Abstract
Human ethnocentrism—the tendency to view one's group as centrally important and superior to other groups—creates intergroup bias that fuels prejudice, xenophobia, and intergroup violence. Grounded in the idea that ethnocentrism also facilitates within-group trust, cooperation, and coordination, we conjecture that ethnocentrism may be modulated by brain oxytocin, a peptide shown to promote cooperation among in-group members. In double-blind, placebo-controlled designs, males self-administered oxytocin or placebo and privately performed computer-guided tasks to gauge different manifestations of ethnocentric in-group favoritism as well as out-group derogation. Experiments 1 and 2 used the Implicit Association Test to assess in-group favoritism and out-group derogation. Experiment 3 used the infrahumanization task to assess the extent to which humans ascribe secondary, uniquely human emotions to their in-group and to an out-group. Experiments 4 and 5 confronted participants with the option to save the life of a larger collective by sacrificing one individual, nominated as in-group or as out-group. Results show that oxytocin creates intergroup bias because oxytocin motivates in-group favoritism and, to a lesser extent, out-group derogation. These findings call into question the view of oxytocin as an indiscriminate “love drug” or “cuddle chemical” and suggest that oxytocin has a role in the emergence of intergroup conflict and violence.
Keywords: hormones, social discrimination, evolution, moral dilemmas, endocrinology
To survive and prosper, individuals need groups whose members contribute information and resources. Because contributing resources and information makes oneself vulnerable to exploitation by others, group members need to know who to trust or to distrust, who can be expected to also contribute to the group and to reciprocate cooperation, and who may abuse in-group generosity and free-ride on others (1). A key mechanism facilitating such in-group cooperation is ethnocentrism, the tendency to view one's own group as centrally important and as superior to other groups. Ethnocentrism manifests itself in positive valuation of (members of) one's in-group. Such in-group favoritism signals loyalty and positive commitment to the group, thus rendering the ethnocentric individual a reliable and trustworthy partner. Ethnocentrism may also show up in negative valuation of (members of) out-groups. Such out-group derogation signals to in-group members who should be excluded from in-group resources and exchanges, and reduces the probability that in-group resources are inadvertently extended to out-groups (1–6).
If in-group favoritism and out-group derogation have adaptive value and sustain in-group functioning, coordination, and cooperation, it follows that (i) throughout evolution those individuals who displayed in-group favoritism and out-group derogation and who detected such tendencies in others were more likely to spread than individuals lacking these capacities (5–8) and (ii) the human brain may have evolved to sustain ethnocentrism through yet-unknown neurobiological systems. Here we conjecture that human ethnocentrism may be motivated by brain oxytocin, a peptide that is produced in the hypothalamus and released into the bloodstream from axon terminals and into the brain from dendrites of hypothalamic neurons (9). Functioning as both a neurotransmitter and a hormone, oxytocin's targets are widespread and include the hippocampus and the amygdala (10–12). Oxytocin interacts with dopaminergic, reward-processing circuits in the nucleus accumbens shell and in the ventral tegmental area (13) and exerts anxiolytic effects via direct activation of oxytocin receptors expressed in serotonergic neurons of the raphe nuclei (14, 15). Indeed, intranasal administration of oxytocin in humans promotes trust and cooperation (11, 16–19), although such effects may be limited to in-group members and do not extend toward out-groups (16, 17). For example, studies on animal cognition show that male rodents engineered to lack forebrain oxytocin receptors recognized but no longer discriminated between familiar (in-group) and novel (out-group) stimulus females (20, 21). If brain oxytocin indeed sustains and motivates human ethnocentrism at the neurobiological level, we should find that humans given oxytocin show more in-group favoritism than those given placebo.
Support for the idea that oxytocin motivates in-group favoritism would qualify work showing that oxytocin associates with (indiscriminate) social approach (22, 23), trust, benevolence, and prosociality (24). However, because in-group loyalty and cooperative motivation may also manifest themselves in out-group derogation (1–4, 16), it cannot be excluded that oxytocin also motivates out-group derogation. Evidence for this possibility would be consistent with work showing that oxytocin in non-human mammals promotes territoriality and aggression toward intruders (25–27) and with studies showing that humans given oxytocin display more schadenfreude when interpersonal competition is lost and more gloating when interpersonal competition is won (28).
