases the synthesis of Ap4A, a pleiotropic signaling molecule (6, 7). In myeloid cells, IFN-γ–induced phosphorylation of glutamyl-prolyl tRNA synthetase (EPRS) initiates transcript-selective translational inhibition of inflammatory mRNAs (8, 9). Stimulus-dependent phosphorylation of EPRS drives the release of EPRS from its residence in the tRNA multisynthetase complex (MSC) to facilitate the noncanonical activity (8, 9). Stimulus- and phosphorylation-dependent release of other MSC components has been reported, supporting the “depot hypothesis” in which proteins are inducibly released from macromolecular complexes to perform auxiliary, “moonlighting” functions (7, 10). These previous studies showed that signal-dependent phosphorylation is critical to the regulation of AARS conformation, activity, intracellular localization, protein (and RNA) partners, and, ultimately, noncanonical function.
EPRS is a component of the GAIT complex that binds the 3′ UTR GAIT element in multiple proinflammatory transcripts (e.g., VEGF-A) and inhibits their translation in human myeloid cells (11, 12). The GAIT complex comprises EPRS, NS1-associated protein (NSAP1), ribosomal protein L13a, and GAPDH (8). A unique GAIT component that recognizes and directly binds target mRNAs, EPRS consists of distinct glutamyl tRNA synthetase (ERS) and prolyl tRNA synthetase (PRS) domains joined by a noncatalytic linker of three highly structured WHEP-TRS repeats separated by putative unstructured spacers (Fig. 1A). The linker by itself is sufficient to bind the other GAIT components and reconstitute a functional GAIT complex in vitro (9, 13).
Fig. 1.
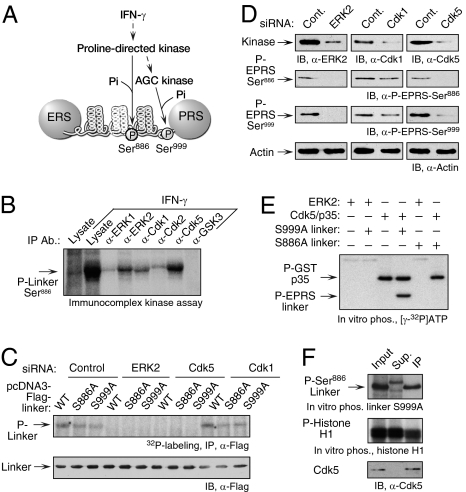
ERK2 and Cdk5 are required for phosphorylation of EPRS at Ser886. (A) Schematic of the IFN-γ–activated kinase pathway directing the phosphorylation of human EPRS at Ser886 and Ser999. (B) Immunocomplexes containing ERK2, Cdk1, and Cdk5 phosphorylate EPRS at Ser886. U937 cells were treated for 4 h with IFN-γ, and candidate proline-directed kinases were immunoprecipitated from lysates. The immunocomplexes were used to phosphorylate recombinant EPRS linker bearing a Ser999-to-Ala mutation in the presence of [γ-32P]ATP. (C) ERK2- and Cdk5-knockdown block 32P incorporation into EPRS linker. U937 cells were cotransfected with Flag-tagged EPRS linker (WT), Ser886-to-Ala (S886A) and Ser999-to-Ala (S999A) mutants, and siRNAs (or control) against ERK2, Cdk5, and Cdk1. Transfected cells were incubated for 4 h with 32P-orthophosphate and IFN-γ. Lysates were immunoprecipitated with anti-Flag antibody, and EPRS linker phosphorylation was determined by autoradiography (Upper). Total transfected EPRS linker was determined by immunoblot analysis (IB) with anti-Flag antibody (Lower). (D) ERK2 and Cdk5 are required for phosphorylation of endogenous EPRS. U937 cells were transfected with the siRNAs shown. Scrambled siRNAs were used as controls (Cont). After 24 h of recovery, cells were treated with IFN-γ for an additional 4 h. Phosphospecific antibodies were used to detect Ser886 and Ser999 phosphorylation. Immunoblot analysis with anti-actin antibody served as a loading control. (E) Recombinant Cdk5 phosphorylates EPRS at Ser886. Recombinant ERK2 and CDK5 activated by GST-tagged p35 were used for in vitro phosphorylation of recombinant EPRS linker substrates bearing either a S999A (Ser886 substrate) or S886A (Ser999 substrate) mutation. (F) Phosphorylation of EPRS linker at Ser886 by endogenous Cdk5. Cdk5 from IFN-γ–activated U937 cells was immunoprecipitated with anti-Cdk5 antibody. Lysate (input), immunoprecipitated fraction (IP), and supernatant (Sup) were used for in vitro phosphorylation of recombinant S999A linker (Top) and histone H1 (Middle) substrates. Cdk5 was detected with anti-Cdk5 antibody (Bottom).
IFN-γ induces early release of EPRS from the MSC and consequent binding to NSAP1 to form the pre-GAIT complex, which lacks GAIT element RNA-binding activity. After ∼14 h, phosphorylated L13a and GAPDH interact with the pre-GAIT complex to form the four-protein GAIT complex that binds and inhibits translation of GAIT element–bearing RNA. These events are mediated by IFN-γ–inducible, two-site phosphorylation of EPRS in the linker domain at Ser886 and Ser999 after ∼1 and 2 h, respectively (9). Both phosphorylation events are required for the release of EPRS from the parent MSC. Negative regulation by NSAP1 specifically requires phosphorylation at Ser886. Ser999 phosphorylation is essential for conformationally correct interaction with phospho-L13a and GAPDH to generate the active GAIT complex that binds target RNA and interacts with eIF4G of the translation initiation machinery, thereby inhibiting translation.
