Abstract
In Saccharomyces cerevisiae, silent chromatin inhibits the expression of genes at the HML, HMR, and telomeric loci. When silent chromatin forms de novo, the rate of its establishment is influenced by different chromatin states. In particular, loss of the enzyme Dot1, an H3 K79 methyltransferase, leads to rapid silencing establishment. We tested whether silencing establishment was antagonized by H3 K79 methylation or by the Dot1 protein itself competing with Sir3 for binding sites on nucleosomes. To do so, we monitored fluorescence activity in cells containing a GFP gene within the HML locus during silencing establishment in a series of dot1 and histone mutant backgrounds. Silencing establishment rate was correlated with Dot1’s enzymatic function rather than with the Dot1 protein itself. In addition, histone mutants that mimicked the conformation of unmethylated H3 K79 increased the rate of silencing establishment, indicating that the H3 K79 residue affected silencing independently of Dot1 abundance. Using fluorophore-based reporters, we confirmed that mother and daughter cells often silence in concert, but in instances where asymmetric silencing occurs, daughter cells established silencing earlier than their mothers. This noninvasive technique enabled us to demonstrate an asymmetry in silencing establishment of a key regulatory locus controlling cell fate.
Keywords: silent information regulator 3, disruptor of telomeric silencing 1, lysine methyltransferase 4
The transcriptional output of a gene must integrate both activating and repressing forces. To turn off transcription, repressors and silencing proteins must override activating signals and vice versa. In the yeast Saccharomyces cerevisiae, transcriptional silencing of the cryptic mating genes at HML and HMR requires the localization of silent information regulatory (Sir) proteins across the silenced loci (1–4). HML and HMR loci are constitutively repressed in wild-type cells. However, conditional alleles of the SIR genes have led to a greater understanding of heterochromatin establishment, loss, and maintenance (5–9). Investigating the dynamics of transitions between heterochromatin and euchromatin in mutants with altered chromatin structure should reveal how chromatin modifications impact the formation of these two states.
Silencing establishment is thought to occur in multiple steps with Sir proteins initially recruited to the silencers flanking HML and HMR and subsequently enriched throughout the silenced loci (3, 8, 10). The apparent spreading of Sir proteins from silencers requires Sir2-dependent deacetylation of the critical histone residue H4 K16 and possibly other acetylated lysines as well (3, 11–14). Because nucleosomes composed of unacetylated histones have a higher affinity for Sir protein complexes, histone deacetylation promotes the localization of Sir proteins throughout HML and HMR, leading to loss of transcription at those loci (9, 15, 16).
Unlike transcriptional activation that can occur on the order of a few minutes, transcriptional silencing at the HML and HMR loci is a longer process that requires multiple generations for full population-wide completion. Many events occur concurrently with silencing establishment such as DNA replication, cell-cycle progression, movement of the silent locus from an internal nuclear space to a more peripheral nuclear space, and an alteration of the chromatin status across the silenced region. Interestingly, DNA replication and nuclear localization are not required for silencing establishment (17–19). Cell-cycle progression through M-phase, however, is required for silencing establishment, but progression through S-phase is required only at HMR and not at HML (5, 20, 21). Further, chromatin modifications influence the speed of the silencing establishment process.
Previously, we showed that when Sir3 protein is first introduced to sir3Δ cells, typically two cell divisions are required before HML is silenced (22). In that study, we used a phenotypic output, the sensitivity of matΔ cells to α-factor, to report functional silencing at HML in a dividing population. The expression status of the HML locus was assayed by monitoring a cell's ability to adopt the a-cell mating type and respond to α-factor, a feat that is possible only when HMLα is silenced. For this current study, we monitored the progress of silencing using a destabilized GFP reporter at HML (23). This approach complemented our previous technique that could evaluate a cell's expression only during G1 of the cell cycle, when the cells are competent to respond to α-factor. In contrast, the expression of the GFP can be monitored continuously throughout one or more cell cycles and can, in principle, provide a graded signal rather than a binary response. Further, the GFP technique is noninvasive as it does not require micromanipulations or α-factor exposure.
