Abstract
Pathogenic Yersinia species suppress the host immune response by using a plasmid-encoded type III secretion system (T3SS) to translocate virulence proteins into the cytosol of the target cells. T3SS-dependent protein translocation is believed to occur in one step from the bacterial cytosol to the target-cell cytoplasm through a conduit created by the T3SS upon target cell contact. Here, we report that T3SS substrates on the surface of Yersinia pseudotuberculosis are translocated into target cells. Upon host cell contact, purified YopH coated on Y. pseudotuberculosis was specifically and rapidly translocated across the target-cell membrane, which led to a physiological response in the infected cell. In addition, translocation of externally added YopH required a functional T3SS and a specific translocation domain in the effector protein. Efficient, T3SS-dependent translocation of purified YopH added in vitro was also observed when using coated Salmonella typhimurium strains, which implies that T3SS-mediated translocation of extracellular effector proteins is conserved among T3SS-dependent pathogens. Our results demonstrate that polarized T3SS-dependent translocation of proteins can be achieved through an intermediate extracellular step that can be reconstituted in vitro. These results indicate that translocation can occur by a different mechanism from the assumed single-step conduit model.
Keywords: bacterial pathogenesis, Yop effector, Ca2+-signaling, neutrophil
Many enteropathogenic bacteria harbor common type III secretion systems (T3SSs) that are essential for virulence. These systems enable productive interactions between the bacteria and the eukaryotic target cells by translocation of bacterial virulence proteins into the cytosol of the host cells (1–3). Depending on the lifestyle of the pathogen, the translocated effector proteins interfere with different signaling and regulatory pathways of the host innate immune defense, which tips the balance in favor of the bacteria (3, 4).
Pathogenic Yersinia species use a plasmid-encoded T3SS to translocate virulence proteins into the cytosol of target cells, and thereby suppress the host immune response. The translocated effectors are called Yersinia outer proteins (Yops) (5), and they block uptake by professional phagocytes, which allows extracellular replication of the bacteria in lymphatic tissue (6, 7). It has been suggested that upon intimate contact between Yersinia and the target cell (2, 8), the Yop effectors are secreted and translocated in a single step from the bacterial cytosol to the target-cell cytoplasm through a continuous channel built up by the ysc/yop T3SS (2, 3, 9). In Yersinia, two secreted T3SS substrates, YopB and YopD, have been shown to be involved in the translocation of effector proteins into the target cell and these proteins are defined as translocators to distinguish them from the effector class of Yops that have a more direct effect on eukaryotic cell function (3). Both YopB and YopD have hydrophobic domains (10) and can be inserted into the membranes of erythrocytes and nucleated cells, where they form pores (11, 12). It is assumed that binding of Yersinia to a target cell triggers secretion and insertion of these proteins into the target-cell plasma membrane, completing the conduit between the bacterium and the target cell through the T3SS needle complex. The Yop effectors, in turn, are believed to be transferred into the host-cell cytoplasm through this pore during the translocation step (13). Investigators using different methods have previously demonstrated the occurrence of polarized, T3SS-dependent translocation of proteins into target cells (2, 14–16), but the detailed mechanisms underlying that process are still largely unknown.
In the present study, we investigated the mechanisms leading to T3SS-dependent protein translocation and succeeded in establishing conditions that allow effector translocation in vitro. Here, we demonstrate that effector proteins associated with the surface of bacterial cells can be translocated in a T3SS-dependent manner. However, our results do not exclude that T3SS-mediated protein translocation also occurs by the currently accepted conduit model of translocation, which was originally suggested by our laboratory (2).
Results
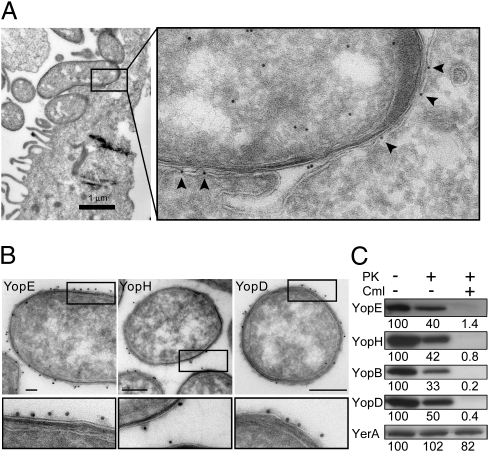
In an attempt to visualize Yop translocation, we performed immunoelectron microscopy of HeLa cells infected with Yersinia pseudotuberculosis to analyze the spatial localization of effectors upon host-cell interaction. In these experiments, the effector protein YopE was evenly distributed in the bacterium-target cell interface and did not occur in distinct foci or associated with clear macromolecular structures (Fig. 1A). The YopE in the bacterium-target cell interface was detected primarily within or on the inside of the plasma membrane of the HeLa cell (arrowheads in Fig. 1A), suggesting that the protein had been translocated into the target cell. Furthermore, the outer membrane of the bacterium appeared to be in close contact with the HeLa cell plasma membrane, but this did not involve any bridging structures. These observations were interesting but surprising, because we had expected to find some spatial organization or clustering of YopE in Y. pseudotuberculosis that were actively translocating Yops, either within the bacterial cell or within the contact zone between the bacterium and the target cell.