In-group favoritism and out-group derogation conspire to create intergroup bias: the unfair response toward another group that devalues or disadvantages the other group and its members by valuing or privileging members of one's in-group (29). Here we predicted that (i) oxytocin creates such intergroup bias because (ii) oxytocin promotes in-group favoritism and, possibly, (iii) out-group derogation. These hypotheses were tested in five experiments, all using double-blind, randomized placebo-controlled between-subjects designs (Materials and Methods). Indigenous Dutch males sat in individual cubicles, and were guaranteed anonymity. Following established practice (10, 16, 18), they self-administered intranasally 24 IU of oxytocin or placebo. After 40 min, they received computer instructions for the experimental tasks that enabled independent assessments of in-group favoritism and out-group derogation. We exposed our indigenous Dutch males to images of in-group targets (Dutch males) and one of two natural but distinct out-group targets: immigrants from Middle Eastern descent (henceforth “Arabs”; experiments 1, 3, and 4) and German citizens (henceforth “Germans”; experiments 2 and 5) (refs. 30–34; Materials and Methods).
Experiments 1 and 2
Experiments 1 (n = 63) and 2 (n = 70) used the Implicit Association Test (IAT), an established technique to assess implicit social valuation (35–37). The IAT asks participants to categorize positive words/in-group names with one key and negative words/out-group names with one key. In a different block, they are then asked to categorize positive words/out-group names with one key and negative words/in-group names with the other key. Four trial blocks are obtained by crossing words (positive/negative valence) and name (in-group/out-group) (35–37). In-group favoritism is computed by subtracting latencies corrected for SD across within-block trials for out-group/positive blocks from the in-group/positive blocks. Thus, a negative IAT score indicates that in-group/positive associations are faster than out-group/positive associations. The same procedure applies to computing an index of out-group negativity (with a positive IAT score indicating out-group derogation). This computation allows one to test whether oxytocin promotes (i) in-group favoritism and (ii) out-group derogation (see Materials and Methods for an alternative computation with identical conclusions).
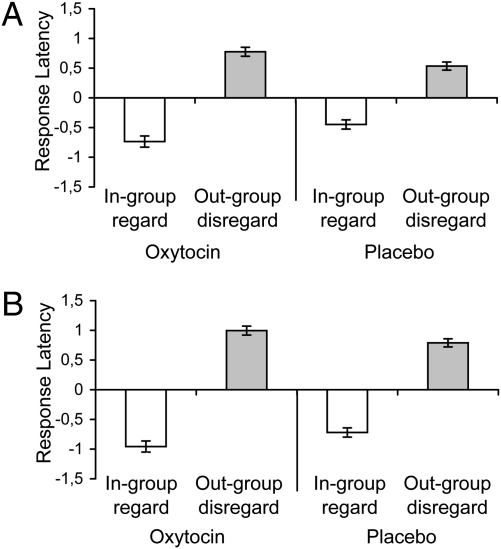
In-group favoritism and out-group derogation were submitted to a 2 (treatment: placebo/oxytocin) × 2 (target: in-group/out-group) ANOVA with the first factor between subjects. In experiment 1 (with Arabs as out-group), effects were found for target, F(1, 61) = 155.61, P < 0.001, and target × treatment, F(1, 61) = 6.95, P < 0.011 (Fig. 1A). Compared with placebo, oxytocin increased in-group favoritism, F(1, 61) = 5.51, P < 0.022, and out-group derogation, F(1, 61) = 4.95, P < 0.030. Experiment 2 (with Germans as out-group) replicated these effects for target, F(1, 68) = 323.29, P < 0.001, and target × treatment, F(1, 68) = 5.08, P < 0.027 (Fig. 1B). Compared with placebo, oxytocin increased in-group favoritism, F(1, 68) = 4.50, P < 0.038; the oxytocin-driven increase in out-group derogation just fell short of statistical significance, F(1, 68) = 3.46, P < 0.067. Thus, there is support for the hypothesis that (i) oxytocin creates intergroup bias because (ii) oxytocin promotes in-group favoritism. Mixed support was obtained for the hypothesis (iii) that oxytocin promotes out-group derogation.