Yeast euchromatin is enriched for nucleosomes that contain acetylation and methylation marks, whereas silent chromatin lacks these modifications (24, 25). When silent chromatin is formed, the levels of H4 K16 acetylation decline across the HMR locus followed soon after by a decline in H3 K4 methylation and H3 K79 methylation (8). Interestingly, the removal of methylation at these positions by deletion of the genes encoding their methyltransferases allows silencing to establish more rapidly as measured either by the association of Sir proteins with chromatin or by phenotypic changes (8, 22). Thus, the removal of methyl marks seems to be an integral step in silent chromatin formation rather than a passive consequence of silencing. By inference, H3 K79 and H3 K4 methyl marks antagonize silent chromatin formation in some way.
Among the sites of histone methylation, H3 K79 methylation (catalyzed by the Dot1 methyltransferase) has the largest antagonistic effect on silent chromatin formation (26–29). Recent biochemical evidence suggests that Dot1 influences silencing in two ways. First, Dot1 catalyzes the mono-, di-, and trimethylation states of H3 K79 (27, 28, 30, 31). This particular lysine is located on the loss of rDNA silencing (LRS) face of H3, a surface whose electrostatic properties are important for association between the nucleosome and the BAH domain of Sir3 (32–36). H3 K79 methylation, therefore, interferes with the nucleosome's ability to adequately bind Sir3 (9). Dot1 can impact silencing formation in a second manner. Dot1 and Sir3 also compete directly for binding to a basic patch on the N-terminal tail of histone H4. Sir3 proteins at the telomeres can reduce Dot1 enrichment there by this competition (37, 38).
We are interested in determining the extent to which Dot1 impacts the kinetics of silent chromatin formation either indirectly through H3 K79 methylation or directly via competition with Sir3. To determine which possible mechanism of Dot1 exerts a greater impact, we combined the tools developed to monitor silencing dynamics continuously and at the single-cell level (23) with classics of the genetics toolkit including DOT1 overexpression vectors (37) and mutant alleles of histone genes (27). In addition, to determine whether cell-cycle dynamics or history within a pedigree affected silencing, we continuously monitored dividing populations of cells that encoded a destabilized GFP allele at HML, building on the foundation laid by Xu and Broach (23) for combining fluorescent reporters and flow cytometry in studies of transcriptional silencing.
Results
Kinetics of Silent Chromatin Formation.
A reporter gene encoding a fast-folding, high-turnover GFP protein at the HML locus allows real-time monitoring of silencing-dependent expression patterns (23). The stability of fluorophores like GFP can limit their use in the detection of dynamic changes. By fusing a Cln2 PEST degradation tag to the carboxyl terminus of GFP, the half-life of GFP was significantly reduced. Cln2 undergoes constant 26S-proteosome-dependent degradation throughout the cell cycle due, in part, to its PEST sequence (39–41). It is estimated that the folding time of the degron-tagged GFP is on the order of 20 min, whereas its half-life estimates range from 20 to 50 min (23, 40). Because a trade-off in reducing fluorophore stability is lower signal accumulation, a nuclear localization sequence (NLS) was fused to GFP to concentrate GFP to the nucleus, thereby improving detection. All strains generated for this study are listed in Table S1.
When placed within the HML locus, the GFP::PEST::NLS construct is subject to Sir-protein–mediated silencing. We verified that GFP fluorescence intensity was not detected unless silencing was disrupted by either mutation of one of the Sir proteins (23) or chemical inhibition of Sir2 using nicotinamide (NAM) (Fig. 1). The expression output of GFP in unsilenced conditions was visualized and quantified by both flow cytometry (Fig. 1 B–D) and quantitative microscopy (Fig. 1 E and F). In both instances, cells with silenced HML loci exhibited minimal fluorescence, whereas those with expressed HML had a 3-fold increase in fluorescence intensity. This fold difference is narrower than molecular measurements of silencing such as qRT-PCR of the a1 mRNA (1,000-fold dynamic range) or microscopy experiments with a stable GFP reporter (10-fold dynamic range). However, the high turnover rate of GFP is optimized to assay for dynamic sensitivity at the cost of signal strength. Despite the more restricted signal intensity, measurements of fluorescence intensity yielded narrow 95% confidence intervals and were reproducible across replicates (Fig. S1).