Significant advances have been made in understanding the role of phosphorylation in inducing noncanonical functions of AARSs. Information on the upstream signaling events and the proximal kinases that induce these phosphorylation events in cells is limited, however. Here we report an IFN-γ–induced signaling event that activates cyclin-dependent kinase (Cdk)-5 and its regulatory protein, Cdk5R1 (p35), which directly catalyzes the initial EPRS Ser886 phosphorylation event and indirectly induces Ser999 phosphorylation through activation of a distinct Ser999 kinase. Cdk5/p35-mediated induction of EPRS phosphorylation and GAIT system activation might be an important link between kinase and macrophage inflammatory gene expression.
Results
Cdk5 Phosphorylates EPRS at Ser886.
Previous pharmacologic inhibitor studies have suggested that an unidentified proline-directed kinase is responsible for EPRS Ser886 phosphorylation (9) (Fig. 1A). Site-specific mutagenesis demonstrated the requirement for S/T-P-X-K/R, a proline-directed kinase recognition motif, in the linker sequence 886SPTR. Inhibitor studies also showed that a distinct AGC kinase phosphorylates EPRS at Ser999 (9). To identify the specific Ser886 kinase, candidate proline-directed kinases were subjected to immunoprecipitation using antibodies against multiple MAPKs and Cdks. Site-specific phosphorylation of Ser886 was measured by in vitro phosphorylation of recombinant S999A linker, which permits phosphorylation of the upstream site only. Robust phosphorylation of Ser886 by immunoprecipitated Cdk5 and lesser activity of ERK2 and Cdk1 were detected (Fig. 1B).
Candidate kinases were investigated by siRNA-mediated knockdown and metabolic labeling of EPRS linker targets mutated to permit single-site phosphorylation. In cells transfected with control siRNA, both single-site targets incorporated approximately half of the 32P compared with WT (Fig. 1C). Knockdown of ERK2 or Cdk5 blocked phosphorylation at both sites, but knockdown of Cdk1 was ineffective. The inhibited phosphorylation of both Ser886 and Ser999 suggests that ERK2 and Cdk5 are upstream activators of the AGC group kinase that phosphorylates Ser999, consistent with the results of previous experiments with pharmacologic inhibitors (9). The roles of ERK2 and Cdk5 in EPRS phosphorylation were verified by probing endogenous protein with phosphospecific EPRS antibodies. siRNA targeting ERK2 and Cdk5, but not Cdk1, significantly inhibited both Ser886 and Ser999 phosphorylation (Fig. 1D). Identical results were obtained using primary human peripheral blood monocytes (PBMs) (Fig. S1).
To identify the proximal Ser886 kinase, recombinant ERK2 and Cdk5 were allowed to in vitro phosphorylate EPRS linker mutated to permit phosphorylation of Ser886 or Ser999. Cdk5 preactivated with p35 activator protein phosphorylated EPRS at Ser886, suggesting that Cdk5 is the proximal kinase (Fig. 1E). Cdk5 did not phosphorylate Ser999, consistent with its role as an upstream activator of a distinct kinase. ERK2 did not phosphorylate either site, indicating that it is upstream of Cdk5. The phosphorylation of p35 by Cdk5 has been reported previously (14). To show that cellular Cdk5 phosphorylates Ser886, the kinase was immunoprecipitated from IFN-γ–activated U937 cell lysates and used to in vitro phosphorylate the linker target. The immunoprecipitate and supernatant phosphorylated histone H1, a general Cdk substrate (15) (Fig. 1F); however, only Cdk5-immunoprecipitate phosphorylated Ser886-containing linker, to an extent comparable to the total activity in IFN-γ–activated cell lysate. These results provide compelling evidence that Cdk5 is the proximal kinase responsible for IFN-γ–stimulated EPRS phosphorylation at Ser886.
Cdk5 Activation by p35 Is Essential for IFN-γ–Stimulated EPRS Phosphorylation and Translation Inhibition.
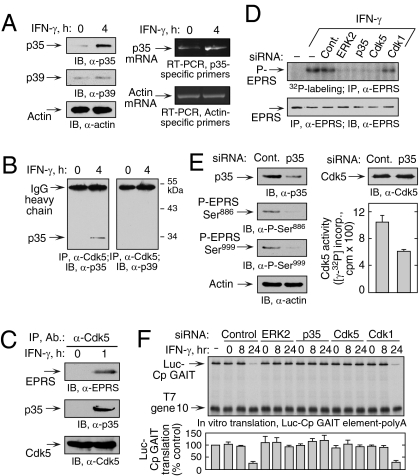
Cdk5 is a ubiquitously expressed, essential prodirected Ser/Thr kinase of the Cdk family of kinases (16, 17). Cdk5 activity is restricted primarily to neurons due to cell type–specific expression of its requisite binding partners, Cdk5 regulatory subunits 1 (Cdk5R1) and 2 (Cdk5R2), commonly referred to as p35 and p39 activator proteins, respectively (18, 19). However, recent studies have found p35 and p39 expression in nonneuronal cells, indicating a role for Cdk5 outside the nervous system (20). Double p35/p39 knockout mice exhibit a phenotype identical to Cdk5-deficient mice, suggesting that p35 and p39 are the principal Cdk5 activators (21). Immunoblot analysis revealed low-level expression of p35 and p39 in U937 cells, but only p35 protein and mRNA were induced by treatment with IFN-γ (Fig. 2A). IFN-γ induced a specific interaction of Cdk5 with p35, but not with p39 (Fig. 2B). Coimmunoprecipitation of lysate from cells treated with IFN-γ for 1 h, a time period in which only Ser886 is phosphorylated (9), revealed interactions of Cdk5 with EPRS and p35 (Fig. 2C). Furthermore, siRNA-mediated knockdown of p35 inhibited IFN-γ–induced phosphorylation of endogenous EPRS as determined by 32P-labeling (Fig. 2D) and by phosphospecific antibody (Fig. 2E, Left). Knockdown of p35 reduced Cdk5 activity, confirming p35’s role in Cdk5 activation (Fig. 2E, Right). The functional requirement for constituents of the Cdk5 activation pathway was investigated. U937 cells were activated with IFN-γ for 8 or 24 h, and cytosolic lysates were tested for their inhibition of in vitro translation of luciferase cDNA upstream of the ceruloplasmin (Cp) 3′ UTR GAIT element (Luc-Cp GAIT). As shown previously, 24-h lysates specifically suppressed translation of the reporter but not of the T7 gene 10 control transcript lacking the GAIT element (Fig. 2F). Pretreatment of cells with siRNA targeting p35, Cdk5, or ERK2 (but not Cdk1) overcame the inhibition by active 24-h lysates. These results demonstrate that the Cdk5/p35 complex is essential for the initial phosphorylation of Ser886, subsequent phosphorylation of Ser999, and translational suppression activity of the GAIT complex.
