Abstract
Aberrant transcriptional regulation in the brain is thought to be one of the key components of the pathogenesis and pathophysiology of neuropsychiatric disorders. Heat shock factors (HSFs) modulate cellular homeostasis through the control of gene expression. However, the roles of HSFs in brain function have yet to be elucidated fully. In the present study, we attempted to clarify the role of HSF1-mediated gene regulation in neuronal and behavioral development using HSF1-deficient (HSF1−/−) mice. We found granule neurons of aberrant morphology and impaired neurogenesis in the dentate gyrus of HSF1−/− mice. In addition, HSF1−/− mice showed aberrant affective behavior, including reduced anxiety and sociability but increased depression-like behavior and aggression. Furthermore, HSF1 deficiency enhanced behavioral vulnerability to repeated exposure to restraint stress. Importantly, rescuing the HSF1 deficiency in the neonatal but not the adult hippocampus reversed the aberrant anxiety and depression-like behaviors. These results indicate a crucial role for hippocampal HSF1 in neuronal and behavioral development. Analysis of the molecular mechanisms revealed that HSF1 directly modulates the expression of polysialyltransferase genes, which then modulate polysialic acid–neural cell adhesion molecule (PSA-NCAM) levels in the hippocampus. Enzymatic removal of PSA from the neonatal hippocampus resulted in aberrant behavior during adulthood, similar to that observed in HSF1−/− mice. Thus, these results suggest that one role of HSF1 is to control hippocampal PSA-NCAM levels through the transcriptional regulation of polysialyltransferases, a process that might be involved in neuronal and behavioral development in mice.
Keywords: emotion, spine density, neuronal maturation, polysialylation
There is increasing evidence that aberrant transcriptional regulation is one of the key components of the pathogenesis and pathophysiology of neuropsychiatric disorders (1, 2). It has been suggested that neuronal activity regulates a complex program of gene expression involved in structural and functional plasticity (3). Recent evidence has indicated that aberrant gene regulation in early brain development can affect brain function and subsequent affective behavior, as well as behavioral responses to stress during adulthood in rodents (4, 5).
Heat shock factors (HSFs) bind to the conserved heat shock element (HSE) consensus sequence and facilitate the transcriptional activation or repression of HSE-containing target genes (6). In mammals, the HSF family consists of four members (HSF1–4) that are considered functionally distinct. HSF1 is an essential molecule for facilitating the response to cellular stress (e.g., elevated temperature, oxidative stress, and increased protein misfolding) and also is required for developmental processes, whereas HSF2 and HSF4 are involved in cell differentiation and development (7). Among the mammalian HSFs, HSF1 is the master transactivator of heat shock proteins, which function as molecular chaperones at various stages in protein biogenesis and degradation (6). To date, however, the developmental and functional roles of HSF1 in the brain remain largely unknown.
Hippocampal formation is vulnerable to damage from a variety of cellular and psychological stressors (8, 9). Hippocampal functions are necessary for emotional regulation, neuroendocrine control, and memory formation (9). Moreover, aberrant structural, functional, and neurogenic changes to this brain structure have been suggested as being involved in the pathogenesis and/or pathophysiology of stress-related neuropsychiatric disorders (10–12). However, the roles of HSF1-mediated gene regulation in the hippocampus in neuronal and behavioral development remain unclear.
In the present study, we attempted to clarify the roles of HSF1 in neuronal and behavioral development using HSF1-deficient (HSF1−/−) mice. We show here that the transcription factor HSF1 is essential for neurogenesis and spinogenesis in the dentate gyrus of the hippocampus and for the development of normal emotional and social behavior. We also demonstrate the molecular mechanisms underlying the aberrant affective behavior in HSF1−/− mice.
Results
Aberrant Morphology of Dentate Gyrus Granule Neurons in HSF1-Deficient Mice.
We first confirmed the expression levels of HSF1 mRNA and protein in the forebrains of HSF1−/− mice. Neither HSF1 mRNA nor HSF1 protein could be detected in the hippocampus or medial prefrontal cortex on postnatal day 2 or postnatal day 56 in HSF1−/− mice (Fig. S1 A and B). In HSF1-heterozygous (HSF1+/−) mice, HSF1 mRNA expression was reduced to ≈50% of the level in HSF1+/+ mice (Fig. S1A). The brain/body weight ratio was comparable among the three genotypes (Fig. S1C). Nissl staining showed enlarged lateral ventricles in HSF1−/− mice (Fig. S1D), and this result is in agreement with previous reports using HSF1-null mice of a different genetic background (13, 14). We did not find any differences in the neuronal densities in the hippocampus or medial prefrontal cortex among the three genotypes (Fig. S1E).
