Abstract
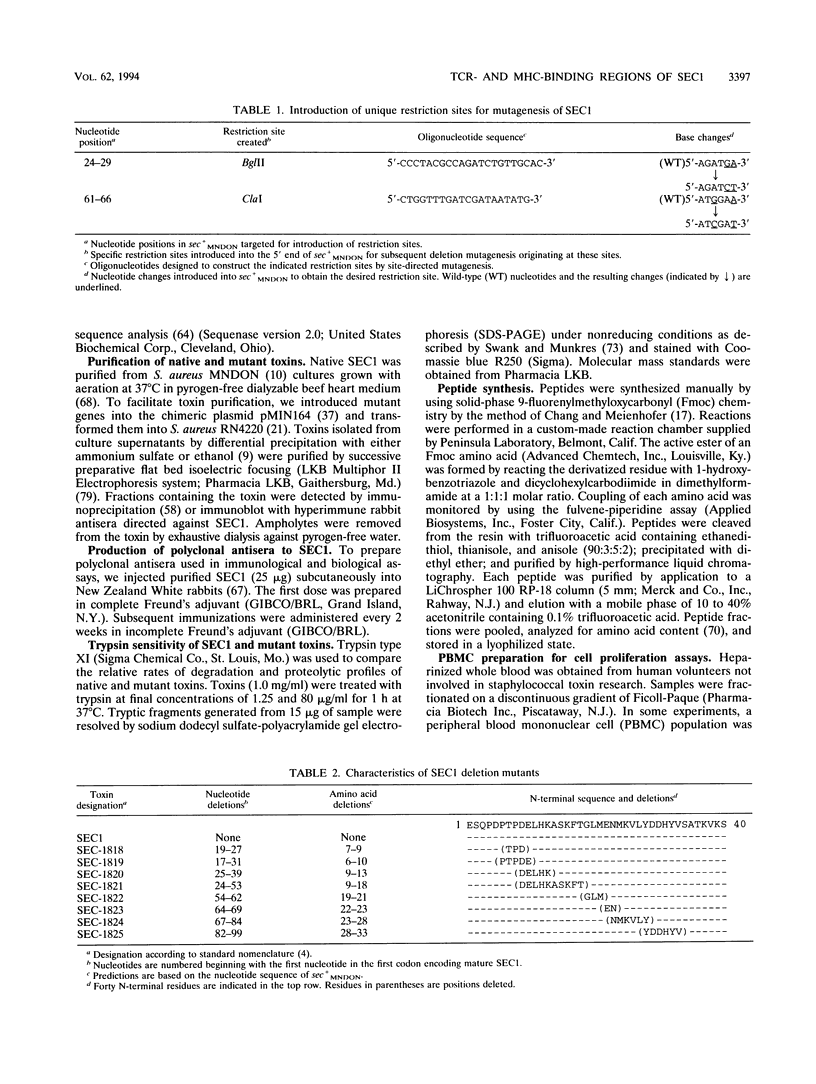
We have focused on regions of staphylococcal enterotoxin C1 (SEC1) causing immunomodulation. N-terminal deletion mutants lacking residues 6 through 13 induced T-cell proliferation similar to that induced by native toxin. However, mutants with residues deleted between positions 19 and 33, although nonmitogenic themselves, were able to inhibit both SEC1-induced T-cell proliferation and binding of the native toxin to major histocompatibility complex (MHC) class II. Presumably, these deletions define a part of SEC1 that interacts with the T-cell receptor. Three synthetic peptides containing residues located in a region analogous to the alpha 5 groove of SEC3 had residual mitogenic activity or blocked T-cell proliferation induced by SEC1 and appear to recognize the same site as SEC1 on a receptor for the toxin, presumably MHC class II. We conclude that isolated portions of the SEC1 molecule can retain residual mitogenic activity but that the entire protein is needed to achieve maximal superantigenic stimulation. Our results, together with the results of other investigators, support a model in which SEC1 binds to an alpha helix of MHC class II through a central groove in the toxin and thereby promotes or stabilizes the interaction between antigen-presenting cells and T cells.