Fig. 2.
Induction of p35 activator protein by IFN-γ is required for Ser886 phosphorylation by Cdk5. (A) IFN-γ induces p35 activator protein. (Left) Lysates from U937 cells treated with IFN-γ for 4 h were immunoblotted. (Right) p35 and β-actin mRNA was determined by RT-PCR of total cellular RNA. (B) IFN-γ induces Cdk5 binding to p35. Lysates from U937 cells treated with IFN-γ for 4 h were coimmunoprecipitated with anti-Cdk5 antibody and probed with anti-p35 (Left) and anti-p39 (Right) antibodies. (C) Cdk5/p35 phosphorylates EPRS at Ser886. Lysates from U937 cells treated with IFN-γ for 1 h were immunoprecipitated and immunoblotted as shown. (D) Knockdown of p35 blocks EPRS phosphorylation. (Upper) siRNAs were transfected into U937 cells and incubated with 32P-orthophosphate and IFN-γ for 4 h. Lysates were immunoprecipitated with anti-EPRS antibody, and EPRS phosphorylation was determined by autoradiography. (Lower) Total EPRS was detected with anti-EPRS antibody. (E) Knockdown of p35 activator protein blocks two-site EPRS phosphorylation. U937 cells were transfected with siRNA against p35 and then treated with IFN-γ as in Fig. 1D. (Left) Knockdown efficiency and EPRS phosphorylation were determined by immunoblot analysis.(Right) Lysates were incubated with anti-Cdk5 antibody, and precipitates were used for in vitro phosphorylation of Cdk5-specific peptide substrate. Aliquots were spotted on phosphocellulose P-81, and the incorporation of 32P into peptide was determined by scintillation counting (mean ± SEM; n = 3 experiments). (F) ERK2, p35, and Cdk5 are required for GAIT complex–mediated translational suppression. U937 cells were transfected with the siRNAs shown and incubated with IFN-γ for up to 24 h. Lysates were added to RRL for in vitro translation of capped, polyadenylated luciferase (Luc) reporter transcript bearing the Cp GAIT element in the presence of 35S-Met. T7 gene 10 RNA lacking the GAIT element was cotranslated as a specificity control. Luc translation was quantified by densitometry, normalized by T7 gene 10, and expressed as percentage of control (mean ± SEM; n = 3 experiments).
Induction of p35 by IFN-γ Activates Cdk5 and EPRS Phosphorylation in Myeloid Cells.
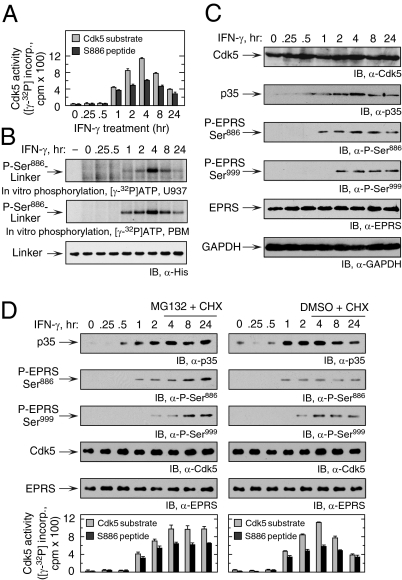
We investigated the regulation of Cdk5 activity and its temporal relationship to EPRS phosphorylation by in vitro phosphorylation of Cdk5-specific peptide substrate, or a peptide containing the Ser886 site, after immunoprecipitation of lysates with anti-Cdk5 antibody (9). Phosphorylation of both peptide substrates was observed after a 1-h lag, with maximal activity seen at 4 h, followed by a gradual decline up to 24 h (Fig. 3A). Cdk5 immunoprecipitated from maximally active cell lysate robustly phosphorylated Cdk5 and EPRS Ser886 peptide substrates, but not S999 peptide (Fig. S2). A similar temporal phosphorylation pattern was observed for lysates from IFN-γ–activated U937 cells and PBMs used to phosphorylate EPRS linker containing only the Ser886 phosphorylation site (S999A) (Fig. 3B) or Ser886 peptide substrate (Fig. S3).
Fig. 3.