Fig. 1.
Oxytocin promotes implicit in-group regard and out-group disregard (displayed ± SE). Negative scores indicate that in-group associations are faster; positive scores indicate that out-group associations are faster. (A) Results for experiment 1 with Arabs as out-group. (B) Results for experiment 2 with Germans as out-group.
Experiment 3
In additional to social valuation, ethnocentrism manifests itself in infrahumanization—the tendency to associate in-group members more than out-group members with secondary emotions that are commonly seen as uniquely human (e.g., delight, embarrassment; as opposed to primary emotions like joy and sadness) (38–41). Such intergroup bias may be the result of an increased tendency to associate uniquely human, secondary emotions with the in-group (in-group favoritism) with a reduced tendency to associate such secondary emotions with the out-group (out-group derogation), or both. In experiment 3, 66 Dutch males given oxytocin or placebo rated whether a target individual would be able to experience six secondary emotions and six primary emotions (1 = not at all to 5 = very much). Two blocks were created, one in which emotions were rated for a typical NEDERLANDER (Dutch) and one in which these emotions were rated for a typical MOSLIM (Muslim). Blocks were presented in random order, and within blocks emotions were randomized. Each block included three negative primary emotions (fear, exhaustion, and pain), three negative secondary emotions (embarrassment, contempt, and humiliation), three positive primary emotions (affection, pleasure, and attraction), and three positive secondary emotions (admiration, hope, and surprise) (38). Within each set of three (positive/negative valence × secondary/primary emotion), ratings were averaged (interitem reliabilities 0.75 < α < 0.84).
Preliminary analyses revealed no effects for the valence factor, all F(1, 64) < 1.02, P > 0.450, or for the order in which blocks were presented, all F(1, 64) < 1, P > 0.782. The lack of order effects indicates that possible effects of oxytocin on in-group favoritism and out-group derogation emerge regardless of the salience of an intergroup comparison, an issue we return to in Discussion. Final analyses collapsed across valence and order, resulting in a 2 (emotion type: primary vs. secondary) × 2 (target: in-group vs. out-group) × 2 (treatment: oxytocin vs. placebo) mixed-model ANOVA with the first two factors within subjects and the last factor between subjects. This analysis revealed effects for emotion, F(1, 64) = 88.44, P < 0.001; target, F(1, 64) = 135.97, P < 0.001; emotion × target, F(1, 64) = 132.59, P < 0.001; and emotion × target × treatment, F(1, 64) = 12.92, P < 0.001. For primary emotions, treatment and target effects were not significant, F < 1, P > 0.451 (overall M = 4.276, SD = 0.634). For secondary emotions, however, there were effects for target, F(1, 64) = 340.45, P < 0.001, and for the treatment × target interaction, F(1, 64) = 7.95, P < 0.006 (Fig. 2). Oxytocin created intergroup bias: compared with placebo, males given oxytocin associated secondary emotions more with their in-group than with the out-group, F(1, 64) = 4.73, P < 0.034. Follow-up analyses showed that this effect of oxytocin on intergroup bias was driven by in-group favoritism: associations between secondary emotions and in-group targets were stronger when participants received oxytocin rather than placebo, F(1, 64) = 4.654, P < 0.035. Treatment did not influence the extent to which out-group targets were associated with secondary emotions, F(1, 64) = 1.319, P < 0.225. Experiment 3 thus supports the hypothesis that (i) oxytocin creates intergroup bias because (ii) oxytocin promotes in-group favoritism. There was no support for the hypothesis that (iii) oxytocin promotes out-group derogation.
Fig. 2.
Oxytocin strengthens the association between uniquely human emotion words and in-group targets, but not out-group targets. Results range from very weak (1) to very strong (5) (displayed ± SE).