Full text
PDF

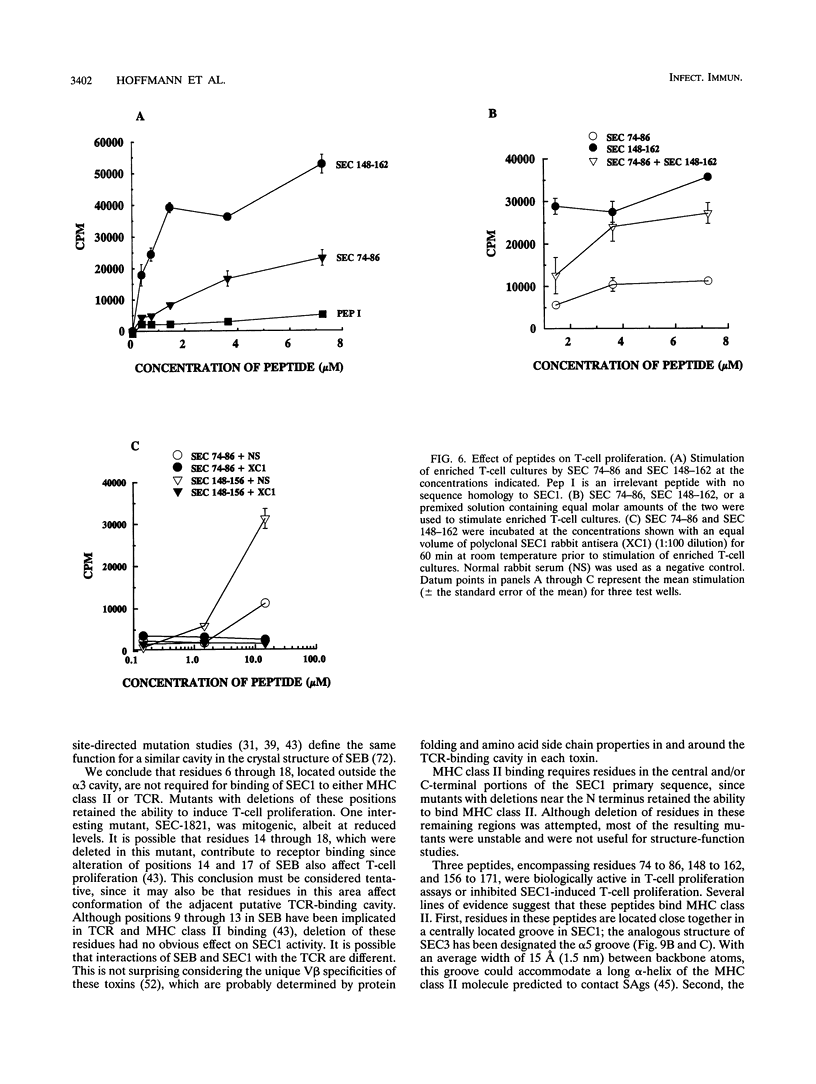



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley M. J., Mekalanos J. J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988 Jan;170(1):34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binek M., Newcomb J. R., Rogers C. M., Rogers T. J. Localisation of the mitogenic epitope of staphylococcal enterotoxin B. J Med Microbiol. 1992 Mar;36(3):156–163. doi: 10.1099/00222615-36-3-155. [DOI] [PubMed] [Google Scholar]
- Blanco L., Choi E. M., Connolly K., Thompson M. R., Bonventre P. F. Mutants of staphylococcal toxic shock syndrome toxin 1: mitogenicity and recognition by a neutralizing monoclonal antibody. Infect Immun. 1990 Sep;58(9):3020–3028. doi: 10.1128/iai.58.9.3020-3028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Bohach G. A., Fast D. J., Nelson R. D., Schlievert P. M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- Bohach G. A., Handley J. P., Schlievert P. M. Biological and immunological properties of the carboxyl terminus of staphylococcal enterotoxin C1. Infect Immun. 1989 Jan;57(1):23–28. doi: 10.1128/iai.57.1.23-28.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Conservation of the biologically active portions of staphylococcal enterotoxins C1 and C2. Infect Immun. 1989 Jul;57(7):2249–2252. doi: 10.1128/iai.57.7.2249-2252.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Expression of staphylococcal enterotoxin C1 in Escherichia coli. Infect Immun. 1987 Feb;55(2):428–432. doi: 10.1128/iai.55.2.428-432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohach G. A., Schlievert P. M. Nucleotide sequence of the staphylococcal enterotoxin C1 gene and relatedness to other pyrogenic toxins. Mol Gen Genet. 1987 Aug;209(1):15–20. doi: 10.1007/BF00329830. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Buelow R., O'Hehir R. E., Schreifels R., Kummerehl T. J., Riley G., Lamb J. R. Localization of the immunologic activity in the superantigen Staphylococcal enterotoxin B using truncated recombinant fusion proteins. J Immunol. 1992 Jan 1;148(1):1–6. [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Chang C. D., Meienhofer J. Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res. 1978 Mar;11(3):246–249. doi: 10.1111/j.1399-3011.1978.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Chintagumpala M. M., Mollick J. A., Rich R. R. Staphylococcal toxins bind to different sites on HLA-DR. J Immunol. 1991 Dec 1;147(11):3876–3881. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch J. L., Soltis M. T., Betley M. J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988 Jul;170(7):2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin C., Kass E. H. Identification of functional antigenic segments of toxic shock syndrome toxin 1 by differential immunoreactivity and by differential mitogenic responses of human peripheral blood mononuclear cells, using active toxin fragments. Infect Immun. 1989 Jul;57(7):2230–2236. doi: 10.1128/iai.57.7.2230-2236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin C., Swack J. A., Williams K., Bonventre P. F., Kass E. H. Activation of in vitro proliferation of human T cells by a synthetic peptide of toxic shock syndrome toxin 1. J Infect Dis. 1991 Mar;163(3):524–529. doi: 10.1093/infdis/163.3.524. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H., Conradt P. T lymphocyte activation by staphylococcal enterotoxins: role of class II molecules and T cell surface structures. Cell Immunol. 1989 Apr 15;120(1):92–101. doi: 10.1016/0008-8749(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Goshorn S. C., Schlievert P. M. Nucleotide sequence of streptococcal pyrogenic exotoxin type C. Infect Immun. 1988 Sep;56(9):2518–2520. doi: 10.1128/iai.56.9.2518-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs N. D., Pontzer C. H., Jarpe M. A., Johnson H. M. Mapping of multiple binding domains of the superantigen staphylococcal enterotoxin A for HLA. J Immunol. 1992 Apr 15;148(8):2516–2521. [PubMed] [Google Scholar]
- Harris T. O., Grossman D., Kappler J. W., Marrack P., Rich R. R., Betley M. J. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect Immun. 1993 Aug;61(8):3175–3183. doi: 10.1128/iai.61.8.3175-3183.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. O., Hufnagle W. O., Betley M. J. Staphylococcal enterotoxin type A internal deletion mutants: serological activity and induction of T-cell proliferation. Infect Immun. 1993 May;61(5):2059–2068. doi: 10.1128/iai.61.5.2059-2068.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund G., Dohlsten M., Herrmann T., Buell G., Lando P. A., Segrén S., Schrimsher J., MacDonald H. R., Sjögren H. O., Kalland T. A recombinant C-terminal fragment of staphylococcal enterotoxin A binds to human MHC class II products but does not activate T cells. J Immunol. 1991 Dec 15;147(12):4082–4085. [PubMed] [Google Scholar]
- Herman A., Croteau G., Sekaly R. P., Kappler J., Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990 Sep 1;172(3):709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Herman A., Labrecque N., Thibodeau J., Marrack P., Kappler J. W., Sekaly R. P. Identification of the staphylococcal enterotoxin A superantigen binding site in the beta 1 domain of the human histocompatibility antigen HLA-DR. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9954–9958. doi: 10.1073/pnas.88.22.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovde C. J., Hackett S. P., Bohach G. A. Nucleotide sequence of the staphylococcal enterotoxin C3 gene: sequence comparison of all three type C staphylococcal enterotoxins. Mol Gen Genet. 1990 Jan;220(2):329–333. doi: 10.1007/BF00260504. [DOI] [PubMed] [Google Scholar]
- Hufnagle W. O., Tremaine M. T., Betley M. J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect Immun. 1991 Jun;59(6):2126–2134. doi: 10.1128/iai.59.6.2126-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. J., Hudson K. R., Fraser J. D., Gascoigne N. R. Enterotoxin residues determining T-cell receptor V beta binding specificity. Nature. 1992 Oct 29;359(6398):841–843. doi: 10.1038/359841a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Jones C. L., Khan S. A. Nucleotide sequence of the enterotoxin B gene from Staphylococcus aureus. J Bacteriol. 