Temporal correspondence of IFN-γ–stimulated p35 expression, Cdk5 activation, and EPRS phosphorylation. (A) IFN-γ–mediated induction of Cdk5 activity is maximal at 4 h. Lysates from U937 cells treated with IFN-γ for up to 24 h were incubated with anti-Cdk5 antibody, and the precipitates were used for in vitro phosphorylation of Cdk5-specific (gray bars) or Ser886-containing EPRS (black bars) peptide substrates (mean ± SEM; n = 3 experiments). (B) Lysates of IFN-γ–treated U937 cells (Top) or PBMs (Middle) were used for in vitro phosphorylation of recombinant His-tagged EPRS linker containing a Ser999-to-Ala mutation that permits only Ser886 phosphorylation. Immunoblot analysis with anti-His antibody served as a loading control (Bottom). (C) IFN-γ induces p35 expression. Lysates from IFN-γ–treated U937 cells were immunoblotted as shown. (D) IFN-γ–induced p35 expression is down-regulated by proteasomal degradation. U937 cells were pretreated with IFN-γ for 4 h and then with IFN-γ, MG132 (10 μM), and cycloheximide (CHX; 30 μg/mL) for up to 24 h (Left). Control cells were treated with DMSO and CHX (Right). Lysate expression of p35, Cdk5, and phosphorylated and total EPRS was determined by immunoblot analysis. Cdk5 activity was determined in lysates after precipitation with anti-Cdk5 antibody as described in Fig. 2E.
Cdk5 expression was not substantially altered after IFN-γ treatment for up to 24 h in U937 cells (Fig. 3C) and human PBMs (Fig. S4). In contrast, p35 was induced with kinetics closely matching those of Ser886 phosphorylation of EPRS linker. This finding was expected, because Cdk5 activity is generally determined by transcriptional or posttranscriptional regulation of p35 (18, 19). In some cases, Cdk5 is induced by a signaling pathway driven by ERK1/2 and Egr-1 (22, 23). Induction of p35 by IFN-γ during neuronal differentiation also requires ERK1/2 and Egr-1 (24). Phosphorylation by Cdk5 targets p35 to polyubiquitination and degradation, forming a negative feedback pathway that represses Cdk5 activity (14). We investigated the role of proteasomal degradation in regulating p35 expression. p35 was determined in IFN-γ–treated U937 cells in the presence of cycloheximide to block protein synthesis and MG-132 to inhibit proteasome activity. MG-132 prevented the decline of p35 protein observed after 4 h, modestly increased EPRS phosphorylation, and sustained Cdk5 activity for 24 h, consistent with tight control of Cdk5 activity by a negative-feedback loop with p35 (Fig. 3D).
Cdk5-Mediated Ser886 Phosphorylation Regulates EPRS Release from MSC and RNA- and Protein-Binding Activities.
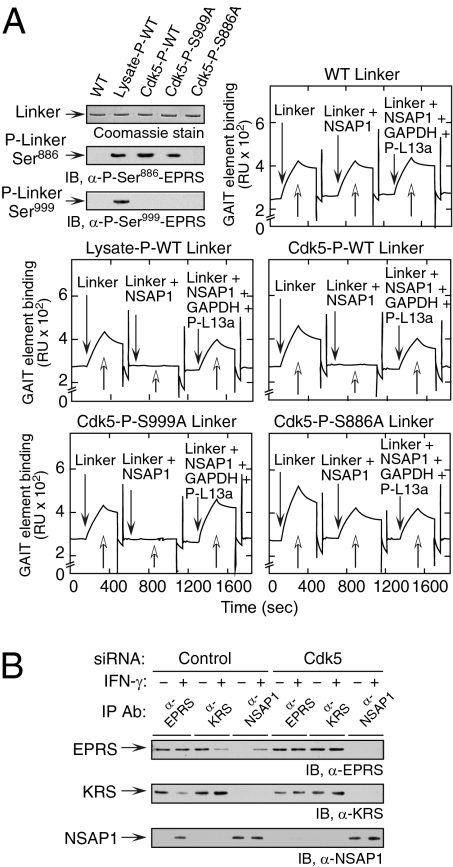
EPRS is responsible for GAIT complex recognition and binding to GAIT element–bearing mRNA (8). NSAP1 binding to Ser886-phosphorylated EPRS negatively regulates this binding, which is subsequently restored by binding of GAPDH and phosphorylated L13a (9, 13). To determine whether Cdk5/p35-mediated phosphorylation regulates EPRS binding to GAIT mRNA, we performed surface plasmon resonance (SPR) using immobilized, biotinylated Cp GAIT RNA. Purified, His-tagged EPRS linker was phosphorylated at Ser886 by incubation with lysate from IFN-γ–activated cells or with Cdk5/p35, and verified with phosphospecific antibodies (Fig. 4A, Top, Left). Unmodified, WT linker efficiently bound GAIT RNA, and binding was not affected by preincubation with NSAP1 or with NSAP1, GAPDH, and P-L13a (Fig. 4A, Top, Right). As shown previously, the RNA-binding activity of linker phosphorylated by cell lysate was completely blocked by preincubation with NSAP1, and the binding was restored on the addition of GAPDH and P-L13a (Fig. 4A, Middle, Left). WT (Fig. 4A, Middle, Right) and S999A mutant linker (Fig. 4A, Bottom, Left) phosphorylated with Cdk5/p35 exhibited binding activities identical to those of WT linker phosphorylated with lysate. In contrast, Cdk5/p35-mediated phosphorylation of S888A linker exhibited binding activity comparable to that of unmodified linker (Fig. 4A, Bottom, Right). These results confirm the regulation of RNA- and protein-binding activities of EPRS by Cdk5/p35-mediated phosphorylation at Ser886.