Experiments 4 and 5
Although the tasks used in experiments 1–3 assessed ethnocentric attitudes, experiments 4–5 considered intergroup bias in the way people treat in-group versus out-group members. Both experiments used the Moral Choice Dilemma Task (42, 43), which presents participants with a series of choice dilemmas. A famous example is that of a trolley running toward five people, who will be killed if nothing is done. Hitting a switch will divert the trolley to another track, where it will kill only one person. Thus, whatever one decides is, in a sense, wrong (it kills one or more human beings), but, at the same time, given the unattractiveness of the alternatives and the forced choice, each decision is defensible. Here we used five such dilemmas (e.g., the trolley dilemma, a case of blowing up a person who was stuck in a hole in a cave in order for five other people to escape, a case of denying a person access to a lifeboat to prevent the boat from sinking), and intermixed these dilemmas with five non-moral judgment problems (e.g., taking a coastal route or a route through the mountains, choosing a coffee or milkshake) (42, 44). Participants were randomly assigned to either the in-group target condition or the out-group target condition. In the in-group target condition, the target person was referred to by a typical Dutch male name (e.g., Dirk, Peter; different names were used across trials); in the out-group target condition, the target person had an Arab name (e.g., Ahmed, Youssef; different names were used across trials); or, in experiment 5, a German name (e.g., Markus, Helmut) was used. In all conditions, the collective saved by sacrificing the target was nameless and the stories were neutral as to the (in-group or out-group) identity of the collective. For instance, in the trolley dilemma, hitting the switch would mean that Maarten (Dutch name) or, in the out-group target condition, Mohammed (Arab name) would be killed, and five other unnamed people would live. The 10 choice problems (five moral, five control) were presented in random order. For each choice problem, participants indicated their decision (0 = no, 1 = yes). Intergroup bias shows up in a greater tendency to sacrifice out-group rather than in-group members (43) because of in-group favoritism (decreased willingness to sacrifice in-group targets), out-group derogation (increased readiness to sacrifice out-group targets), or both.
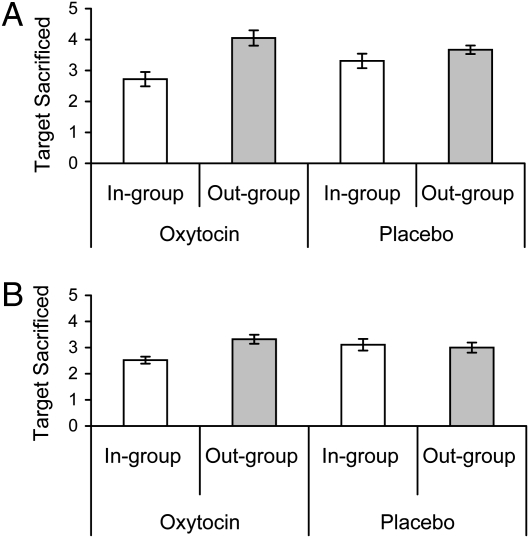
The number of “sacrifice” responses (range 0–5) and “yes” responses (0–5 for the control choices) in experiment 4 (n = 71) were submitted to a 2 (placebo/oxytocin) × 2 (in-group/out-group target) × 2 (sacrifice/control choice) ANOVA with the last factor within subjects. Results showed effects for target, F(1, 67) = 8.14, P < 0.006; choice type, F(1, 67) = 53.40, P < 0.001; target × choice type, F(1, 67) = 6.78, P < 0.011; and target × choice type × treatment, F(1, 67) = 4.80, P < 0.032. Target or treatment did not influence control choices, all F(1, 67) < 1.98, P > 0.17. For sacrifice choices, however, the predicted target × treatment interaction was significant, F(1, 67) = 12.08, P < 0.001 (Fig. 3A). Oxytocin promoted intergroup bias: Under oxytocin, males were more likely to sacrifice out-group targets than in-group targets [F(1, 68) = 17.27, P < 0.001]; under placebo, this tendency was not significant [F(1, 68) = 1.17, P < 0.284]. This overall intergroup bias was driven by in-group favoritism: Compared with placebo, oxytocin reduced the sacrifice of in-group targets, F(1, 68) = 4.11, P < 0.047. It was not driven by out-group derogation, in that treatment did not influence the readiness to sacrifice out-group targets, F(1, 68) = 1.37, P < 0.247. Experiment 5 (n = 77) replicated these effects for choice type, F(1, 73) = 46.86, P < 0.001; target × choice type, F(1, 73) = 2.76, P < 0.10; and target × choice type × treatment, F(1, 73) = 4.43, P < 0.039 with German instead of Arab names. Target or treatment did not influence control choices, all F(1, 73) < 1.39, P > 0.21. For sacrifice choices, the target × treatment interaction was significant, F(1, 73) = 9.33, P < 0.003 (Fig. 3B). Again, oxytocin created intergroup bias: Under oxytocin, males were more likely to sacrifice out-group targets than in-group targets [F(1, 74) = 9.33, P < 0.003]; under placebo, this tendency was not significant [F(1, 74) = 0.16, P < 0.694]. This overall intergroup bias was driven by in-group favoritism: Compared with placebo, oxytocin reduced the sacrifice of in-group targets, F(1, 74) = 4.95, P < 0.029. It was not because of out-group derogation, in that treatment did not increase the readiness to sacrifice out-group targets, F(1, 74) = 1.36, P < 0.248. Together, these results provide additional support for the hypothesis that (i) oxytocin creates intergroup bias because (ii) oxytocin promotes in-group favoritism. There was no support for the hypothesis that (iii) oxytocin promotes out-group derogation.