Fig. 1.
YopE is evenly distributed in the bacterium-target cell interface during infection of HeLa cells and Yops are found on the surface of Y. pseudotuberculosis before target cell contact. (A) Immunoelectron micrograph showing localization of YopE in Y. pseudotuberculosis during infection of a HeLa cell. Arrowheads indicate YopE detected on the surface of or within the plasma membrane of the target cell. (B) Immunoelectron micrographs showing surface localization of YopE, YopH, and YopD on Y. pseudotuberculosis before target-cell contact. (Scale bars, 100 nm for YopE and YopH; 200 nm for YopD.) (C) Western blot analysis of YopE, YopH, YopB, YopD, and the cytoplasmic T3SS chaperone YerA (SycE) in Y. pseudotuberculosis treated with chloramphenicol (Cml) or proteinase K (PK). Relative signal intensities are indicated below each panel.
Yops Are Localized on the Surface of Y. pseudotuberculosis Cells Before Target Cell Contact.
Because we were unable to observe any spatial organization of YopE in Y. pseudotuberculosis cells that were actively translocating Yops, we investigated the localization of YopE in the Y. pseudotuberculosis wild type strain YPIII/pIB102 before target cell contact. For this purpose, we grew the bacteria at 37 °C in Luria-bertani (LB) medium with ≥ 0.5 mM Ca2+. In this case, it was clear that YopE was present primarily on the surface of the bacteria (Fig. 1B), and not in the cytoplasm, as was predicted by the one-step injection model. Surface-localized YopE did not colocalize with the needle protein YscF in double-stained sections (Fig. S1A), even though three to five YscF needles were detected in each sectioned bacterium, and the YscF antibodies were shown to be specific for the T3SS needle structure in single-labeled sections (Fig. S1B). YopH and the translocator YopD were also detected mainly on the surface of wild-type Y. pseudotuberculosis cells in immunogold-labeled sections (Fig. 1B). To assess the relative levels of surface localized Yops in the whole bacterial population, we conducted proteinase K protection experiments. In logarithmically growing bacteria, 50 to 70% of YopE, YopH, YopB, and YopD, but not YerA (SycE), was accessible to proteinase K degradation, which indicates that a large proportion of the Yops were present on the surface of the bacteria before target cell contact, whereas the cytosolic chaperone YerA (SycE) was not (Fig. 1C). Notably, after exposing the bacteria to chloramphenicol to block de novo protein synthesis, the vast majority of the Yops were sensitive to the protease, which indicates that the Yops are located on the surface of the bacteria under these conditions (Fig. 1C). It has previously been shown that YopE induces a cytotoxic response in HeLa cells infected with Y. pseudotuberculosis in the presence of chloramphenicol (17), suggesting that surface-localized YopE can be translocated. To further investigate this possibility, we used an in vitro assay where rapid physiological responses in Y. pseudotuberculosis target cells could be monitored at single-cell resolution.
Surface-Localized YopH Can Block the Immediate-Early Ca2+ Response in Infected Neutrophils.
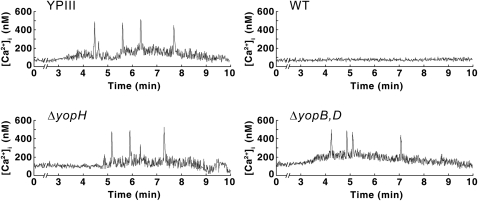
Neutrophils are a major target of Yop translocation during infection (18), and we have previously shown that the phosphotyrosine phosphatase (PTPase) YopH is responsible for inhibiting the immediate-early Ca2+ response (spikes) in these cells (19). Each Ca2+ spike corresponds to the binding of a single YopH-deficient Y. pseudotuberculosis bacterium to β1-integrins on a neutrophil, and YopH-mediated inhibition occurs within seconds after binding (19). This system provides a suitable assay for monitoring YopH translocation at single-cell resolution. To determine whether surface-localized YopH can generate a functional effect in this system, we monitored intracellular Ca2+ levels in neutrophils that were infected with bacteria in which de novo protein synthesis had been blocked with chloramphenicol. Interestingly, chloramphenicol did not affect the capacity of the Y. pseudotuberculosis wild-type strain to inhibit the Ca2+ spikes in neutrophils (Fig. 2). In accordance with previous results, the yopH mutant (YPIII/pIB29) and the plasmid-cured YPIII strain (lacking the yop/ysc virulon) induced the expected Ca2+ spikes (Fig. 2), which confirms that YopH is necessary for the observed effect. The YopH-dependent inhibition of Ca2+ spiking in neutrophils was also shown to be YopB- and YopD-dependent because infections with a double yopByopD mutant (YPIII/pIB619) induced the spikes (Fig. 2). These results suggest that surface-localized YopH is translocated in a T3SS-dependent manner and that this assay can be used to monitor the translocation of YopH at single-cell resolution.
Fig. 2.