To investigate the dendritic morphology of granule neurons of the dentate gyrus in adult HSF1−/− mice, we performed Golgi staining. The total dendritic length in HSF1−/− mice was significantly reduced compared with the length in HSF1+/+ mice (Fig. 1 A and B). We also evaluated the density of dendritic spines of the dentate gyrus granule neurons in HSF1−/− mice and found a significant reduction relative to the density in HSF1+/+ mice (Fig. 1 C and D). Moreover, the total dendritic lengths and the number of dendrites in both the apical and the basal CA3 pyramidal neurons of the hippocampus were significantly lower in HSF1+/− and HSF1−/− mice than in HSF1+/+ mice (Fig. S2). Thus, HSF1 deficiency causes alterations in the structure of hippocampal neurons.
Fig. 1.
Reduced hippocampal spine density in HSF1-deficient mice. (A) Representative images of dentate gyrus granule neurons in HSF1+/+ and HSF1−/− mice. (Scale bars: 50 μm.) (B) Bar graph shows the total dendrite length of dentate gyrus granule neurons in HSF1+/+, HSF1+/−, and HSF1−/− mice (n = 5 for each group). *P < 0.05. (C) Representative images of dendritic spines from dentate gyrus granule neurons in HSF1+/+ and HSF1−/− mice. (Scale bars: 20 μm.) (D) Bar graph shows the spine densities of dentate gyrus granule neurons in HSF1+/+, HSF1+/−, and HSF1−/− mice (n = 5 for each group). *P < 0.05.
Impaired Neurogenesis in the Dentate Gyrus of Adult HSF1-Deficient Mice.
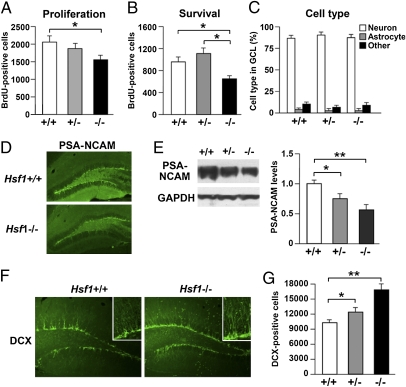
To examine the effect of HSF1 deficiency on adult hippocampal neurogenesis, we performed BrdU staining. Relative to HSF1+/+ mice, HSF1−/− mice showed significantly fewer BrdU+ cells in the subgranular zone of the dentate gyrus 2 h after BrdU injection (Fig. 2A). Four weeks after BrdU labeling, the number of BrdU+ cells in the granule cell layer of the dentate gyrus was significantly lower in HSF1−/− mice than in either HSF1+/− or HSF1+/+ mice (Fig. 2B). In all groups, most BrdU+ cells expressed the neuronal nuclei (NeuN) marker at 4 wk (Fig. 2C). In contrast, a small proportion of BrdU+ cells were colabeled with the astrocyte marker glial fibrillary acidic protein (GFAP) at this time point (Fig. 2C). There were no significant differences among the three genotypes regarding these cell types in the dentate gyrus granule neurons of newborns (Fig. 2C). In addition, immunostaining for polysialic acid-neural cell adhesion molecule (PSA-NCAM), a late progenitor and immature neuron marker, revealed reduced expression in HSF1−/− mice compared with HSF1+/+ mice in adulthood (Fig. 2D). Western blotting revealed that PSA-NCAM levels in the hippocampus were reduced in HSF1+/− and HSF1−/− mice on postnatal day 2 (Fig. 2E), with no difference in the Ncam1 levels (Fig. S3). Previous reports have demonstrated that PSA removal promotes the differentiation of neural progenitor cells into mature neurons in the subgranular zone (15), suggesting that in HSF1 mutants cells may differentiate into neurons prematurely. To test this possibility, we examined granule neuron development using doublecortin (DCX). We found that the number of DCX+ cells was significantly greater in HSF1 mutants than in HSF1+/+ mice (Fig. 2 F and G). Thus, HSF1−/− mice showed reduced neural progenitor proliferation and survival and enhanced premature neuronal differentiation in the dentate gyrus, suggesting that HSF1 deficiency inhibits dentate gyrus maturation.
Fig. 2.