Fig. 3.
Oxytocin reduces the willingness to sacrifice in-group targets to save a larger collective but not the readiness to sacrifice out-group targets. Results range from 0 to 5 (displayed ± SE). (A) Results for experiment 4 with Arabs as out-group. (B) Results for experiment 5 with Germans as out-group.
Discussion
Results show that oxytocin creates intergroup bias because it motivates in-group favoritism and, in some cases, out-group derogation. These findings provide evidence for the idea that neurobiological mechanisms in general, and oxytocinergic systems in particular, evolved to sustain and facilitate within-group coordination and cooperation. This notion is further supported by the fact that oxytocin modulated in-group favoritism across different measures and generalizes across the two natural out-groups studied here. There are notable differences in the characteristics of and stereotypic perceptions vis-à-vis out-groups of Arab immigrants and Germans (refs. 30–33; Materials and Methods). However, across experiments, effects for oxytocin on in-group favoritism were strikingly similar. It thus seems that oxytocin's effects on in-group favoritism are relatively immune to cultural norms, exposure, between-group differences in socio-economic status, and the like.
Although results provided consistent support for the hypothesis that oxytocin motivates in-group favoritism, limited support was found for the hypothesis that oxytocin drives out-group derogation. Evidence was obtained in experiment 1, and to a lesser extent in experiment 2, where we gauged ethnocentrism through implicit measures that tap into biases operating outside of volitional control (45). This finding resonates with work showing that testosterone in humans reduces automatic fear responses but not volitionally controlled, self-reports of anxiety (46). Alternatively, it may be that intergroup bias is driven more by in-group favoritism and that out-group derogation plays a relatively minor role. In-groups are psychologically primary—people live in them and, sometimes, for them (47)—and in-group favoritism has strong adaptive value and facilitates within-group coordination and survival. Allport (47) already conjectured that there is good reason to believe that the in-group love–prejudice effect is far more basic to human life than is the out-group hate–prejudice effect, and research on human ethnocentrism supported this positive–negative asymmetry of social discrimination (29, 48). Current findings on the role of oxytocin fit this positive–negative asymmetry, showing that oxytocin creates intergroup bias primarily because it motivates in-group favoritism and not because it motivates out-group derogation.
The research designs in experiments 1 and 2 assessed in-group favoritism in the context of intergroup comparisons—participants responded to both in-group and out-group targets. In experiment 3, participants either first responded to in-group targets and then to out-group targets or vice versa. Importantly, order of target presentation did not qualify our findings, suggesting that in-group favoritism emerged regardless of whether judgments were rendered on in-group targets before or after judgments had been rendered on out-group targets. Experiments 4 and 5 exposed participants to either in-group targets or to out-group targets (but not both) and thus provided an assessment of in-group favoritism in absence of an explicit intergroup comparison. Again, support was found for the hypotheses that oxytocin creates intergroup bias because it motivates in-group favoritism. Put differently, oxytocin motivated in-group favoritism both when an intergroup comparison was salient (experiments 1 and 2) and when such an intergroup comparison was substantially more implicit (experiments 3–5). Together, these results suggest that in-group favoritism emerges regardless of whether an explicit out-group comparison is rendered salient. However, we should be cautious in concluding that oxytocin motives in-group favoritism in the pure absence of intergroup comparisons, and future research could invest in examining oxytocin's effects on in-group favoritism (and out-group derogation) in the explicit absence of intergroup comparisons (see ref. 43 for a guiding example).