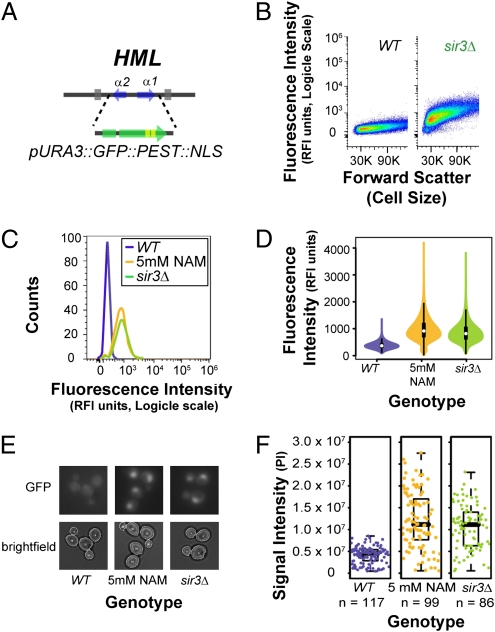
Fig. 1.
The expression status of HML visualized using fluorescent markers. (A) A gene encoding a nuclear-localized, destabilized version of GFP expressed from the URA3 promoter was integrated into the HML locus replacing α1 and α2 genes. (B) Fluorescence intensity of cells containing the hml::GFP reporter in either SIR3 or sir3Δ backgrounds was measured using a flow cytometer. Fluorescence intensity is plotted against forward scatter, a measurement proportional to cell size. (C) Fluorescence intensity measurements of cells containing an hml::GFP reporter are shown as a scaled histogram. Both sir3Δ and 5-mM nicotinamide profiles are shown. (D) The same information in C is here illustrated as “violin plots,” which are symmetrized kernel density plots with a small box plot illustrated inside them. In these plots, the wider the violin shape is at a given fluorescence intensity, the more cells are associated with that value. The white dot indicates the geometric mean of the full population and the thick and thin lines are a box plot. (E) Cells containing the hml::GFP reporter imaged by microscopy. (F) Signal intensities tabulated by quantitative microscopy for hml::GFP cells in three cultures.
To determine whether our reporter strains were capable of visualizing dynamic changes in expression status, we used flow cytometry to measure the fluorescence intensity of hml::GFP::PEST::NLS strains after silencing was disrupted by nicotinamide and then reestablished (Fig. 2 and Fig. S1). To do so, we grew cells in nicotinamide to produce a silencing-compromised condition in which GFP was expressed from the HML locus. By washing out the nicotinamide and resuspending cells in fresh media, we could observe the loss of GFP signal as a proxy for the loss of transcription occurring at HML. The kinetics of silencing establishment measured using this approach were consistent with previous studies (22, 42) showing that silencing is reestablished within 6 h following restoration of Sir protein function. Interestingly, between the first and the second time points taken after washing, a small elevation of GFP fluorescence was observed before a drop of signal intensity. Although the reason for this initial elevation in signal intensity was unknown, it is possible that fresh media itself may be stimulatory or that nicotinamide may have off-target effects that dampen transcriptional activity or growth in general. We also observed slight differences in the starting signal intensities of some replicates. Some starting point signal intensities were slightly variable across replicates whereas others appeared characteristic of a given strain (Fig. S1).
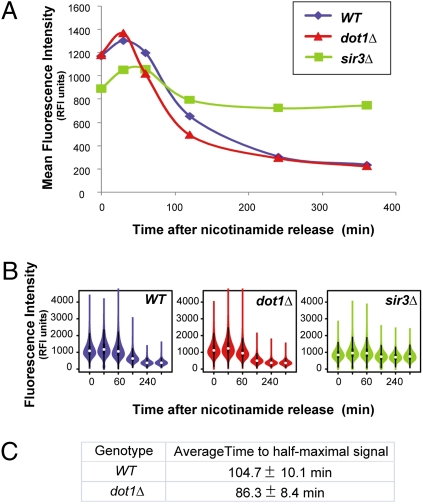
Fig. 2.
The establishment of silencing visualized by flow cytometry. (A) Isogenic cultures of genotypes DOT1 (JRY9101), dot1Δ (JRY9104), and sir3Δ (JRY9103) were grown in 5 mM nicotinamide to derepress hml::GFP::PEST::NLS. Silencing establishment was measured in cultures washed of nicotinamide. The mean fluorescence intensity in relative fluorescence units (RFI units) is shown plotted against time for the three cultures. Ninety-five percent confidence intervals for these datasets are tabulated in Fig. S1. (B) Violin plots of the same data in A are illustrated to show the spread of the data. (C) The time to half-maximal signal intensity was calculated for each strain in each replicate as illustrated in Fig. S2. These values were averaged over the three replicates and SDs were calculated and tabulated.