Impaired neuronal maturation in the dentate gyrus of HSF1-deficient mice. (A) BrdU was given 2 h before mice were killed to examine the effects of HSF1 deficiency on cell proliferation. BrdU+ cells were counted from six animals per group. (B) BrdU was given once a day for 4 d before mice were killed to examine the effects of HSF1 deficiency on cell survival. BrdU+ cells were counted from six animals per group. (C) Graph shows the percentages of BrdU+ neurons, astrocytes, and other cells in the granule cell layer. (D) Images of PSA-NCAM staining in brain sections of HSF1+/+ and HSF1−/− mice. (E) Western blotting of PSA-NCAM levels in the hippocampus of HSF1+/+, HSF1+/−, and HSF1−/− mice (n = 6 for each group). (F) Images of DCX staining in brain sections of HSF1+/+ and HSF1−/− mice. (Insets) High-magnification images of DCX+ cells. (G) Effect of HSF1 deficiency on the total number of DCX+ cells (n = 6 for each group). *P < 0.05; **P < 0.01.
Effects of HSF1 Deficiency on Affective Behavior.
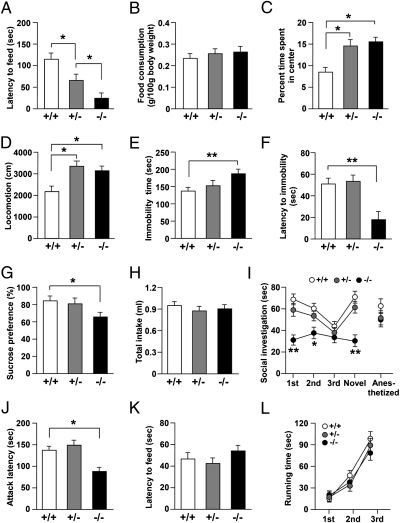
To investigate the effects of HSF1 deficiency on behavior, we first examined anxiety behavior using a novelty-suppressed feeding test and an open field test. We found that the latency to the first feeding episode was significantly shorter in HSF1+/− mice than in HSF1+/+ mice (Fig. 3A). Moreover, HSF1−/− mice showed significantly shorter latency to the first feeding episode than HSF1+/− mice (Fig. 3A), with no difference in feeding activity after the end of the test (Fig. 3B). Additionally, HSF1+/− and HSF1−/− mice spent a significantly greater proportion of time in the center area of the open field than did HSF1+/+ mice (Fig. 3C). These results suggest reduced anxiety behavior in HSF1+/− and HSF1−/− mice. However, the locomotor activity of both HSF1 mutants was significantly higher than that of the HSF1+/+ mice (Fig. 3D).
Fig. 3.
Aberrant affective behavior in HSF1-deficient mice. Bar graphs show (A) latency to feeding and(B) level of food consumption in the novelty-suppressed feeding test (n = 11–15 for each group), (C) percent time spent in the center area and (D) locomotor activity in the open field test (n = 9–13 for each group), (E) immobility time and (F) latency to first immobility bout in the forced swim test (n = 11–15 for each group), and (G) sucrose preference and (H) total intake in the sucrose preference test (n = 11–15 for each group). (I) Graph shows interaction time in the social investigation test (n = 12–16 for each group). (J and K) Bar graphs show latency (J) to the first attack bout in the social resident–intruder test (n = 11–14 for each group) and (K) to finding the hidden food in the hidden food test (n = 9–13 for each group). (L) Graph shows running time in the Rotarod test (n = 9–13 for each group). *P < 0.05; **P < 0.01.
Next, using a forced swim test and a sucrose preference test, we investigated the depression-like behavior of mice. The immobility time of HSF1−/− mice was significantly longer than that of HSF1+/+ mice (Fig. 3E), and the latency to the first immobility bout of the mice was significantly shorter in HSF1−/− mice than in HSF1+/+ or HSF1+/− mice (Fig. 3F). In the sucrose preference test, HSF1−/− mice showed significantly lower sucrose preference than did HSF1+/+ mice (Fig. 3G), with no difference in total fluid consumption (Fig. 3H). These results suggest increased depression-like behavior in HSF1−/− mice.