YopH-mediated inhibition of the immediate-early Ca2+ response in human neutrophils. Real-time monitoring of intracellular Ca2+ in neutrophils infected with chloramphenicol-treated Y. pseudotuberculosis strains: plasmid-cured mutant (YPIII), wild-type bacteria (WT), a yopH deletion mutant (ΔyopH), and a double yopByopD-deletion mutant (ΔyopB,D). The mean numbers (± SEM) of Ca2+ peaks (300–600 nM) generated within 10 min of infection in nine independent experiments were as follows: YPIII, 3.41 ± 0.62; wild type, 0.22 ± 0.10; ΔyopH, 3.39 ± 0.53; ΔyopB+D, 3.28 ± 0.81.
Y. pseudotuberculosis Strains Coated with Purified YopH in Vitro Block the Immediate-Early Ca2+ Response in Infected Neutrophils.
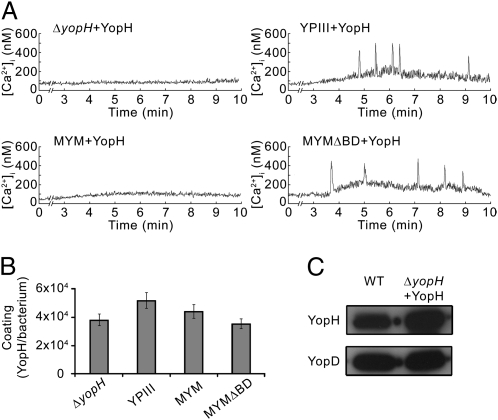
Our next aim was to determine whether external addition of purified YopH in vitro would suppress the phenotype of yopH mutants. YopH was purified to homogeneity (Fig. S2) and used to coat Y. pseudotuberculosis strains. Remarkably, the coated yopH mutant strain regained the ability to block the Ca2+ spikes (Fig. 3A), which demonstrates that the externally added YopH reversed the yopH mutant phenotype with respect to the Ca2+-blocking activity. The Ca2+-blocking ability of the YopH-coated mutant strain was shown to be dependent on a functional T3SS and the YopH PTPase activity, as neither coating of the plasmid-cured YPIII strain with YopH nor coating of the yopH mutant strain with purified inactive YopHC403A could suppress the yopH mutant phenotype (Fig. 3A and Fig. S3). The folding status of the purified YopH added to the bacteria did not influence the ability of coated strains to block the Ca2+ spikes because infections after coating with denaturated YopH in 4 M urea also blocked the response. The added YopH per se did not affect the Ca2+ spiking in neutrophils, as neutrophils pretreated with purified YopH exhibited Ca2+ spikes after being infected with the plasmid-cured YpIII strain. Moreover, the recorded Ca2+ spikes were also shown to be the result of intracellular release of Ca2+ by the neutrophil, because addition of 1 mM EGTA in the extracellular medium had no effect.
Fig. 3.
Y. pseudotuberculosis yopH mutant strains coated with purified YopH block the immediate-early Ca2+ response in human neutrophils. (A) Real-time monitoring of intracellular Ca2+ in neutrophils infected with YopH-coated Y. pseudotuberculosis strains: yopH deletion mutant (ΔyopH + YopH), plasmid-cured mutant (YPIII + YopH), multiple yop mutant (MYM + YopH), and an isogenic multiple yop mutant deleted for yopB and yopD (MYMΔBD + YopH). The mean numbers (± SEM) of Ca2+ peaks (300–600 nM) generated within 10 min of infection in nine independent experiments were as follows for the various bacteria strains: ΔyopH + YopH, 0.38 ± 0.21; YPIII + YopH, 3.21 ± 0.58; MYM + YopH, 0.40 ± 0.18; MYMΔBD + YopH, 3.24 ± 0.74. (B) Coating of externally added purified YopH on different Y. pseudotuberculosis strains presented as number of molecules per cell (mean ± SEM of four independent experiments). (C) Western blot analysis of YopH in chloramphenicol-treated wild-type Y. pseudotuberculosis (WT) and YopH-coated yopH mutant (ΔyopH + YopH). YopD is shown as a loading control.
To further investigate the role of the translocators YopB and YopD, and to exclude the possibility that other effector proteins can have an impact on the translocation of externally added YopH, we performed coating experiments using a pair of isogenic multiple-yop mutants, YPIII/pIB29MEK and YPIII/pIB29MEKBD (20) (hereafter called MYM and MYMΔBD). Both these strains encode functional T3SSs but have in-frame deletions in yopH, yopM, yopE, yopK (MYM and MYMΔBD), and yopB and yopD (MYMΔBD), which renders them incapable of inhibiting the Ca2+ spiking in neutrophils. The results revealed that the Ca2+ spiking was blocked by YopH-coated MYM but not by YopH-coated MYMΔBD (Fig. 3A). Separate control experiments showed that the coating efficiency was similar for all of the bacterial strains we used (Fig. 3B), and notably, it was similar to the YopH levels seen in chloramphenicol-treated bacteria (Fig. 3C), which shows that the coating done in vitro did not result in significantly elevated levels of YopH on the surface of the bacteria. These data are unique in suggesting that effector proteins that are added in vitro and subsequently found on the surface of Yersinia are functional and can be translocated into target cells through a T3SS-dependent mechanism.
Purified YopH-Bla Fusions Are Translocated Across the Target-Cell Plasma Membrane in a T3SS-Dependent Manner by Coated Y. pseudotuberculosis Strains.