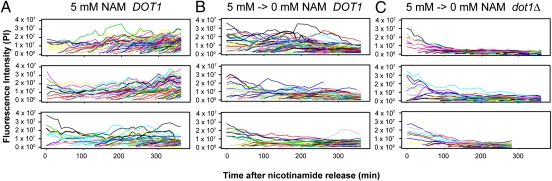
Our flow-cytometry–based assays of silencing establishment confirmed earlier observations that the loss of chromatin-modifying enzymes alters the rate of silencing establishment (8, 22). Cells deficient in the H3 K79 methyltransferase encoded by DOT1 establish silencing more rapidly than wild-type cells. In the flow cytometry experiments, dot1Δ cells reached half-maximal signal intensity by an average of 86.3 min after release from nicotinamide compared with 104.7 min in DOT1 cells (Fig. 2 and Fig. S2). Whereas previous studies measured silencing using mating-type-dependent α-factor sensitivity, a binary readout, these new data indicated that the rapid rate of silencing establishment in dot1Δ cells was also evidenced by a graded output (GFP intensity) and was free from potential complications of α-factor treatment. By 240 min, the dot1Δ and wild-type strains were indistinguishable with respect to the extent of silencing.
Mechanisms of Dot1 Antagonism on Silencing.
dot1Δ cells establish silencing up to one cell division faster than DOT1 cells as measured by phenotypic outputs in single cells (22), 1–2 h faster as measured by Sir3 occupancy in ChIP studies (8), and up to 20 min faster in the GFP-reporter experiments (Fig. 2 and Fig. S2). One explanation for the accelerated silencing observed in dot1 cells may involve H3 K79 methylation impeding Sir3 assocation with histone H3. Alternatively, Dot1 protein may directly slow silencing by competing with Sir3 for association with a basic patch on histone H4 (37). Both mechanisms impact Sir3 protein binding in telomeric regions of the genome, but their impact on silencing at HML and HMR is not known, and their impact on the rate of silencing establishment has never been investigated. To test which mechanisms are responsible for Dot1’s ability to antagonize silencing establishment, we evaluated dot1Δ cells with an overexpression plasmid containing either a functional copy of the DOT1 gene or a dot1 point mutant (Fig. 3 A and B, Table S2). This dot1 mutant allele replaced a single amino acid critical for catalytic function but still allowed stable association with histone H4 (37). If Dot1 catalytic activity were required to antagonize silencing, then the catalytically inactive mutant should phenocopy the accelerated kinetics of silencing establishment observed in dot1Δ cells. Conversely, if Dot1 protein were impeding the kinetics of silencing establishment due to its competition with Sir3 for histone H4, the overexpression of Dot1—catalytically active or not—should impede silencing establishment.
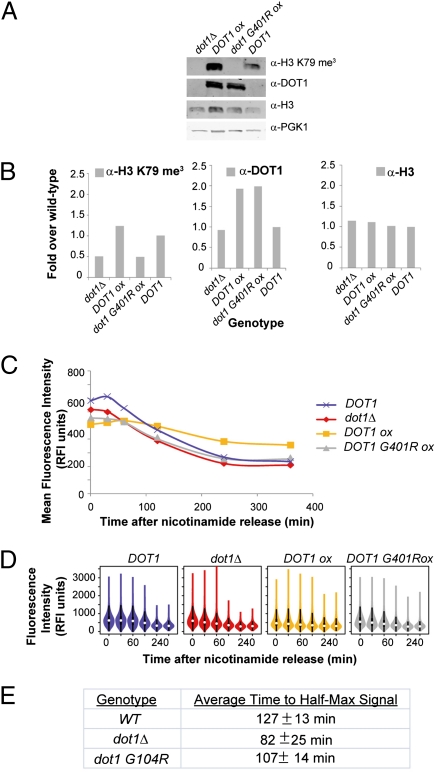
Fig. 3.