Through its influence on in-group favoritism, oxytocin contributes to the development of intergroup bias and preferential treatment of in-group over out-group members. Because such unfair treatment triggers negative emotions, violent protest, and aggression among disfavored and excluded individuals (49), by stimulating in-group favoritism, brain oxytocin may trigger a chain reaction toward intense between-group conflict. This possibility questions the rather widespread view of oxytocin as a “cuddle chemical” or “love drug” (24). There is no doubt that oxytocin is implicated in the development of trust (10, 18), empathy, and prosociality (11), but these tendencies appear limited to individuals belonging to one's in-group (16, 17). Thus, rather than making humans prosocial, oxytocin functions to strengthen an evolutionary evolved and rather functional tendency to discriminate between in-group and out-group as well as to give members of one's own group preferential treatment. Such ethnocentrism has adaptive value to individuals and their groups but, unfortunately, also paves the way for intergroup bias, conflict, and violence.
Materials and Methods
Subjects.
Experiments were approved by the University of Amsterdam ethics committee and complied with American Psychological Association guidelines. Two-hundred eighty male participants (M = 21.31 y) were recruited via an on-line recruiting system and offered a monetary reward of €10 (∼$13 US) for participating in a study on the effects of medication on test scores and decision-making. They filled out an on-line medical screening; exclusion criteria were significant medical or psychiatric illness, medication, smoking more than five cigarettes per day, and drug or alcohol abuse. Participants were instructed to refrain from smoking or drinking (except for water) for 2 h before the experiment. All experimental sessions were conducted between 1200 hours and 1600 hours. Informed consent was obtained from all participants before the experiment.
Test Medication.
Participants self-administered a single intranasal dose of 24 IU of oxytocin (Syntocinon spray; Novartis; three puffs per nostril, each with 4 IU of oxytocin) or placebo 40 min before the start of the experimental tasks. To avoid any subjective effects (for example, olfactory effects) other than those caused by oxytocin, the placebo contained all of the active ingredients except for the neuropeptide. The placebo was manufactured by Stichting Apotheek der Haarlemse Ziekenhuizen in coordination with the pharmacy at the Amsterdam Medical Centre, adhering to European Union guidelines on good manufacturing practice and good clinical practice. The placebo was produced by using the exact same recipes and procedures used by Novartis to produce the carrier of Syntocinon, the synthetic analog of oxytocin. Placebos were delivered in the same bottles as Syntocinon. In short, the only difference between the placebo and treatment was the absence versus presence of the active neuropeptide.
Experimental Procedures and Materials.
In all experiments, participants came to the laboratory individually and were seated in individual cubicles preventing them from seeing and communicating with others. Participants read and signed an informed consent and were instructed to self-administer the medication (placebo or oxytocin, double-blind randomized) under experimenter supervision. The experimenter left, and participants completed a series of unrelated tests. Instructions guaranteed complete anonymity. To conform with prior research showing that effects of oxytocin peak after ∼30–40 min (10, 16, 18), after 38–42 min, the computer switched to the experimental task (lasting 15–20 min). Sixty-six participants in experiment 5 also participated in experiment 3, with experimental sessions being counterbalanced in order of appearance and intersected by a series of questionnaires on political values and opinions (order of presentation had no effects). In all tasks, participants keyed in their responses to questions, were thanked, and dismissed. Upon completion of the entire experiment, participants were paid and debriefed.