1986 Apr;166(1):29–33. doi: 10.1128/jb.166.1.29-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Herman A., Clements J., Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Feb 1;175(2):387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kotzin B., Herron L., Gelfand E. W., Bigler R. D., Boylston A., Carrel S., Posnett D. N., Choi Y., Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989 May 19;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Jenkins R. N., Long E. O. Distinct binding sites on HLA-DR for invariant chain and staphylococcal enterotoxins. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9657–9661. doi: 10.1073/pnas.89.20.9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp D. R., Long E. O. Identification of HLA-DR1 beta chain residues critical for binding staphylococcal enterotoxins A and E. J Exp Med. 1992 Feb 1;175(2):415–424. doi: 10.1084/jem.175.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb M., Majumdar G., Tomai M., Beachey E. H. Accessory cell-independent stimulation of human T cells by streptococcal M protein superantigen. J Immunol. 1990 Sep 1;145(5):1332–1336. [PubMed] [Google Scholar]
- Kotb M., Ohnishi H., Majumdar G., Hackett S., Bryant A., Higgins G., Stevens D. Temporal relationship of cytokine release by peripheral blood mononuclear cells stimulated by the streptococcal superantigen pep M5. Infect Immun. 1993 Apr;61(4):1194–1201. doi: 10.1128/iai.61.4.1194-1201.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Labrecque N., Thibodeau J., Sékaly R. P. Interactions between staphylococcal superantigens and MHC class II molecules. Semin Immunol. 1993 Feb;5(1):23–32. doi: 10.1006/smim.1993.1004. [DOI] [PubMed] [Google Scholar]
- Labrecque N., Thibodeau J., Sékaly R. P. T-cell receptor recognition of superantigens: another view. Res Immunol. 1993 Mar-Apr;144(3):175–180. doi: 10.1016/0923-2494(93)80113-d. [DOI] [PubMed] [Google Scholar]
- Marr J. C., Lyon J. D., Roberson J. R., Lupher M., Davis W. C., Bohach G. A. Characterization of novel type C staphylococcal enterotoxins: biological and evolutionary implications. Infect Immun. 1993 Oct;61(10):4254–4262. doi: 10.1128/iai.61.10.4254-4262.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Merryman P., Silver J., Gregersen P. K., Solomon G., Winchester R. A novel association of DQ alpha and DQ beta genes in the DRw10 haplotype. Determination of a DQw1 specificity by the DQ beta-chain. J Immunol. 1989 Sep 15;143(6):2068–2073. [PubMed] [Google Scholar]
- Metzroth B., Marx T., Linnig M., Fleischer B. Concomitant loss of conformation and superantigenic activity of staphylococcal enterotoxin B deletion mutant proteins. Infect Immun. 1993 Jun;61(6):2445–2452. doi: 10.1128/iai.61.6.2445-2452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Noskova V. P., Ezepchuk YuV, Noskov A. N. Topology of the functions in molecule of staphylococcal enterotoxin Type A. Int J Biochem. 1984;16(2):201–206. doi: 10.1016/0020-711x(84)90073-9. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Ohnishi H., Tanaka T., Takahara J., Kotb M. CD28 delivers costimulatory signals for superantigen-induced activation of antigen-presenting cell-depleted human T lymphocytes. J Immunol. 1993 Apr 15;150(8 Pt 1):3207–3214. [PubMed] [Google Scholar]