Overexpression of catalytically active and inactive Dot1. Cells carrying the overexpression construct pGAL1::DOT1 (labeled DOT1-ox) or the catalytically inactive overexpression construct pGAL1::dot1 G401R were tested for their kinetics of silencing establishment. dot1Δ cells and DOT1 cells were used as controls in these assays. (A) Strains grown in galactose medium were tested for global H3 K79 me3 levels by protein immunoblot with α-H3 K79 me3 antibody. Due to the overexpression of Dot1 proteins, the wild-type levels of Dot1 cannot be markedly observed at these exposure levels. However, the presence of wild-type Dot1 is confirmed by its enzymatic property, H3 K79 trimethylation. (B) H3 K79 me3 signals were quantified and normalized to H3 levels. Dot1 and H3 signals were quantified and normalized to PGK1. (C) Silencing establishment time-course assays of strains were performed under inducing (galactose) conditions. Mean fluorescence intensities are plotted against time after release from nicotinamide. (D) Violin plots indicate the distribution of the data from C. (E) The time to half-maximal signal intensity was calculated for each strain in each replicate and averaged over the three replicates.
The overexpression of a catalytically inactive version of dot1 accelerated silencing establishment to the same extent as the dot1Δ mutation (Fig. 3 C and D and Fig. S3A). In contrast, overexpression of functional DOT1 slowed silencing establishment and altered levels of silencing at both the start and the finish of the time course (Fig. 3 C and D and Fig. S3A). Therefore, it was the modification status of H3 K79 that affected silencing and not the overall concentration of Dot1. Together, these results indicated that Dot1 antagonized silencing via H3 K79 methylation.
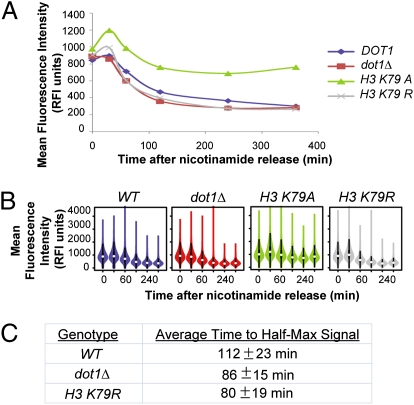
To provide an independent test of whether H3 K79 methylation was the critical feature affecting the kinetics of silencing, we performed silencing establishment time-course assays in yeast strains containing mutated histone proteins. Dimethylation (and by inference, trimethylation) of lysine at residue H3 K79 causes the lysine side chain to assume an alternative conformation that alters the local electrostatic potential by partially uncovering H4 V70 and H3 L82 (43). The replacement of lysine with arginine prevents this electrostatic alteration, thereby mimicking the unmethylated conformation. If H3 K79 methylation were critical in antagonizing silencing, then the H3 K79R mutant would be expected to phenocopy dot1Δ cells with respect to the kinetics of silencing. For comparison, mutating H3 K79 to alanine abrogates silencing potentially by disrupting the charge-based interaction between either H3 and Sir3 or H3 and H4 (32, 44). We replaced plasmids carrying HHT2::HHF2 alleles with plasmids carrying the appropriate K79R mutation (hht2K79R::HHF2). Confirming the dot1 mutant analysis above, the H3 K79R mutant cells phenocopied the dot1Δ strains by accelerating the establishment of silencing (Fig. 4 and Fig. S3). Because Dot1 itself was unchanged in these experiments, H3 K79 methylation status was the main feature that correlated with silencing establishment efficacy.
Fig. 4.
Dot1 antagonized silencing through H3 K79 methylation. (A) Wild-type cells (JRY9121), dot1Δ cells (JRY9123), cells containing H3 K79A histone mutations (JRY9125), and cells containing H3 K79R histone mutations (JRY9127) were grown in 5 mM nicotinamide and then rinsed of nicotinamide at the start of a time-course assay. The mean fluorescence intensity is plotted against time after release from nicotniamide. (B) Violin plots are illustrated to show the distributions. (C) The time to half-maximal signal intensity was calculated for each strain in each replicate and averaged over the three replicates.
Silencing Establishment in Dividing Populations of Cells.