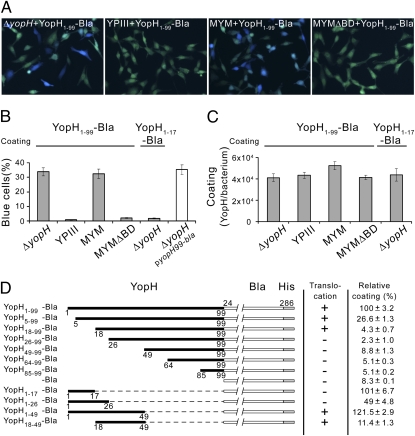
In the next step of our investigation, we used the previously described β-lactamase reporter system as a direct assay of translocation of Yop effectors into eukaryotic cells (21, 22). We constructed a fusion protein containing the first 99 amino acids of YopH [previously shown to be sufficient for translocation of YopH in vivo (9)] and β–lactamase lacking the N-terminal secretion signal sequence. The resulting reporter protein, YopH1–99-Bla, was purified to homogeneity from Escherichia coli (Fig. S2) and used to coat Y. pseudotuberculosis strains. After 10 min of infection, a coated yopH mutant and a coated MYM strain showed efficient translocation of YopH1–99-Bla, whereas the coated isogenic translocator mutant MYMΔBD and the coated plasmid-cured YPIII strain failed to promote translocation of this hybrid protein (Fig. 4 A and B). Importantly, the efficiency in translocating YopH1–99-Bla added in vitro was comparable to that observed in vivo using yopH1–99-bla expressed in trans (Fig. 4B). The coating efficiency achieved with the hybrid protein was similar for the different bacteria strains (Fig. 4C), and no translocation was observed in the absence of bacteria. Coating per se was not T3SS-dependent, and the plasmid-cured YPIII strain bound YopH and YopH1–99-Bla to essentially the same extent as the other strains did (Figs. 3B and 4C). All translocation experiments were carried out in the presence of cytochalasin D to avoid any unspecific uptake of the added protein fusions that could have occurred through internalization of coated bacterial cells. However, control experiments showed that translocation of the fusion protein in vitro was T3SS-specific in the absence of cytochalasin D, even though all of the coated bacteria strains were internalized by HeLa under these conditions (Fig. S4). Importantly, translocation experiments using Y. pseudotuberculosis strains coated with a partially purified YopK1–182-Bla fusion protein (Fig. S5A) also resulted in T3SS-dependent translocation of this hybrid protein (Fig. S5B), which shows that translocation of externally added effectors does not apply exclusively to YopH.
Fig. 4.
Translocation of purified YopH-Bla fusions coated on Y. pseudotuberculosis strains is dependent on a functional T3SS and a translocation domain in YopH. (A) Translocation of purified YopH1–99-Bla into HeLa cells infected with coated Y. pseudotuberculosis strains: a yopH deletion mutant (ΔyopH + YopH1–99-Bla), plasmid-cured mutant (YPIII + YopH1–99-Bla), a multiple yop mutant (MYM + YopH1–99-Bla), and an isogenic multiple yop mutant deleted for yopB and yopD (MYMΔBD + YopH1–99-Bla). Translocation is seen as blue fluorescence. (B) Translocation of indicated purified YopH-Bla fusions into HeLa-cells by coated Y. pseudotuberculosis strains (filled bars) and YopH1–99-Bla expressed in trans (open bar). The data represent mean ± SEM of six separate experiments corresponding to more than 1,000 cells. (C) Coating of externally added, purified YopH-Bla fusions on different Y. pseudotuberculosis strains presented as number of molecules per cell (mean ± SEM of four independent experiments). (D) Coating and translocation of purified YopH-Bla fusions by a multiple yop mutant (MYM). Relative coating with the YopH-Bla variants is presented as mean ± SEM of four individual experiments. Based on the results of two individual infection experiments, the cut-offs used to judge the degree of translocation of extracellularly coated variants were as follows: 25% detected blue HeLa cells was regarded as positive (+) and less than 2% blue HeLa cells as negative (−).
Translocation of YopH-Bla Fusions Coated on Y. pseudotuberculosis Strains in Vitro Requires a Translocation Domain That Is Distinct from the Secretion Signal.
To further investigate the mechanism and specificity of translocation of extracellular YopH, we constructed and purified truncated versions of YopH1–99-Bla and then analyzed these fusion proteins with regard to their ability to coat and to be translocated by coated Y. pseudotuberculosis strains in vitro (Fig. 4D). This process showed that amino acids 18 to 49 in YopH were sufficient for T3SS-dependent translocation of YopH-Bla fusions coated on Y. pseudotuberculosis strains, which agrees entirely with the previously reported YopH translocation domain (9). Translocation in vitro required the translocators YopB and YopD and was not promoted by a MYMΔBD strain coated with any of the fusion proteins. The extent of coating of the bacteria achieved with the different YopH-Bla fusions varied, but the efficiency in this respect was not correlated with translocation. In fact, purified YopH1–17-Bla (Fig. S2) coated Yersinia stains to a similar level as YopH1–99-Bla did (Fig. 4 C and D), but, it was not translocated into HeLa cells when it was coated on the yopH mutant strain (Fig. 4B) or the MYM strain (Fig. 4D). Compared with YopH1–17-Bla, purified YopH18–99-Bla and YopH18–49-Bla coated Yersinia strains less efficiently, but they were translocated (Fig. 4D). In accordance with results reported by Sory et al. (9), expression of yopH1–17-bla in trans resulted in T3SS-dependent secretion of the fusion protein into the extracellular medium, whereas no translocation was achieved in infection experiments in vivo. On the other hand, YopH18–99-Bla and YopH18–49-Bla were not secreted to the extracellular medium or translocated into HeLa cells when expressed in trans, although these fusions were translocated when coated on bacteria in vitro. Taken together, these observations show that secretion and translocation can be uncoupled in T3SS-dependent protein translocation in Y. pseudotuberculosis.