We examined the social behavior of the mice using the social investigation and social resident–intruder tests. HSF1+/+ and HSF1+/− mice showed decreasing social investigation time with repeated exposure to a stimulus mouse (Fig. 3I). A subsequent dishabituation trial with an unfamiliar mouse elicited an increased response, showing individual recognition. In contrast, HSF1−/− mice showed shorter social investigation times than HSF1+/+ mice (Fig. 3I). There was no significant difference in the social investigation time of HSF1−/− mice when an anesthetized adult mouse was used as the unfamiliar mouse (Fig. 3I). In the social resident–intruder test, HSF1−/− mice showed shorter attack latency to the intruder mice than did HSF1+/+ and HSF1+/− mice (Fig. 3J), suggesting increased offensive aggression in HSF1−/− mice. We did not find any difference among the three genotypes in their latencies to find the hidden food in the hidden food test (Fig. 3K) or their running times in the Rotarod test (Fig. 3L), suggesting that the aberrant affective behaviors in the behavioral paradigms used in this study are not caused by anosmic effects or motor problems in HSF1 mutants.
We also investigated whether HSF1 deficiency could affect behavioral responses to repeated stress exposure. Mice were subjected to 2 h of restraint stress per day for 14 consecutive days. Then their anxiety- and depression-like behaviors were examined. Although HSF1+/+ mice did not show any behavioral alteration after repeated exposure to restraint stress, HSF1 mutants showed enhanced vulnerability/susceptibility to the same stress exposure (Fig. S4). These results suggest that the reduced expression of HSF1 increases vulnerability to repeated stress.
Aberrant Affective Behavior in HSF1-Deficient Mice Could Be Partially Reversed by Overexpression of an Active Form of HSF1 in the Neonatal Hippocampus.
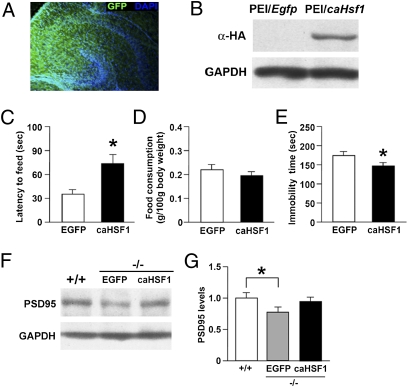
We investigated whether rescuing the HSF1 deficiency in the hippocampus could reverse the aberrant behavior of HSF1−/− mice. A constitutively active form of HSF1 (caHSF1) (16) was overexpressed in the hippocampus of neonatal HSF1−/− pups (postnatal day 1 or 2) using the polyethylenimine (PEI) gene delivery system, and behavioral assays were performed in young adults. In the novelty-suppressed feeding test, the overexpression of caHSF1 in HSF1−/− mice enhanced the latency to feeding compared with EGFP controls with no change in feeding activity (Fig. 4 C and D). In the forced swim test, the overexpression of caHSF1 in HSF1−/− mice reduced the immobility time compared with EGFP controls (Fig. 4E). In contrast, caHSF1 overexpression had no effect on the sucrose preference, social investigation, or social resident–intruder tests (Fig. S5 A–C). We found that the overexpression of caHSF1 in the hippocampus of neonatal wild-type mice did not influence their latency to feed (Fig. S5D) or their immobility time (Fig. S5E) during adulthood. We also examined the behavioral effects of direct caHSF1 overexpression in the hippocampus of adult HSF1−/− mice; caHSF1 overexpression in adults had no significant effects on the behavioral assays performed (Fig. S6). We next examined the expression levels of postsynaptic density protein 95 (PSD95), a postsynaptic marker, by Western blotting to investigate whether the overexpression of caHSF1 also would reverse the aberrant synaptic formation that occurs in HSF1−/− mice. We found reduced expression of PSD95 in the hippocampus of adult HSF1−/− mice, and this reduction was reversed by overexpression of caHSF in neonatal HSF1−/− mice (Fig. 4 F and G). These results suggest that hippocampal HSF1 has a crucial role in the normal development of certain types of behavior, such as anxiety and depression-like behaviors, as well as in synapse formation, during the early postnatal period.
Fig. 4.
Effect of rescuing HSF1 deficiency in the neonatal hippocampus on behavioral and neuronal development. (A) Fluorescence micrograph of a neonatal mouse hippocampus after injection on postnatal day1–2 with EGFP expression plasmid complexed with PEI. EGFP fluorescence was detected in the hippocampal region 3 d after the injection. (B) Western blotting with anti-HA antibody shows the transduction of HA-caHSF1 in the hippocampus of mice 3 d after the injection of PEI/caHSF1 complex. (C–E) Bar graphs showing (C) latency to feeding and (D) food consumption in the novelty-suppressed feeding test (n = 14–16 for each group) and (E) immobility time in the forced swim test (n = 14–16 for each group). *P < 0.05. (F and G) Western blotting with anti-PSD95 antibody (F) and bar graph (G) showing that the reduced expression of PSD95 in the hippocampus of HSF1−/− mice was reversed by the overexpression of caHSF1 (n = 4–6 for each group). *P < 0.05.