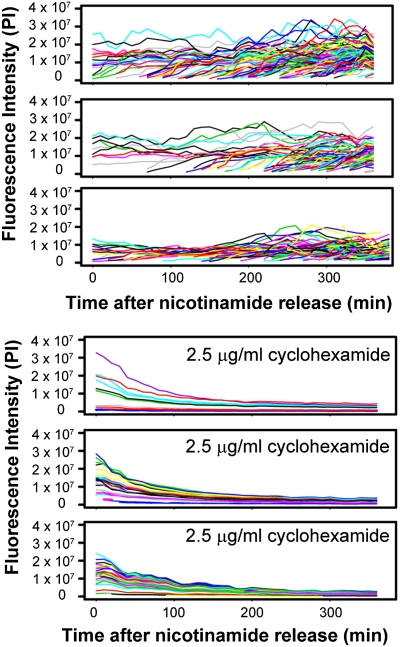
An advantage of the GFP reporter strain is its ability to report expression from the HML locus continuously throughout the cell cycle whereas earlier studies evaluated the expression status of HML only at G1. To determine whether quantitative microscopy could be used to image HML expression dynamics in dividing populations of cells, we measured the fluorescence intensity in sir3Δ hml::GFP::PEST::NLS cells using quantitative, time-lapse microscopy. This technique is distinct from flow cytometry in that cell-cycle, cell history, and pedigree information can be determined in addition to GFP reporter intensity measurements. To visualize the range of GFP expression over time in the absence of silencing, we quantified the GFP fluorescence of 10–20 cells and all their progeny using an automated system. The total GFP intensities of each cell were graphed over time as a series of line traces (Fig. 5) that illustrate the variation in fluorescence intensity over time.
Fig. 5.
Transcriptional variation and GFP degradation. (Upper) To measure fluorescent variability over time, cells of the genotype hml::GFP::NLS::PEST sir3Δ (JRY9103) were measured for fluorescence intensity using quantitative microscopy. Traces of the fluorescence intensity of each cell are displayed as line plots where each line represents the intensity of a given cell over the time course. Three replicates are depicted. (Lower) To determine the degradation rate of the GFP fluorophore, hml::GFP::NLS::PEST sir3Δ cells (JRY9103) were exposed to 2.5 μg/mL cycloheximide 10 min before the start of a similar time course. The loss of fluorescence intensity over time is shown for three replicates. Fluorescence intensities are plotted in pixel intensity units.
The level of GFP fluorescence in a given cell integrates the rates of GFP transcription, translation, folding, and degradation. Because the rates of GFP protein folding and degradation have been shown to remain constant throughout the cell cycle and are routinely used as a negative control for proteins with altered cell-cycle folding or degradation (23, 40, 45), we assumed that the impact of translation, folding, and degradation should be constant and that altered transcription would have the biggest impact on fluorescence intensity variation. Still, we measured the half-life of our GFP proteins over time by tracking the decline of fluorescence intensity in cells treated with 2.5 μg/mL cycloheximide, an inhibitor of translation. Indeed, GFP signal intensity dropped precipitously in response to cycloheximide addition (Fig. 5, Lower), reaching a half-maximal level after just 50 min. Cells exposed to cycloheximide showed synchronous loss of fluorescence intensity even though they were grown asynchronously, further confirming that cell-cycle impacts on degradation rate were minimal.
To determine whether quantitative microscopy could be used to monitor silencing establishment, we conducted time-course experiments in which SIR3 hml::GFP::PEST::NLS-containing cells were grown overnight in 5 mM nicotinamide, rinsed of nicotinamide, and mounted on a microscope slide for observations of silencing reestablishment. Cells grown continuously in 5 mM nicotinamide retained relatively high GFP signal intensities (Fig. 6A) similar to those of sir3Δ cells (Fig. 5). Consistent with previous experiments, GFP signal intensity decreased over time when nicotinamide was washed from the media (Fig. 6B). The loss of Dot1 function accelerated silencing establishment at the HML reporter (Fig. 6C), confirming that assays using quantitative microscopy of an HML-encoded destabilized GFP reporter were consistent with previous techniques (8, 22). Moreover, this technique was uniquely able to report fluctuations in signal intensity in individual cells over time. The highly variable or “bumpy” quality of the signal traces during expression and establishment was noteworthy.
Fig. 6.
Establishment of silencing over time visualized continuously by quantitative microscopy. DOT1 (JRY9101) or dot1Δ (JRY9104) cells were grown in 5 mM nicotinamide to derepress the hml::GFP::PEST::NLS reporter gene, and silencing establishment was then initiated by washing nicotinamide out of the media. As a control, DOT1 cells were maintained in 5 mM nicotinamide media for the duration of the time course. The fluorescence intensity of each cell is illustrated as a line plot. Each graph represents ∼10–20 starting cells and all their subsequent descendants.