Two natural out-groups were selected for hypothesis testing. Our choice for Arabs and Germans as natural out-groups was based on a number of considerations. In 2005, the Pew Global Project found that 51% of Dutch citizens had unfavorable opinions about Muslims (30), job applicants were more often rejected immediately when their resume listed them with an Arab rather than Dutch name (31), and Moroccan adolescent males were the target of negative stereotyping and more or less subtle derogation by their indigenous Dutch counterparts (32). Although images of Germans tend to be seen as less threatening (33), Germans have been shown to be seen by indigenous Dutch as aggressive, arrogant, and cold (33, 34). Such negative stereotypes and prejudice against Germans may be traced back to a long history of rivalry and intergroup competition between Germany and The Netherlands (34).
Complementary Analyses.
In experiments 1 and 2, we computed IAT scores so that independent measurements of in-group favoritism and out-group derogation were obtained. However, this computational strategy allows for the possibility that people are more familiar with Dutch names and react to them more quickly than to Arab or German names. To check this possibility, we computed the commonly used overall bias score by subtracting in-group/positive from in-group/negative, and out-group/positive from out-group/negative. In-group bias and out-group bias were submitted to a 2 (bias) × 2 (treatment) ANOVA with the last factor between subjects. In experiment 1 (with Arabs as out-group), we found a main effect for bias, F(1, 61) = 155.61, P < 0.001, and a bias × treatment interaction, F(1, 61) = 6.95, P < 0.011. Compared with placebo, participants given oxytocin displayed more in-group positivity (M = −0.832 vs. M = −0.587) and more out-group negativity (M = 0.679 vs. M = 0.397). These effects were replicated in experiment 2 (with Germans as out-group): Both the bias and the bias × treatment effects were significant, F(1, 68) = 323.29, P < 0.0001, and F(1, 68) = 5.08, P < 0.027, respectively. Compared with placebo, participants given oxytocin displayed more in-group positivity (M = −0.993 vs. M = −0.846) and more out-group negativity (M = 0.929 vs. M = 0.673). These complementary findings point to the same conclusion as those reported above, namely that oxytocin promotes ethnocentric valuation of the in-group and devaluation of the out-group. These additional analyses rule out the possibility that these effects are attributable to familiarity.
Acknowledgments
We thank M. Kemper and S. W. W. Feith for assistance in preparing trial medication and H. Aaldering and H. Ter Kuilen for assistance during the experiments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Brewer MB. The psychology of prejudice: Ingroup love or outgroup hate? J Soc Issues. 1999;55:429–444. [Google Scholar]
- 2.Efferson C, Lalive R, Fehr E. The coevolution of cultural groups and ingroup favoritism. Science. 2008;321:1844–1849. doi: 10.1126/science.1155805. [DOI] [PubMed] [Google Scholar]
- 3.Fiske ST. What we know now about bias and intergroup conflict, the problem of the century. Curr Dir Psychol Sci. 2002;11:123–128. [Google Scholar]
- 4.Yzerbyt V, Demoulin S. Intergroup relations. In: Fiske ST, et al., editors. Handbook of Social Psychology. 5th Ed. Vol. 1. New York: Wiley; 2010. pp. 1024–1083. [Google Scholar]
- 5.Neuberg S, Kenrick DT, Schaller M. Evolutionary social psychology. In: Fiske ST, et al., editors. Handbook of Social Psychology. 5th Ed. Vol. 2. New York: Wiley; 2010. pp. 761–797. [Google Scholar]
- 6.Hammond A, Axelrod RA. The evolution of ethnocentrism. J Conflict Resolut. 2006;50:926–936. [Google Scholar]
- 7.Darwin C. The Descent of Man. New York: Appleton; 1873. [Google Scholar]
- 8.Brown DE. Human universals, human nature, and human culture. Daedalus. 2004;133:47–54. [Google Scholar]
- 9.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: The hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 10.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamer M, Zurowski B, Büchel Ch. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–9405. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur J Neurosci. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- 16.De Dreu CKW, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 17.Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Horm Behav. 2010;57:368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 19.Israel S, et al. The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS ONE. 2009;4:e5535. doi: 10.1371/journal.pone.0005535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macbeth AH, Lee HJ, Edds J, Young WS., III Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SE, et al. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Miller G. The prickly side of oxytocin. Science. 2010;328:1343. doi: 10.1126/science.328.5984.1343-a. [DOI] [PubMed] [Google Scholar]