SPI-1–Dependent Translocation of YopH-Bla Fusions Coated on Salmonella typhimurium Strains.
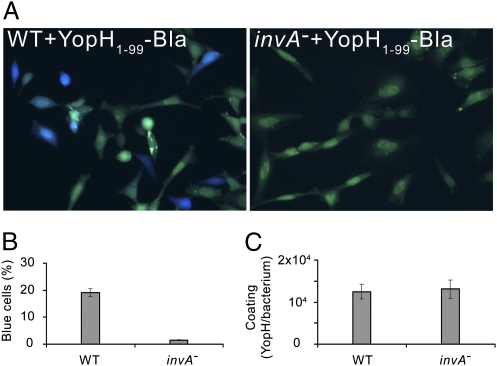
Our finding that Y. pseudotuberculosis can translocate surface-localized YopH and YopH-Bla fusions into target cells raised the question of whether other T3SS-dependent pathogens also have this ability. To address that issue, we studied translocation of YopH-Bla fusions coated on S. typhimurium strains in vitro, as this bacterial species has previously been shown to promote translocation of Yersinia effectors expressed in trans (1). We conducted translocation experiments in which the S. typhimurium wild-type strain (LT2) was coated with purified YopH1–99-Bla, which showed that the hybrid protein was indeed efficiently translocated into target cells within 10 min (Fig. 5 A and B). Furthermore, coating a strain with a polar insertion in invA (STA1) resulted in essentially no translocation (Fig. 5 A and B), indicating that the ability of S. typhimurium to translocate YopH1–99-Bla added in vitro depends on a functional SPI-1 T3SS. The coating efficiency of YopH1–99-Bla on S. typhimurium strains was similar (Fig. 5C) and, in accordance with that seen in Yersinia, in vitro translocation of YopH1–17-Bla coated on S. typhimurium in vitro was not promoted. Notably, coating S. typhimurium strains with YopH18–49-Bla gave the same result as that seen with YopH1–99-Bla (Fig. 5 A and B), showing that translocation of YopH-Bla fusions by S. typhimurium is dependent on the same domain in YopH as that seen in Yersinia. Thus we conclude that S. typhimurium can promote translocation of extracellularly added YopH1–99-Bla in a SPI-1–dependent manner.
Fig. 5.
SPI-1–dependent translocation of purified YopH1–99-Bla into HeLa cells infected with coated S. typhimurium strains. (A) Translocation of purified YopH1–99-Bla into HeLa cells infected with coated S. typhimurium strains; LT2 (WT + YopH1–99-Bla) and a polar invA insertional mutant (invA– + YopH1–99-Bla). Translocation is seen as blue fluorescence. (B) Translocation of purified YopH1–99-Bla into HeLa cells by coated S. typhimurium strains. Data are presented as mean ± SEM of six separate experiments corresponding to more than 1,000 cells. (C) Coating of externally added, purified YopH1–99-Bla on S. typhimurium strains presented as number of molecules per cell (mean ± SEM of four independent experiments).
Discussion
The main conclusion that can be drawn from the present study is that effectors located on the surface of Y. pseudotuberculosis can be translocated in a T3SS-dependent manner. In short, our results show that YopH and YopE, as well as the translocators YopB and YopD, are found primarily on the surface of the bacterium before it comes in contact with a target cell. These presecreted T3SS proteins are functional and induce a physiological response after translocation in to the host cell. In addition, coating yopH mutant strains with purified YopH in vitro complements the mutant phenotype with respect to the ability to block Ca2+ activity in infected neutrophils, resulting in the same physiological response as that induced by the wild-type Y. pseudotuberculosis strain. We also conclude that rapid, specific, and T3SS-dependent translocation of YopH-Bla and YopK-Bla hybrid proteins into HeLa cells occurs after infection with coated Y. pseudotuberculosis strains. Finally, our observation that YopH-Bla fusions coated on S. typhimurium was translocated in an SPI-1–dependent fashion indicates that the ability to translocate extracellular effector proteins is a general feature of T3SS-dependent pathogens.