Fig. 4.
Cdk5-mediated EPRS phosphorylation is required for the release of EPRS from the MSC and for binding to GAIT element RNA. (A) EPRS linker phosphorylated at Ser886 by Cdk5 reconstitutes the GAIT complex assembly and binding to GAIT element RNA. His-tagged, WT, and mutant (S886A and S999A) EPRS linker proteins were phosphorylated by incubation with lysate from IFN-γ–treated U937 cells or with recombinant Cdk5/p35, and then repurified using Ni-affinity resin (Top, Left). Specific phosphorylation was confirmed using anti-phosphospecific antibodies. Unmodified (Top, Right), lysate-phosphorylated (Middle, Left), or Cdk5-phosphorylated WT (Middle, Right), S999A (Bottom, Left), and S886A (Bottom, Right) EPRS linkers were preincubated with NSAP1 or with NSAP1, GAPDH, and phospho-L13a (P-L13a), and binding to biotinylated Cp GAIT element RNA immobilized on a streptavidin sensor chip was determined by SPR and expressed as resonance units (RU). (B) Cdk5-mediated phosphorylation induces EPRS release from MSC. U937 cells were transfected with siRNA targeting Cdk5 (and control siRNA) and then treated with IFN-γ for 4 h. Lysates were immunoprecipitated with antibodies against EPRS, LysRS (KRS, MSC constituent), and NSAP1 (pre-GAIT complex constituent), and evaluated by immunoblot analysis.
Both Ser886 and Ser999 phosphorylation events are required for EPRS escape from the MSC (9). The role of Cdk5/p35 in the release of EPRS from the MSC was assessed by immunoprecipitation with antibodies against a MSC constituent, LysRS (KRS), and the pre-GAIT complex protein NSAP1. The induction of p35 protein ∼1–2 h after IFN-γ treatment coincided not only with Cdk5 activation and EPRS phosphorylation, but also with EPRS translocation from the MSC to the pre-GAIT complex (Fig. S5). The role of Cdk5/p35 in EPRS translocation was tested directly by knockdown. In untreated control cells, IFN-γ induced translocation of about half of the EPRS from the MSC to the pre-GAIT complex, as reported previously (8) (Fig. 4B). However, after Cdk5 knockdown, translocation of EPRS was completely blocked, verifying the critical role of Cdk5.
Cdk5-Mediated Phosphorylation of EPRS Regulates VEGF-A Expression.
We investigated the effect of Cdk5 knockdown on expression of an established GAIT target, VEGF-A (11). In untreated cells, VEGFA mRNA and protein expression was induced after 8 h of IFN-γ treatment; however, protein expression was significantly repressed after 24 h despite abundant VEGFA mRNA (Fig. 5A), as reported previously (11, 25). Knockdown of Cdk5, but not of Cdk1, overcame the suppression of VEGF-A expression at 24 h, confirming the specific function of Cdk5 in GAIT pathway activation. Expression of double-phosphomimetic (S886D/S999D) EPRS linker in Cdk5 knockdown cells reconstituted GAIT-mediated inhibition of VEGF-A, thereby establishing the specific role of Cdk5-mediated phosphorylation of EPRS in gene expression (Fig. 5B).
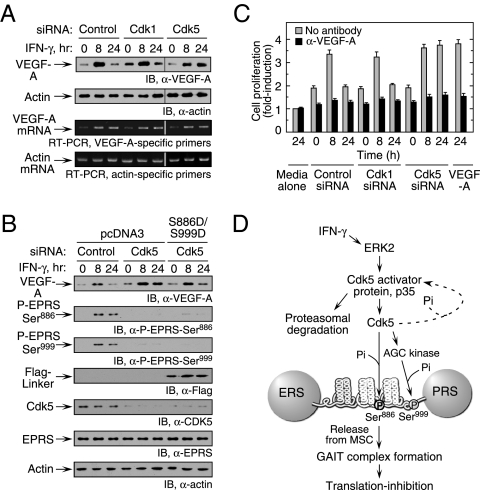
Fig. 5.
Cdk5-mediated EPRS phosphorylation regulates VEGF-A expression. (A) Inhibition of Cdk5 expression enhances VEGF-A expression. U937 cells were transfected with siRNA as shown and then incubated with IFN-γ for up to 24 h. (Upper) Lysates were probed with anti–VEGF-A or actin antibodies. (Lower) RT-PCR analysis of total cellular RNA was performed using gene-specific primers. (B) Expression of phosphomimetic EPRS linker in Cdk5 knockdown cells restores inhibition of VEGF-A expression. U937 cells cotransfected with siRNAs and Flag-tagged, double-phosphomimetic EPRS linker (or vector control) were incubated with IFN-γ for up to 24 h. VEGF-A, Cdk5, endogenous EPRS phosphorylation, Flag-tagged EPRS linker, and actin loading control were determined by immunoblot analysis. (C). U937 cell Cdk5 activity influences VEGF-A–directed EC proliferation. Conditioned medium from siRNA-transfected and IFN-γ–treated U937 cells was added to bovine aortic ECs. ECs treated with recombinant VEGF-A (10 ng/mL) served as a positive control. Proliferation was determined by an MTT assay and expressed as fold induction compared with ECs treated with medium alone (gray bars). The activity of conditioned medium preincubated with anti–VEGF-A antibody was assessed as well (black bars). Values shown are mean ± SEM (n = 3 experiments in triplicate). (D) Schematic of IFN-γ–induced signaling events that activate Cdk5/p35 to induce EPRS phosphorylation and suppress gene expression by translation control.