Molecular Mechanism of Reduced PSA-NCAM Expression in HSF1-Deficient Mice.
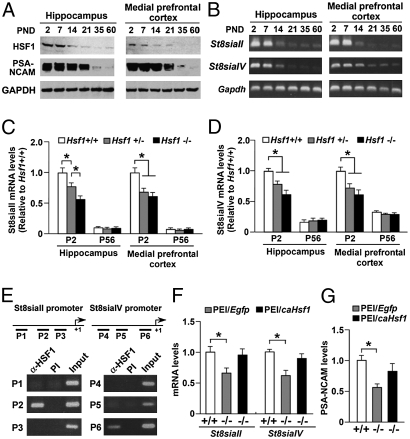
We next investigated the molecular mechanisms underlying the aberrant behavior observed in adult HSF1−/− mice. As mentioned earlier, we found that the levels of hippocampal PSA-NCAM were dependent on the expression of HSF1 in neonatal mice (Fig. 2E). PSA promotes synaptogenesis and synaptic plasticity (17, 18). PSA biosynthesis is realized by two polysialyltransferases, ST8SiaII and ST8SiaIV (19). Importantly, these polysialyltransferases are required for hippocampal formation, synaptic plasticity, memory formation, and anxiety behavior (20–22). We therefore speculated that HSF1 deficiency could affect the transcriptional regulation of St8siaII and St8siaIV, leading to reduced PSA-NCAM expression and subsequent behavioral changes in HSF1-deficient mice. This hypothesis is supported by findings of developmental changes in hippocampal HSF1 and PSA-NCAM protein levels and in hippocampal St8siaII and St8siaIV mRNA levels (Fig. 5 A and B). HSF1 expression and PSA-NCAM expression were abundant in neonatal mice but gradually decreased with brain development (Fig. 5A). Similarly, RT-PCR experiments revealed that the expression of St8siaII and St8siaIV mRNA decreased with brain development (Fig. 5B). Next, we quantified the mRNA levels of St8SiaII and St8SiaIV in the hippocampus and medial prefrontal cortex of HSF1 mutants. We found reduced expression of St8siaII (Fig. 5C) and St8siaIV (Fig. 5D) mRNA in HSF1 mutants on postnatal day 2. To assess the role of HSF1 in the regulation of St8siaII and St8siaIV transcription more directly, we performed ChIP assays of hippocampal DNA. The ChIP assays showed that HSF1 binds exclusively to the P2 and P6 regions of the St8siaII and St8siaIV promoters, respectively, in the hippocampus of 1-wk-old mice (Fig. 5E). The binding of HSF1 to these promoter regions was not observed in HSF1−/− mice (Fig. S7 A and B), indicating the specificity of the ChIP protocol. In addition, we found that there were potential HSEs in the St8siaII and St8siaIV promoters (Fig. S7 C and D). Furthermore, the direct overexpression of caHSF1 in the hippocampus of neonatal HSF1−/− mice increased the levels of St8siaII and St8siaIV mRNA (Fig. 5F), as well as PSA-NCAM levels (Fig. 5G), to control levels, which were assessed 5 d after injection of PEI/DNA complexes. These results strongly suggest that HSF1 deficiency down-regulates St8siaII and St8siaIV transcription and concomitantly reduces PSA-NCAM levels in the hippocampus.
Fig. 5.
HSF1 regulates the expression of St8siaII and St8siaIV. (A) Western blotting showing the expression levels of HSF1 and PSA-NCAM proteins in the hippocampus and the medial prefrontal cortex of HSF1+/+ mice at different developmental stages. (B) Real-time PCR analysis showing the expression levels of St8siaII and St8siaIV mRNA in the hippocampus and the medial prefrontal cortex of HSF1+/+ mice at differential developmental stages. PND, postnatal day. (C and D) Real-time PCR analysis showing the expression levels of (C) St8siaII and (D) St8siaIV mRNA in the hippocampus and the medial prefrontal cortex of HSF1+/+, HSF1+/−, and HSF1−/− mice on postnatal days 2 (P2) and 56 (P56) (n = 6 for each group). (E) ChIP assay with HSF1 antibody (α-HSF1) or preimmune serum (PI) as well as with input DNA shows the recruitment of HSF1 to the St8siaII and St8siaIV promoters in the neonatal hippocampus of mice (postnatal day 2). The transcription start site is denoted as +1. Underscoring indicates the positions of the PCR primers (P1–P6) used in the ChIP assay. (F and G) Overexpression of caHSF1 in the neonatal hippocampus of HSF1−/− mice restored the expression of (F) St8siaII and St8siaIV mRNA and (G) PSA-NCAMs (n = 8 for each group). *P < 0.05.