- Poindexter N. J., Schlievert P. M. Toxic-shock-syndrome toxin 1-induced proliferation of lymphocytes: comparison of the mitogenic response of human, murine, and rabbit lymphocytes. J Infect Dis. 1985 Jan;151(1):65–72. doi: 10.1093/infdis/151.1.65. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Structural basis for differential binding of staphylococcal enterotoxin A and toxic shock syndrome toxin 1 to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):125–128. doi: 10.1073/pnas.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. K., Pontzer C. H., Johnson H. M. The I-A beta b region (65-85) is a binding site for the superantigen, staphylococcal enterotoxin A. Biochem Biophys Res Commun. 1990 Apr 30;168(2):696–701. doi: 10.1016/0006-291x(90)92377-c. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Schlievert P. M., Bettin K. M., Watson D. W. Purification and characterization of group A streptococcal pyrogenic exotoxin type C. Infect Immun. 1977 May;16(2):673–679. doi: 10.1128/iai.16.2.673-679.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M. Role of superantigens in human disease. J Infect Dis. 1993 May;167(5):997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M., Schoettle D. J., Watson D. W. Purification and physicochemical and biological characterization of a staphylococcal pyrogenic exotoxin. Infect Immun. 1979 Mar;23(3):609–617. doi: 10.1128/iai.23.3.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlievert P. M., Shands K. N., Dan B. B., Schmid G. P., Nishimura R. D. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981 Apr;143(4):509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978 Dec 25;253(24):8787–8791. [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Taub D. D., Rogers T. J. Direct activation of murine T cells by staphylococcal enterotoxins. Cell Immunol. 1992 Apr;140(2):267–281. doi: 10.1016/0008-8749(92)90195-u. [DOI] [PubMed] [Google Scholar]
- Weeks C. R., Ferretti J. J. Nucleotide sequence of the type A streptococcal exotoxin (erythrogenic toxin) gene from Streptococcus pyogenes bacteriophage T12. Infect Immun. 1986 Apr;52(1):144–150. doi: 10.1128/iai.52.1.144-150.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Smith H. P., Surman S., Le P., Wen R., Blackman M. A. Major histocompatibility complex-specific recognition of Mls-1 is mediated by multiple elements of the T cell receptor. J Exp Med. 1993 Feb 1;177(2):433–442. doi: 10.1084/jem.177.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J., Baron J., Buxser S., Janeway C. A., Jr Bacterial proteins that mediate the association of a defined subset of T cell receptor:CD4 complexes with class II MHC. J Immunol. 1990 Feb 1;144(3):892–901. [PubMed] [Google Scholar]
- de Azavedo J. C., Foster T. J., Hartigan P. J., Arbuthnott J. P., O'Reilly M., Kreiswirth B. N., Novick R. P. Expression of the cloned toxic shock syndrome toxin 1 gene (tst) in vivo with a rabbit uterine model. Infect Immun. 1985 Oct;50(1):304–309. doi: 10.1128/iai.50.1.304-309.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Newman W., Shimizu Y., Nutman T. B., Tanaka Y., Horgan K. J., Gopal T. V., Ennis E., O'Sullivan D., Grey H. Analysis of T cell stimulation by superantigen plus major histocompatibility complex class II molecules or by CD3 monoclonal antibody: costimulation by purified adhesion ligands VCAM-1, ICAM-1, but not ELAM-1. J Exp Med. 1991 Oct 1;174(4):901–913. doi: 10.1084/jem.174.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]