Cell History Impacted Silencing Establishment.
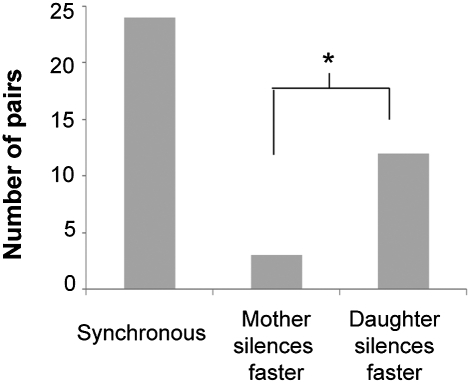
Previously, we observed a slight bias in the rate of silencing establishment such that daughter cells seemed slightly more likely to establish silencing before their mother cells. However, in the earlier study, a reliance on a G1-specific response to α-factor pheromone or micromanipulation itself may have, in some manner, caused or affected the bias and thus complicated its interpretation. Therefore, to test whether mother and daughter cells establish silencing with the same probability, we surveyed roughly 40 mother/daughter pairs from the silencing establishment assays in Fig. 6 for their relative patterns of silencing establishment (Fig. 7 and Fig. S4). In these graphs, the mother cell's trace (blue) began earlier and the daughter cell's trace (red) arose after the daughter cell had grown to >326 pixels in size. Depending on the concordance between the mother and daughter fluorescence intensity traces, we estimated whether the mother cell had greater signal than the daughter or vice versa. If the traces overlapped, we called the pair “synchronous.” Of the 39 traces, 24 had near perfect synchronicity between the mother and daughter fluorescence intensity traces. Twelve pairs showed a slight bias in silencing dynamics in which the daughter cell silenced HML before the mother (daughter silences faster). And in three cases, the mother cell silenced HML before the daughter (mother silences faster). The data mirrored and extended the trends previously documented in single-cell pedigrees (22), independently confirming that mother and daughter cells usually established silencing in the same cell division but in cases of asymmetry, the daughter cells established silencing more rapidly than the mothers.
Fig. 7.
Silencing establishment in mother–daughter pairs. Silencing establishment between mother and daughter cells was investigated using data from Fig. 6B. (A) The fluorescence intensity for mother–daughter pairs is shown in Fig. S4 and bar plots of the data are shown here. Binomial tests performed on different categories yielded the following scores: synchronous vs. mother, P value = 4.9 × 10−5; synchronous vs. daughter, P value = 0.065; and mother vs. daughter, P value = 0.035.
HML Expression Variation Was Independent of Cell-Cycle Phase.
It was possible that the observed fluctuations in HML transcription and silencing might be cell-cycle dependent. Indeed the URA3 promoter used to drive expression of the GFP reporter gene at HML has been reported to be more easily expressed within heterochromatin when cells are at G2/M than at other phases of the cell cycle (46). To test whether this variability correlated with cell-cycle phase, we used the data from the sir3Δ time courses (Fig. 5) and manually annotated the time of bud emergence (an approximation of S-phase) and of mother/daughter separation (an approximation of G1). By normalizing and stacking the traces of these different cells about the point of bud emergence, we could potentially capture phase-dependent rises or falls in expression. However, when these stacked traces were viewed en masse or averaged, we detected no dependence of bud emergence on fluorescence intensity. The same was true for the point of mother/daughter separation (Fig. S5). In short, there was no obvious dependence on cell cycle for the GFP expression trends in these assays. The variability in expression could instead be attributed to stochastic aspects of gene expression or to microenvironmental factors beyond our control.
Discussion
Single-cell–based assays have provided insight into variation, stochasticity, and dynamics of gene expression within populations of cells. Quantitative real-time microscopy has revealed dynamic aspects of cell and molecular biology. In this study, we combined these two approaches to illuminate the pattern and kinetics of silencing establishment as a function of chromatin modification, cell history, and the cell cycle.
Dot1 Antagonized Silencing Through H3 K79 Methyl Status.