Enzymatic Removal of PSA from the Neonatal Hippocampus Impaired Behavioral Development.
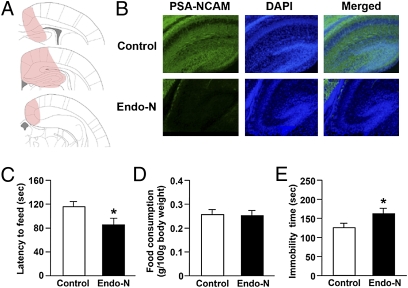
To investigate the functional consequences of the down-regulation of PSA-NCAMs by HSF1 deficiency in the hippocampus, we injected endoneuraminidase (endo-N) bilaterally into the neonatal hippocampus. Endo-N is a phage enzyme that specifically cleaves α2,8-linked sialic acid polymers and diffuses rapidly in tissues, thereby selectively removing PSA (23). Fig. 6B shows the PSA-NCAM immunoreactivity in representative mice treated with endo-N or heat-inactivated endo-N. Successful removal of PSA was observed in the endo-N–treated mice. Then we performed behavioral assays in adult mice. In the novelty-suppressed feeding test, endo-N infusion decreased the latency to feeding (Fig. 6C) without altering feeding activity (Fig. 6D). In the forced swim test, infusion of endo-N increased the immobility time (Fig. 6E). However, in the sucrose preference, social investigation, and social resident–intruder tests, endo-N injections had no effects on sucrose preference, social interaction time, or attack latency, respectively (Fig. S8). Finally, we injected endo-N bilaterally into the adult hippocampus and performed behavioral assays. Endo-N injection into the adult hippocampus had no significant effects on the types of behavior examined (Fig. S9). These results suggest that PSA-NCAMs during early postnatal development have an important influence on anxiety- and depression-related behaviors in adults.
Fig. 6.
Effect of enzymatic removal of PSA-NCAMs from the neonatal hippocampus on behavioral development. Endo-N or heat-inactivated endo-N (control) was injected bilaterally into the hippocampus of mice at postnatal day 1–2. Behavioral assays were performed in young adults. (A) The distribution of PSA-NCAM immunoreactivity 4 d after endo-N injection. The colored areas in the brain template represent the diffusion of the enzyme. (B) Representative immunofluorescence images of PSA-NCAMs (green) and DAPI (blue) in mice injected with heat-inactivated endo-N (Upper) or endo-N (Lower). (C–E) Bar graphs showing (C) latency to feeding and (D) food consumption in the novelty-suppressed feeding test (n = 14–16 for each group) and (E) immobility time in the forced swim test (n = 14–16 for each group). *P < 0.05.
Discussion
In the present study, we investigated the effects of HSF1 deficiency on neuronal and behavioral development. We found that HSF1 deficiency resulted in impaired hippocampal spinogenesis and neurogenesis, suggesting essential roles for HSF1 in normal brain development even under nonstressed conditions in vivo. We also found that the direct overexpression of caHSF1 in the hippocampus of neonatal but not adult HSF1−/− mice partially reversed this aberrant behavior. Furthermore, HSF1+/− mice showed increased vulnerability to repeated restraint stress exposure. At the molecular level, HSF1 deficiency reduced St8siaII and St8siaIV mRNA levels and subsequently reduced PSA-NCAM expression in the hippocampus. Moreover, St8siaII and St8siaIV mRNA levels were modulated directly by HSF1 in vivo. Strikingly, enzymatic removal of PSA from the neonatal hippocampus reduced anxiety but increased depression in mice. Thus, our data suggest that HSF1 regulates PSA-NCAM levels through the transcriptional control of St8siaII and St8siaIV in the hippocampus during the early postnatal period and that this process may be involved in the behavioral and neuronal development of mice.