- 25.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: Link to anxiety. J Neurosci. 2005;25:6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen CA. Biological aspects of social bonding and the roots of human violence. Ann N Y Acad Sci. 2004;1036:106–127. doi: 10.1196/annals.1330.006. [DOI] [PubMed] [Google Scholar]
- 28.Shamay-Tsoory SG, et al. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66:864–870. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Dovidio JF, Gaertner SL. Intergroup bias. In: Fiske ST, et al., editors. Handbook of Social Psychology. 5th Ed. Vol. 2. New York: Wiley; 2010. pp. 1084–1121. [Google Scholar]
- 30.Velasco Gonzalez K, Verkuyten M, Weesie J, Poppe E. Prejudice towards Muslims in the Netherlands: Testing integrated threat theory. Brit J Soc Psychol. 2008;47:667–685. doi: 10.1348/014466608X284443. [DOI] [PubMed] [Google Scholar]
- 31.Derous E, Nguyen H-H, Ryan AM. Hiring discrimination against Arab minorities: Interactions between prejudice and job characteristics. Hum Perform. 2009;22:297–320. [Google Scholar]
- 32.Kamans E, Gordijn EH, Oldenhuis H, Otten S. What I think you see is what you get: Influence of prejudice on assimilation to negative meta-stereotypes among Dutch Moroccan teenagers. Eur J Soc Psychol. 2009;39:842–851. [Google Scholar]
- 33.Otten S, Stapel DA. Who is this Donald? How social categorization affects aggression-priming effects. Eur J Soc Psychol. 2007;37:1000–1015. [Google Scholar]
- 34.Leach CW, Spears R, Branscombe NR, Doosje B. Malicious pleasure: Schadenfreude at the suffering of another group. J Pers Soc Psychol. 2003;84:932–943. doi: 10.1037/0022-3514.84.5.932. [DOI] [PubMed] [Google Scholar]
- 35.Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- 36.Beer JS, et al. The Quadruple Process model approach to examining the neural underpinnings of prejudice. Neuroimage. 2008;43:775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Weisbuch M, Pauker K, Ambady N. The subtle transmission of race bias via televised nonverbal behavior. Science. 2009;326:1711–1714. doi: 10.1126/science.1178358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demoulin S, et al. Dimensions of “uniquely” and non-uniquely” human emotions. Cogn Emotion. 2004;18:71–96. [Google Scholar]
- 39.Leyens J-P, et al. Emotional prejudice, essentialism, and nationalism. Eur J Soc Psychol. 2003;33:703–717. [Google Scholar]
- 40.Vaes J, Paladino MP, Castelli L, Leyens JP, Giovanazzi A. On the behavioral consequences of infrahumanization: The implicit role of uniquely human emotions in intergroup relations. J Pers Soc Psychol. 2003;85:1016–1034. doi: 10.1037/0022-3514.85.6.1016. [DOI] [PubMed] [Google Scholar]
- 41.Harris LT, Fiske ST. Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychol Sci. 2006;10:847–853. doi: 10.1111/j.1467-9280.2006.01793.x. [DOI] [PubMed] [Google Scholar]
- 42.Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 43.Cikara M, Farnsworth RA, Harris LT, Fiske ST. On the wrong side of the trolley track: Neural correlates of relative social valuation. Soc Cogn Affect Neurosci. 2010;5:404–413. doi: 10.1093/scan/nsq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- 45.Fazio RH, Olson MA. Implicit measures in social cognition research: Their meaning and use. Annu Rev Psychol. 2003;54:297–327. doi: 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- 46.van Honk J, Peper JS, Schutter DJ. Testosterone reduces unconscious fear but not consciously experienced anxiety: Implications for the disorders of fear and anxiety. Biol Psychiatry. 2005;58:218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Allport GW. The Nature of Prejudice. Cambridge: Addison-Wesley; 1954. [Google Scholar]
- 48.Otten S, Mummendey A. Valence-dependent probability of in-group favoritism between minimal groups: An integrative review on the positive-negative asymmetry in social discrimination. In: Capozza D, Brown R, editors. Social Identity Processes. London: Sage; 2000. pp. 33–48. [Google Scholar]
- 49.Hewstone M, Rubin M, Willis H. Intergroup bias. Annu Rev Psychol. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]