The present data support a model of T3SS-dependent protein translocation in which polarized translocation proceeds through the formation of an extracellular intermediate that can be complemented by extracellular addition of purified effectors in vitro. Reconstitution of this intermediate requires the T3SS translocator proteins (YopB and YopD in Yersinia) and a specific translocation domain located in the effector protein. We suggest that polarized translocation of T3SS effector proteins is achieved upon close contact between the pathogen and the target cell, and that this occurs via an extracellular effector/translocator intermediate. Interaction between the translocators and the translocation domain in the effector is probably necessary for translocation of the effector across the plasma membrane of the target cell. The actual translocation might be accomplished through a binary AB-toxin–like mechanism (23), where the hydrophobic T3SS translocators resemble the pore-forming B-moiety that mediates the translocation of the catalytic A-moiety (Yop effectors) across the host-cell plasma membrane. The translocation-competent intermediate could be either a preformed effector/translocator complex on the surface of the bacterium or a transient effector/translocator complex that is assembled in the target-cell plasma membrane upon contact with the bacterium.
This two-step model of T3SS-dependent protein translocation might explain the elusive translocation domain, which has been identified in some effectors (9, 24–26). This multifunctional domain is located downstream of the N-terminal secretion signal in many T3SS substrates, and it overlaps the binding site of the cognate chaperone, which has been suggested to be involved in targeting of the effector to the T3SS apparatus in an unfolded, secretion-competent state in the bacterial cytosol (25, 27). Several reports have identified mutations in this domain that result in substrates that are secreted but not translocated into target cells (9, 24, 26). In a one-step injection model, T3SS-dependent secretion of a substrate should be sufficient for translocation, as this model predicts that substrates are directly translocated from the bacterial cytosol to the target cell cytoplasm through the channel that is formed by fusion of the T3SS needle complex and the translocation pore in the target-cell cytoplasm. Nevertheless, secreted substrates that have an altered translocation domain are not translocated. However, in a two-step model, the necessity of this translocation domain becomes apparent, because secreted T3SS substrates must interact to form a translocation-competent intermediate on the outside of the cell. Our data show that amino acids 18 to 49 in YopH, together with the hydrophobic translocators YopB and YopD, are necessary for translocation of externally added YopH-Bla fusions. Moreover, external addition of purified YopH-Bla effectively eliminates any pleiotropic effects of altered chaperone binding, targeting, recognition, or secretion of the substrate by the T3SS machine, and this strategy enables us to be unique in discriminating between secretion and translocation in T3SS-dependent protein translocation. Our data show that the previously reported translocation domain in YopH plays a role in T3SS-dependent translocation of this effector outside the bacterium. Furthermore, the observed translocation of YopH18–49-Bla demonstrates that the N-terminal secretion signal is redundant for translocation, and it also shows, uniquely, that secretion and translocation can be uncoupled in T3SS-dependent protein translocation.
A key feature of the two-step model presented here is surface localization of T3SS substrates during T3SS-dependent protein translocation. We found that both translocators and effectors are present on the surface of Y. pseudotuberculosis before host cell contact. In addition, other investigators (28) have succeeded in isolating extracellular YopBDE complexes from the supernatant of Yersinia pestis cultures in which the bacteria had been induced to secrete effector proteins. Vaccination of mice with these complexes induced protective immunity against Y. pestis strains lacking the F1 capsid, which suggests that the complexes are exposed and recognized by the humoral immune system in the absence of the protective extracellular capsid (28). A recent screen for small-molecule inhibitors of Yop translocation in Y. pseudotuberculosis also identified molecules that inhibit effector translocation but not Yop synthesis or secretion (29), which further supports a two-step mechanism for T3SS-dependent protein translocation in Yersinia.
T3SS translocators have also been demonstrated on the surface of other T3SS-dependent pathogens. For example, there are reports of specific release of surface-localized IpaB, IpaC, and IpaD in Shigella flexneri upon target cell contact (30, 31). Furthermore, specific engulfment of latex beads coated with purified so-called Ipa complexes has been observed (32); briefly, these coated beads were internalized by target cells through a mechanism that resembled the active, T3SS-dependent engulfment of invading Shigella, which indicates that the coated beads induced a T3SS-dependent response in the host cells. Other investigators have observed surface localization of the SPI-1 substrates SipB and SipD in S. typhimurium (33), and several articles have described surface-localized SPI-2 substrates in Salmonella (34–37).
Notwithstanding, considering Salmonella and Shigella, no surface-localized effector proteins have yet been found, and the few studies that have assayed effector proteins in these pathogens have indicated that these proteins are present in the cytoplasm of the bacteria before contact with target cells (15, 38, 39). In an investigation of S. typhimurium, Schlumberger et al. (15) performed real-time imaging of SipA translocation and showed that 26% of the bacterial cells contained detectable amounts of SipA in their cytosol before they came in contact with host cells. Polar translocation of this pool of SipA from the bacterial cytosol to the target-cell cytoplasm was also demonstrated, and it was found that the translocation of SipA was correlated with depletion of SipA in the bacterial cytosol. However, although Schlumberger and et al. demonstrated polar translocation of SipA by infecting Salmonella, they did not investigate the actual translocation mechanism. Our results show that purified YopH-Bla fusions coated on S. typhimurium strains was translocated by the bacteria in a SPI-1–dependent manner, which suggests that T3SS-dependent translocation can occur through formation of an extracellular intermediate that can be complemented in vitro in these bacteria. This finding further indicates that the translocation mechanism is conserved among different T3SS-dependent pathogens, even though, as expected, the regulation and the deployment of the T3SS vary between species that use different infection strategies. The fact that no extracellular effectors have been found in Salmonella and Shigella does not exclude the existence of an extracellular intermediate in T3SS-dependent protein translocation. Such an intermediate might be transient, and it might be assembled only after effectors are secreted to the surface of the bacteria upon contact with target cells.