We found aberrant affective behaviors in HSF1−/− mice. A previous report also demonstrated reduced anxiety behaviors in HSF1−/− mice (in a mixed-strain background of BALB/c and 129SV) (24). Importantly, our data indicate that rescuing the HSF1 deficiency in the neonatal hippocampus reversed the aberrant behavior in the forced swim and novelty-suppressed feeding tests. However, the other types of behavior, such as sucrose preference, aggression, and sociability, were not affected by this manipulation. This selective reversal of anxiety- and depression-like behaviors may have resulted from the broad range of neural dysfunction in HSF1−/− mice. Our data suggest that hippocampal function modulated by HSF1-mediated gene regulation is involved in the behavioral performance in the novelty-suppressed feeding and forced swim tests, whereas the aberrant social and hedonic behaviors in HSF1−/− mice may be caused by the dysregulation of brain structures other than hippocampus. In fact, the activity of the hippocampal local network (the dentate gyrus to CA1) is linked to depression-related behavior in the forced swim test (25). In addition, adult hippocampal neurogenesis is required to observe the behavioral effects of antidepressants in the novelty-suppressed feeding test (26). Moreover, given that adult-generated immature neurons integrate into a preformed neuronal circuit that is associated with hippocampal function (27), the behavioral abnormalities of HSF1 mutants in the forced swim and novelty-suppressed feeding tests likely involve the combination of reduced numbers of newly born neurons and their aberrant integration into the existing circuitry. However, decreased neurogenesis generally is thought to be associated with increased anxiety in the novelty-suppressed feeding test (26), and this supposition is inconsistent with our data. Further studies are needed to clarify the role of HSF1-mediated regulation of neurogenesis on behaviors as well as the brain region-specific role of HSF1 in the regulation of various behaviors.
We found that HSF1deficiency led to increased levels of depression-like behavior but reduced levels of anxiety behavior. Depression and anxiety often are comorbid conditions, but in this case these behavioral effects seem to occur in opposition. Interestingly, those incongruent behaviors also are observed in Clock-knockdown mice, which show a mixed state of manic- and depressive-like behavior: less anxiety and hyperactivity but greater depression-like behavior (28). Additionally, HSF1 is reported to act as a circadian transcription factor (29). Taken together, these findings indicate that HSF1 deficiency may cause a rhythmic change during development, resulting in a mixed state of manic- and depression-like behavior. However, there is a possibility that the reduced anxiety behavior of the HSF1 mutants is related to their hyperactivity, because they showed increased locomotion in the open field test. Another possible reason for the increased depression-like and reduced anxiety behaviors in HSF1−/− mice is an altered responsiveness to stressful situations. HSF1−/− mice may be unable to adapt to unusual stressful events and may show abnormal coping behavior. In support of this notion, we found increased susceptibility to restraint stress in HSF1−/− mice. However, further studies are needed to clarify the complex interactions between anxiety and depressive behaviors in rodents.
PSA is an important posttranslational protein modification of NCAMs that is involved in the development and function of the central nervous system (30). Previous reports have shown that PSA removal enhances neuronal differentiation of hippocampal adult progenitors in vivo (15) but prevents the synapse formation of hippocampal neurons (31), thus supporting our observations of increased expression of DCX and reduced spine densities in the dentate gyrus of HSF1−/− mice. Interestingly, the phenotypes of HSF1-mutant mice are closely related to those of ST8SiaII- and ST8SiaIV-deficient mice: (i) ST8SiaII and ST8SiaII/IV double deficiency reduced and eliminated PSA-NCAM expression, respectively, in the brain (21, 22); (ii) ST8SiaIV-deficient mice had reduced PSA+ and increased DCX+ cell numbers in the hippocampus (32); (iii) ST8SiaII and ST8SiaIV double-deficient mice had enlarged lateral ventricles (22); and (iv) ST8SiaII- and ST8SiaIV-deficient mice showed altered anxiety behavior, locomotor activity, sociability, and aggression (21, 33). These data support the view that the reduced PSA-NCAM level caused by hypoexpression of ST8SiaII and ST8SiaIV is one of the mechanisms underlying the aberrant brain and behavioral development of HSF1-deficient mice.
In conclusion, the data presented here suggest that HSF1-mediated gene regulation in the brain during early postnatal development is involved in neuronal and behavioral development as well as in vulnerability to repeated stress exposure during adulthood. Moreover, our data suggest that HSF1 controls hippocampal PSA-NCAM levels through the transcriptional regulation of genes for polysialyltransferases and that this process might be involved in hippocampal function and subsequent behavioral regulation. Thus, we propose that aberrant HSF1 function in early brain development may increase susceptibility to neuropsychiatric disorders during adulthood.