To investigate the downstream consequence of Cdk5-mediated EPRS phosphorylation, we assessed its role in endothelial cell (EC) proliferation, a major proangiogenic activity of VEGF-A. Subconfluent ECs were incubated with medium conditioned by siRNA-transfected and IFN-γ–treated U937 cells. Conditioned medium from cells treated with siRNA targeting Cdk1 (or control) and IFN-γ for 8 h increased EC proliferation compared with 0-h controls (Fig. 5C). Proliferation was reduced to basal levels after the addition of medium conditioned by cells treated with IFN-γ for 24 h due to the GAIT-mediated suppression of VEGF-A expression. However, 24-h conditioned medium from Cdk5 knockdown cells failed to reduce EC proliferation, as would be expected when the GAIT pathway is blocked. In all cases, stimulatory activity was neutralized by anti–VEGF-A antibody, confirming the role of VEGF-A. Taken together, these results show that Cdk5-mediated EPRS phosphorylation is required for activation of the GAIT system and for posttranscriptional suppression of VEGF-A expression.
Discussion
We have shown that IFN-γ activates Cdk5 via a pathway that includes ERK2 and p35 and that initiates two-site phosphorylation of EPRS and translational control by the GAIT system (Fig. 5D). Cdk5 activity is tightly regulated by p35 expression in monocytic cells. Temporal expression of Cdk5 activity and Ser886 phosphorylation closely parallel the induction of p35 and is maximal at about 4 h. The activity of Cdk5 declines gradually, again paralleling the decline in p35 expression due to proteasomal degradation, forming a negative-feedback loop to restrict Cdk5 activity. This activity of Cdk5 and its activator protein in monocytic cells reveals a unique function that regulates the noncanonical function of a tRNA synthetase to control translation of a proinflammatory posttranscriptional regulon.
Mutation or dysregulation of several AARSs has been linked to disease; however, no such associations have been described for EPRS (26, 27). Charcot–Marie–Tooth disease has been linked to heritable mutations in GlyRS and TyrRS that generally do not affect aminoacylation activity (28, 29). Likewise, LysRS is associated with amyotrophic lateral sclerosis through a mechanism unrelated to its canonical function (30). Whether defective phosphorylation of any of these AARSs plays a role in disease etiology is unclear. However, MAPK-dependent phosphorylation of LysRS is known to modulate the immune response by regulating gene expression (7), and LysRS triggers proinflammatory responses in macrophages (31). Genetic defects in two GAIT system components, GAPDH and death-associated protein kinase (the kinase that phosphorylates L13a), have been implicated in progression of chronic inflammatory conditions (12, 32, 33). Our findings suggest that condition-dependent conformational alterations, or heritable mutations in EPRS that influence the susceptibility to phosphorylation by Cdk5/p35, might negatively influence the translational suppression of proinflammatory gene expression.
Despite sequence and structural similarities to other Cdk kinases, Cdk5 is an anomalous Cdk family member because it plays only a minor role in cell cycle regulation (18). The atypical nature of Cdk5 is also evidenced by its dependence on p35 and p39 as activator proteins instead of the usual cyclin (19). Cdk5 is highly expressed in the nervous system and is indispensable for neural development and function. In addition, Cdk5 is implicated in activities in nonneuronal cells, including exocytosis, apoptosis, wound healing, gene transcription, myocyte senescence, glucose-stimulated insulin secretion in pancreatic β cells, and hematopoetic cell differentiation (20). Our identification of EPRS as a physiological substrate of Cdk5/p35 reveals a unique facet of the kinase in regulating inflammatory gene expression by a transcript-selective translational control mechanism.
Dysregulation of Cdk5/p35 causes neuronal and extraneuronal pathologies in inflammation-related disorders. Defects in Cdk5 and its putative inhibitor, CDKAL1, are linked to type 2 diabetes and Alzheimer's disease (34, 35). Glucose-induced inactivation of Cdk5/p35 in pancreatic β cells is implicated in glucotoxicity and diabetes (36, 37). Likewise, conditional deletion of Cdk5 in the developing forebrain causes macrophage activation and neurodegeneration (38). Single nucleotide polymorphisms in the CDK5 gene promoter contribute to lung cancer risk (39). Ectopic expression of Cdk5/p35 in undifferentiated human myeloid leukemia cells results in their differentiation, identifying them as targets for the treatment of promyeloblastic leukemia (23, 40). Previous studies did not illuminate the molecular mechanisms through which Cdk5/p35 dysregulation influences inflammation, however. Our results reveal a pathway through which Cdk5/p35 modulates the myeloid inflammatory response. Stimulus-dependent activation of Cdk5/p35 by IFN-γ is a critical early event in the activation of EPRS for formation of the GAIT complex. Cdk5/p35 participates in the delayed translational suppression of proinflammatory genes and can be considered a critical regulatory component of the GAIT-mediated “resolution of inflammation” pathway. Pathophysiologic interruption of the Cdk5/p35 activation pathway would be expected to exacerbate the inflammatory response of myeloid cells. Alternatively, because chemical activation of the pathway might reduce inflammation, Cdk5/p35 represents a potential target for new anti-inflammatory therapies.
Materials and Methods
Antibodies, Reagents, and Cell Culture.
Antibodies against EPRS, L13a, and phospho-EPRS-Ser886 and -Ser999 sites were obtained as described previously (9, 11). Recombinant Cdk5/p35, ERK2, and histone H1 were obtained from Upstate/Millipore. siRNAs targeting Cdk1, Cdk5, and ERK2 were obtained from Dharmacon, and those targeting p35 were obtained from Santa Cruz Biotechnology. Human U937 monocytic cells (American Type Culture Collection) and PBMs were cultured in RPMI medium 1640 supplemented with 10% FBS, and then treated with IFN-γ (8, 11). Bovine aortic ECs were cultured in Ham's F-12/DME (1:1) medium with 5% FBS. Cell lysates were prepared with Phosphosafe extraction buffer (Novagen) containing protease inhibitors.