In conclusion, we have demonstrated that T3SS-dependent protein translocation can proceed through an extracellular intermediate that can be reconstituted in vitro. Previous reports have provided data indicating a two-step mechanism for T3SS-dependent protein translocation (9, 24, 26, 29–31), but the current findings are unique in representing direct demonstration that extracellular (secreted) effectors can be translocated by a T3SS-dependent mechanism. Our results cannot exclude the microinjection model, because it is possible that these two translocation systems operate in parallel. However, our data are remarkable when considering that they demonstrate that the translocation of externally added YopH depends on the same factors that would be expected to be involved if the experiments were carried out in vivo, where YopH would be expressed and secreted by the bacteria. We also show that T3SS-dependent protein translocation can be studied using purified proteins in vitro, and this will definitely constitute a basis for extended studies of T3SSs and the translocation process per se.
Materials and Methods
A detailed description of the present materials and methods can be found in SI Materials and Methods.
The bacterial strains and plasmids that were used in this study are listed in Table S1. Induction of T3SS in Yersinia strains for immunogold labeling, infection, and coating experiments was achieved by growing Yersinia strains for 2 h at 37 °C in Luria-Bertani (LB) broth containing ≥ 0.5 mM Ca2+. Induction of T3SS in Salmonella strains for infection and coating experiments was achieved by growing Salmonella strains anaerobically overnight in LB broth. Ultrathin cryosectioning was carried out as described elsewhere (40). Immunolabeling with purified antibodies was performed according to the protein A-gold method (41). Surface localization of Yops was determined by Western blot analysis of samples from wild-type Y. pseudotuberculosis strains treated with 50 μg/mL chloramphenicol or 500 μg/mL proteinase K. Isolation of human neutrophils was done as described elsewhere (42). Intracellular Ca2+ was measured in infected Fura-2-AM–loaded neutrophils by live-cell microscopy (Nikon Eclipse Ti-E) using appropriate filters, and the concentrations were calculated as described by Grynkiewicz et al. (43). Translocation of β-lactamase fusions into CCF2-AM-loaded HeLa cells was determined 10 min after bacterial infection by counting the number of blue cells in images taken with a live-cell imaging microscope (Nikon Eclipse Ti-E) equipped with a true color camera (Nikon DS-2Fi).
Immobilized metal affinity chromatography was performed to purify native YopH and YopHC403A from the culture medium of secretion-induced Y. pseudotuberculosis strains. Bla fusions were cloned using the primers listed in Table S2 and were purified from E. coli using the pET101/D TOPO system. Coating of proteins on bacterial strains for infection and coating experiments was achieved by adding purified proteins (20–60 nM) to T3SS-induced bacteria. Coating efficiencies were determined by quantification of 35S-labeled proteins recovered on washed coated bacterial strains. Relative coating of YopH-Bla fusions on bacteria was determined by measuring the β-lactamase activity recovered on coated bacterial strains. Gentamicin protection assays were carried out to assess internalization of coated bacteria by HeLa cells.
Supplementary Material
Acknowledgments
We thank Prof. J. Klumperman and Ms. Viola Oorschot at the Cell Microscopy Center, Utrecht, The Netherlands for their technical advice. This work was supported by Swedish Research Council VR-M Grants 521-2008-2603 (to H.W.-W.) and K2008-58X-11222-14-3 (to M.F.), the Kempe Foundation (H.W.-W.), Sven och Lilly Lawskis fond för naturvetenskaplig forskning (H.W.-E.), the Carl Trygger Foundation for Scientific Research (R.R.), and the Foundation for Medical Research (Insamlingsstiftelsen) at Umeå University (R.R. and M.F.). The electron microscopy work was started at the Cell Microscopy Center, University Medical Center, Utrecht, The Netherlands, and continued at the Electron Microscopy Platform at the Chemical Biological Centre, Umeå University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013888108/-/DCSupplemental.