Materials and Methods
The generation and maintenance of HSF1-null mice (ICR genetic background) were conducted as described previously (34). Behavioral testing of adult male mice was performed during the light phase of the light/dark cycle, as previously reported (5, 35, 36). PEI-mediated gene transfer was performed as previously described (5, 36). To eliminate PSA-NCAMs, the highly specific PSA-glycosidase endo-N was used (37). Data are presented as means ± SEM. Detailed methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (to S.U. and Y.W.), a grant for research on psychiatric and neurological diseases and mental health from the Japanese Ministry of Health, Labor, and Welfare (to S.U. and Y.W.), and by the Yamaguchi University Research Project on STRESS (Y.W. and A.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016424108/-/DCSupplemental.
References
- 1.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 2.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 3.Flavell SW, Greenberg ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. 2008;31:563–590. doi: 10.1146/annurev.neuro.31.060407.125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver IC, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 5.Uchida S, et al. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- 8.Liu XH, Kato H, Nakata N, Kogure K, Kato K. An immunohistochemical study of copper/zinc superoxide dismutase and manganese superoxide dismutase in rat hippocampus after transient cerebral ischemia. Brain Res. 1993;625:29–37. doi: 10.1016/0006-8993(93)90134-9. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 10.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 13.Santos SD, Saraiva MJ. Enlarged ventricles, astrogliosis and neurodegeneration in heat shock factor 1 null mouse brain. Neuroscience. 2004;126:657–663. doi: 10.1016/j.neuroscience.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Homma S, et al. Demyelination, astrogliosis, and accumulation of ubiquitinated proteins, hallmarks of CNS disease in hsf1-deficient mice. J Neurosci. 2007;27:7974–7986. doi: 10.1523/JNEUROSCI.0006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess A, et al. Polysialic acid regulates the clustering, migration, and neuronal differentiation of progenitor cells in the adult hippocampus. Dev Neurobiol. 2008;68:1580–1590. doi: 10.1002/dneu.20681. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto M, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. J Biol Chem. 2005;280:34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 17.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 19.Eckhardt M, et al. Molecular characterization of eukaryotic polysialyltransferase-1. Nature. 1995;373:715–718. doi: 10.1038/373715a0. [DOI] [PubMed] [Google Scholar]
- 20.Eckhardt M, et al. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20:5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angata K, et al. Sialyltransferase ST8Sia-II assembles a subset of polysialic acid that directs hippocampal axonal targeting and promotes fear behavior. J Biol Chem. 2004;279:32603–32613. doi: 10.1074/jbc.M403429200. [DOI] [PubMed] [Google Scholar]
- 22.Weinhold B, et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280:42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 23.Vimr ER, McCoy RD, Vollger HF, Wilkison NC, Troy FA. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci USA. 1984;81:1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu X, et al. Basal behavioral characterization of hsf1 deficient mice and its cellular and behavioral abnormalities underlying chronic unpredictable stressors. Behav Brain Res. 2008;193:225–229. doi: 10.1016/j.bbr.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Airan RD, et al. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- 26.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee S, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reinke H, et al. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008;22:331–345. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 31.Dityatev A, et al. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nacher J, et al. Divergent impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid expression in immature neurons and interneurons of the adult cerebral cortex. Neuroscience. 2010;167:825–837. doi: 10.1016/j.neuroscience.2010.02.067. [DOI] [PubMed] [Google Scholar]
- 33.Calandreau L, Márquez C, Bisaz R, Fantin M, Sandi C. Differential impact of polysialyltransferase ST8SiaII and ST8SiaIV knockout on social interaction and aggression. Genes Brain Behav. 2010;9:958–967. doi: 10.1111/j.1601-183X.2010.00635.x. [DOI] [PubMed] [Google Scholar]
- 34.Inouye S, et al. Activation of heat shock genes is not necessary for protection by heat shock transcription factor 1 against cell death due to a single exposure to high temperatures. Mol Cell Biol. 2003;23:5882–5895. doi: 10.1128/MCB.23.16.5882-5895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida S, et al. Characterization of the vulnerability to repeated stress in Fischer 344 rats: Possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor. Eur J Neurosci. 2008;27:2250–2261. doi: 10.1111/j.1460-9568.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 36.Uchida S, et al. Epigenetic status of Gdnf in the ventral striatum determines susceptibility and adaptation to daily stressful events. Neuron. doi: 10.1016/j.neuron.2010.12.023. in press. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Fernandez MA, et al. Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J Neurosci. 2007;27:4552–4561. doi: 10.1523/JNEUROSCI.0396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.