RNA Interference and 32P Labeling.
For kinase or p35 knockdown, U937 cells were transiently transfected with siRNAs using the Amaxa Nucleofector V Kit. Cells were transferred to Opti-MEM medium (Invitrogen) for 6 h, and then allowed to recover for 18–24 h in RPMI medium 1640 supplemented with 10% FBS before IFN-γ treatment. For cotransfection, Flag-tagged EPRS linker (WT or mutant) plasmid DNA was nucleofected with siRNA. For 32P labeling, [32P]orthophosphate (300 μci; Perkin-Elmer) was added in the presence of IFN-γ for 4 h in phosphate-free RPMI 1640 medium. Labeled cells were lysed in Phosphosafe buffer and immunoprecipitated with anti-Flag antibody–conjugated agarose (Sigma-Aldrich) or with anti-EPRS antibody conjugated to protein A–Sepharose beads (Sigma-Aldrich).
In Vitro Translation.
Capped, poly(A)-tailed Luc-Cp GAIT and T7 gene 10 template RNAs were prepared using the mMessage mMachine (Ambion). RNAs were translated in rabbit reticulocyte lysate (Promega) in the presence of Met-free amino acids and [35S]Met (8, 13). Cell lysates were added to the translation reaction mixture, which was resolved on 10% SDS/PAGE and radiolabel-detected by autoradiography.
Protein–Protein Interaction by Coimmunoprecipitation and SPR.
Cell lysates (1 mg) were coimmunoprecipitated with specific antibody in detergent-free immunoprecipitation buffer as described previously (9). Binding of EPRS linker proteins to immobilized, biotinylated 29-nt Cp GAIT element RNA (Dharmacon) was determined using a Biacore 3000 SPR system (9, 13).
In Vitro Phosphorylation and Cdk5 Kinase Assays.
Purified, His-tagged EPRS linker protein was phosphorylated in vitro by incubation with cell lysate or with recombinant active kinases (Cdk5/p35 or ERK2) and 5 μci of [γ-32P]ATP (Perkin-Elmer) (9). For the immunocomplex kinase assay, precleared lysate (500 μg) was incubated with antibody against proline-directed kinases. The immunocomplex was captured with protein A–Sepharose beads, washed, suspended in kinase assay buffer [50 mM Tris-HCl (pH 7.6), 1 mM DTT, 10 mM MgCl2, 1 mM CaCl2, and phosphatase inhibitor mixture], and used for in vitro phosphorylation of S999A EPRS linker. For Cdk5 activity assay, lysates were incubated with anti-Cdk5 antibody, and the precipitate was suspended in kinase assay buffer. Precipitates were used for phosphorylation of Cdk5-specific peptide (PKTPKKAKKL) or Ser886 (QRRDR886SPTRNREPA)- or Ser999 (QRGGLH999SSGAGEGQ)-containing EPRS peptide substrates by [γ-32P]ATP as above. Incorporation of 32P into peptide was determined by spotting on phosphocellulose P-81 paper and scintillation counting.
EC Proliferation.
EC proliferation was measured in the presence of U937 cell–conditioned medium essentially as described previously (11). siRNA-transfected U937 cells were treated with IFN-γ for up to 24 h, collected, washed, and incubated in serum-free RPMI medium for 2 h. The conditioned medium was concentrated with a 5-kDa cutoff filter (Millipore). Bovine aortic ECs at 50% confluence were incubated in serum-free Ham's F-12/DME (1:1) medium for 18 h before the addition of conditioned medium for 24 h. Proliferation was measured with an MTT assay (Promega). To determine the role of VEGF-A, conditioned medium was preincubated with anti–VEGF-A antibody (2 μg/mL for 1 h).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HL029582, R01 GM086430, and P01 HL076491 (to P.L.F.), by American Heart Association Ohio Valley Affiliate Postdoctoral Fellowships (to A.A. and J.J.), and by an American Heart Association National Center Scientist Development grant (to A.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011275108/-/DCSupplemental.