References
- 1.Rosqvist R, Håkansson S, Forsberg A, Wolf-Watz H. Functional conservation of the secretion and translocation machinery for virulence proteins of Yersiniae, Salmonellae and Shigellae. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosqvist R, Magnusson KE, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornelis GR, Van Gijsegem F. Assembly and function of type III secretory systems. Annu Rev Microbiol. 2000;54:735–774. doi: 10.1146/annurev.micro.54.1.735. [DOI] [PubMed] [Google Scholar]
- 4.Galán JE, Collmer A. Type III secretion machines: Bacterial devices for protein delivery into host cells. Science. 1999;284:1322–1328. doi: 10.1126/science.284.5418.1322. [DOI] [PubMed] [Google Scholar]
- 5.Bölin I, Portnoy DA, Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985;48:234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosqvist R, Bölin I, Wolf-Watz H. Inhibition of phagocytosis in Yersinia pseudotuberculosis: A virulence plasmid-encoded ability involving the Yop2b protein. Infect Immun. 1988;56:2139–2143. doi: 10.1128/iai.56.8.2139-2143.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis GR. Yersinia type III secretion: Send in the effectors. J Cell Biol. 2002;158:401–408. doi: 10.1083/jcb.200205077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sory MP, Boland A, Lambermont I, Cornelis GR. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Håkansson S, Bergman T, Vanooteghem JC, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmström A, et al. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol Microbiol. 1997;24:73–91. doi: 10.1046/j.1365-2958.1997.3211681.x. [DOI] [PubMed] [Google Scholar]
- 12.Tardy F, et al. Yersinia enterocolitica type III secretion-translocation system: Channel formation by secreted Yops. EMBO J. 1999;18:6793–6799. doi: 10.1093/emboj/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Håkansson S, et al. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector protein/s across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 14.Van Engelenburg SB, Palmer AE. Imaging type-III secretion reveals dynamics and spatial segregation of Salmonella effectors. Nat Methods. 2010;7:325–330. doi: 10.1038/nmeth.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlumberger MC, et al. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc Natl Acad Sci USA. 2005;102:12548–12553. doi: 10.1073/pnas.0503407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enninga J, Mounier J, Sansonetti P, Tran Van Nhieu G. Secretion of type III effectors into host cells in real time. Nat Methods. 2005;2:959–965. doi: 10.1038/nmeth804. [DOI] [PubMed] [Google Scholar]
- 17.Lloyd SA, Norman M, Rosqvist R, Wolf-Watz H. Yersinia YopE is targeted for type III secretion by N-terminal, not mRNA, signals. Mol Microbiol. 2001;39:520–531. doi: 10.1046/j.1365-2958.2001.02271.x. [DOI] [PubMed] [Google Scholar]
- 18.Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol. 2010;12:1064–1082. doi: 10.1111/j.1462-5822.2010.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson K, Magnusson KE, Majeed M, Stendahl O, Fällman M. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect Immun. 1999;67:2567–2574. doi: 10.1128/iai.67.5.2567-2574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Håkansson S, Galyov EE, Rosqvist R, Wolf-Watz H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol Microbiol. 1996;20:593–603. doi: 10.1046/j.1365-2958.1996.5251051.x. [DOI] [PubMed] [Google Scholar]
- 21.Marketon MM, DePaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005;309:1739–1741. doi: 10.1126/science.1114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004;186:5486–5495. doi: 10.1128/JB.186.16.5486-5495.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JA, Collier RJ. Anthrax toxin: Receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 24.Schesser K, Frithz-Lindsten E, Wolf-Watz H. Delineation and mutational analysis of the Yersinia pseudotuberculosis YopE domains which mediate translocation across bacterial and eukaryotic cellular membranes. J Bacteriol. 1996;178:7227–7233. doi: 10.1128/jb.178.24.7227-7233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsot C, Hamiaux C, Page AL. The various and varying roles of specific chaperones in type III secretion systems. Curr Opin Microbiol. 2003;6:7–14. doi: 10.1016/s1369-5274(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 26.Rodgers L, Mukerjea R, Birtalan S, Friedberg D, Ghosh P. A solvent-exposed patch in chaperone-bound YopE is required for translocation by the type III secretion system. J Bacteriol. 2010;192:3114–3122. doi: 10.1128/JB.00113-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galán JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 28.Ivanov MI, et al. Vaccination of mice with a Yop translocon complex elicits antibodies that are protective against infection with F1- Yersinia pestis. Infect Immun. 2008;76:5181–5190. doi: 10.1128/IAI.00189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harmon DE, Davis AJ, Castillo C, Mecsas J. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob Agents Chemother. 2010;54:3241–3254. doi: 10.1128/AAC.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulfide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci USA. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ménard R, Prévost MC, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collazo CM, Galán JE. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravortty D, Rohde M, Jäger L, Deiwick J, Hensel M. Formation of a novel surface structure encoded by Salmonella Pathogenicity Island 2. EMBO J. 2005;24:2043–2052. doi: 10.1038/sj.emboj.7600676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beuzón CR, Banks G, Deiwick J, Hensel M, Holden DW. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol. 1999;33:806–816. doi: 10.1046/j.1365-2958.1999.01527.x. [DOI] [PubMed] [Google Scholar]
- 36.Nikolaus T, et al. SseBCD proteins are secreted by the type III secretion system of Salmonella pathogenicity island 2 and function as a translocon. J Bacteriol. 2001;183:6036–6045. doi: 10.1128/JB.183.20.6036-6045.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein JR, Jones BD. Salmonella pathogenicity island 2-encoded proteins SseC and SseD are essential for virulence and are substrates of the type III secretion system. Infect Immun. 2001;69:737–743. doi: 10.1128/IAI.69.2.737-743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder GN, Hilbi H. Molecular pathogenesis of Shigella spp.: Controlling host cell signaling, invasion, and death by type III secretion. Clin Microbiol Rev. 2008;21:134–156. doi: 10.1128/CMR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winnen B, et al. Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS ONE. 2008;3:e2178. doi: 10.1371/journal.pone.0002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liou W, Geuze HJ, Slot JW. Improving structural integrity of cryosections for immunogold labeling. Histochem Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- 41.Slot JW, Geuze HJ, Gigengack S, Lienhard GE, James DE. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991;113:123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 43.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.