References
- 1.Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 2.Ribas de Pouplana L, Schimmel P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem Sci. 2001;26:591–596. doi: 10.1016/s0968-0004(01)01932-6. [DOI] [PubMed] [Google Scholar]
- 3.Guo M, Schimmel P, Yang XL. Functional expansion of human tRNA synthetases achieved by structural inventions. FEBS Lett. 2010;584:434–442. doi: 10.1016/j.febslet.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SW, Cho BH, Park SG, Kim S. Aminoacyl-tRNA synthetase complexes: Beyond translation. J Cell Sci. 2004;117:3725–3734. doi: 10.1242/jcs.01342. [DOI] [PubMed] [Google Scholar]
- 5.Clemens MJ. Does protein phosphorylation play a role in translational control by eukaryotic aminoacyl-tRNA synthetases? Trends Biochem Sci. 1990;15:172–175. doi: 10.1016/0968-0004(90)90153-3. [DOI] [PubMed] [Google Scholar]
- 6.Dang CV, Traugh JA. Phosphorylation of threonyl- and seryl-tRNA synthetase by cAMP-dependent protein kinase: A possible role in the regulation of P1, P4-bis(5′-adenosyl)-tetraphosphate (Ap4A) synthesis. J Biol Chem. 1989;264:5861–5865. [PubMed] [Google Scholar]
- 7.Yannay-Cohen N, et al. LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol Cell. 2009;34:603–611. doi: 10.1016/j.molcel.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Sampath P, et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: Gene-specific silencing of translation. Cell. 2004;119:195–208. doi: 10.1016/j.cell.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 9.Arif A, et al. Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol Cell. 2009;35:164–180. doi: 10.1016/j.molcel.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray PS, Arif A, Fox PL. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem Sci. 2007;32:158–164. doi: 10.1016/j.tibs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ray PS, Fox PL. A post-transcriptional pathway represses monocyte VEGF-A expression and angiogenic activity. EMBO J. 2007;26:3360–3372. doi: 10.1038/sj.emboj.7601774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: A gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–331. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-Prolyl tRNA synthetase in translational control of gene expression. Mol Cell. 2008;29:679–690. doi: 10.1016/j.molcel.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrick GN, Zhou P, Kwon YT, Howley PM, Tsai LH. p35, the neuronal-specific activator of cyclin-dependent kinase 5 (Cdk5), is degraded by the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:24057–24064. doi: 10.1074/jbc.273.37.24057. [DOI] [PubMed] [Google Scholar]
- 15.Kitzmann M, et al. cdk1- and cdk2-mediated phosphorylation of MyoD Ser200 in growing C2 myoblasts: Role in modulating MyoD half-life and myogenic activity. Mol Cell Biol. 1999;19:3167–3176. doi: 10.1128/mcb.19.4.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai LH, Takahashi T, Caviness VS, Jr., Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- 17.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 19.Lalioti V, Pulido D, Sandoval IV. Cdk5, the multifunctional surveyor. Cell Cycle. 2010;9:284–311. doi: 10.4161/cc.9.2.10466. [DOI] [PubMed] [Google Scholar]
- 20.Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. Bioessays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- 21.Ko J, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 23.Chen F, Wang Q, Wang X, Studzinski GP. Up-regulation of Egr1 by 1,25-dihydroxyvitamin D3 contributes to increased expression of p35 activator of cyclin-dependent kinase 5 and consequent onset of the terminal phase of HL60 cell differentiation. Cancer Res. 2004;64:5425–5433. doi: 10.1158/0008-5472.CAN-04-0806. [DOI] [PubMed] [Google Scholar]
- 24.Song JH, et al. Interferon-γ induces neurite outgrowth by up-regulation of p35 neuron-specific cyclin-dependent kinase 5 activator via activation of the ERK1/2 pathway. J Biol Chem. 2005;280:12896–12901. doi: 10.1074/jbc.M412139200. [DOI] [PubMed] [Google Scholar]
- 25.Ray PS, et al. A stress-responsive RNA switch regulates VEGFA expression. Nature. 2009;457:915–919. doi: 10.1038/nature07598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonellis A, Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet. 2008;9:87–107. doi: 10.1146/annurev.genom.9.081307.164204. [DOI] [PubMed] [Google Scholar]
- 27.Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci USA. 2008;105:11043–11049. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonellis A, et al. Glycyl tRNA synthetase mutations in Charcot-Marie-Tooth disease type 2D and distal spinal muscular atrophy type V. Am J Hum Genet. 2003;72:1293–1299. doi: 10.1086/375039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordanova A, et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet. 2006;38:197–202. doi: 10.1038/ng1727. [DOI] [PubMed] [Google Scholar]
- 30.Kunst CB, Mezey E, Brownstein MJ, Patterson D. Mutations in SOD1 associated with amyotrophic lateral sclerosis cause novel protein interactions. Nat Genet. 1997;15:91–94. doi: 10.1038/ng0197-91. [DOI] [PubMed] [Google Scholar]
- 31.Park SG, et al. Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc Natl Acad Sci USA. 2005;102:6356–6361. doi: 10.1073/pnas.0500226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. Association of late-onset Alzheimer's disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci USA. 2004;101:15688–15693. doi: 10.1073/pnas.0403535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, et al. DAPK1 variants are associated with Alzheimer's disease and allele-specific expression. Hum Mol Genet. 2006;15:2560–2568. doi: 10.1093/hmg/ddl178. [DOI] [PubMed] [Google Scholar]
- 34.Lee KY, et al. Elevated neuronal Cdc2-like kinase activity in the Alzheimer disease brain. Neurosci Res. 1999;34:21–29. doi: 10.1016/s0168-0102(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 35.Saxena R, et al. Diabetes Genetics Initiative of Broad Institute of Harvard and MIT, Lund University, and Novartis Institutes of BioMedical Research. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 36.Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer's disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology. 2004;145:3023–3031. doi: 10.1210/en.2003-1522. [DOI] [PubMed] [Google Scholar]
- 37.Wei FY, et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat Med. 2005;11:1104–1108. doi: 10.1038/nm1299. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, et al. Conditional deletion of neuronal cyclin-dependent kinase 5 in developing forebrain results in microglial activation and neurodegeneration. Am J Pathol. 2010;176:320–329. doi: 10.2353/ajpath.2010.081158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi HS, et al. Single-nucleotide polymorphisms in the promoter of the CDK5 gene and lung cancer risk in a Korean population. J Hum Genet. 2009;54:298–303. doi: 10.1038/jhg.2009.29. [DOI] [PubMed] [Google Scholar]
- 40.Chen F, Studzinski GP. Expression of the neuronal cyclin-dependent kinase 5 activator p35Nck5a in human monocytic cells is associated with differentiation. Blood. 2001;97:3763–3767. doi: 10.1182/blood.v97.12.3763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.