This study evaluated how different mechanisms of Dot1 action impact silencing establishment kinetics and provided independent support of our past results indicating that dot1Δ cells establish silencing faster than DOT1 cells. Moreover, we were able to test two competing models for how Dot1 antagonizes Sir-protein–mediated silencing. Our data indicated that Dot1 impacted silencing establishment through H3 K79 methyl status and not through direct competition of the Dot1 protein with Sir3 for binding sites on nucleosomes. Both the catalytically dead Dot1 protein and the wild-type Dot1 protein bind histone H4 to the same extent (37). However, cells overexpressing a catalytically inactive Dot1 protein phenocopied cells lacking the Dot1 protein altogether, in both cases speeding the establishment of silencing. Furthermore, cells containing H3 K79R, an unmethylated lysine mimic, phenocopied dot1Δ cells in their silencing establishment dynamics, supporting the notion that Dot1’s catalytic activity opposes silencing establishment through the methylation status of H3 K79.
H3 K79 methylation is important for silencing, for cell-cycle–regulated gene expression, and for meiotic checkpoint control. Because Dot1 is a distributive enzyme and because mono-, di-, and trimethylation of H3 K79 disrupt telomeric silencing, the three states were thought to encode overlapping information (30). However, specific mutants can independently disrupt either di- or trimethylation of H3 K79, suggesting that the activity of the Dot1 enzyme may be alterable (31). Further, a lack of overlap between dimethyl and trimethyl marks genome-wide supports the notion that distinct methyl status may inform some processes (31).
Both the loss and the overproduction of Dot1 lead to a decrease in silencing strength measured by reporters located at two telomere loci (26). At those markers, the overexpression of Dot1 is thought to directly antagonize Sir3 binding to H4 and to decrease Sir3’s affinity for H3 via H3 K79 methylation. Conversely, the loss of dot1 is thought to decrease silencing indirectly whereby a global depletion of H3 K79 methylation permits inappropriate Sir protein binding genome-wide and a resulting dilution of Sir protein concentrations at silenced regions (27). Given that dot1Δ mutants have a decreased ability to silence genes at the telomeres, it may seem paradoxical that the dot1Δ mutation increases the rate of silencing establishment at HML and HMR. Although overexpression of DOT1 leads to a reduction of silencing at HML and HMR (22, 36), dot1Δ deletion causes only subtle effects at HML and HMR that are most obvious in silencing-compromised mutants. This difference suggests that “dilution effects” may not impact HML and HMR as strongly as they do reporters at telomeres. Because HML and HMR loci use the combined association of Abf1, Rap1, the ORC complex, and Sir1 to recruit Sir proteins, the rate of Sir protein recruitment may occur at near wild-type levels at these loci even when Sir proteins are diluted by dot1 loss. In that case, the rate of silencing establishment would be a function of a near normal rate of Sir protein recruitment at HML and HMR through interactions with ORC, Rap1, and Abf1, combined with an accelerated rate of Sir protein accumulation on nucleosomes unabated by H3 K79 methylation.
It is still unclear whether Sir protein binding eventually occurs despite H3 K79 methylation albeit slowly, whether H3 K79 methylation is diluted through replication and/or nucleosome exchange, or whether H3 K79 methylation is enzymatically removed by some heretofore unidentified demethylase. We have not ruled out the possibility that the accelerated rate of transcriptional silencing is due to some deficiency in transcription at the HML locus. However, because the levels of transcription at HML are not impacted by H3 K79 methylation, this possibility seems unlikely. Still, our results definitively indicate that H3 K79 methylation impacts silent chromatin dynamics at the HML locus.
Individual Cells and Silencing Establishment.
Although silencing of most cells occurs within two-cell divisions, a substantial decrease in transcripts from HML and HMR has been observed within 30 min following Sir3 restoration in cells previously lacking it (8, 42), and a minority of cells can even establish silencing within one cell cycle (22, 23). We used the power of continuous live-cell imaging to examine silencing dynamics within the first cell-cycle phases following the removal of a silencing inhibitor. Although the time required to transcribe, translate, and fold GFP limits our resolution, several points were clear. First, there was considerable cell-to-cell variation in the level of HML expression measured by GFP fluorescence. This variation between cells was also evident within individual cells over time. Second, we found no correlation between variations in fluorescence intensity at either of two recognizable points in the cell cycle. This analysis argues against models that suggest that low levels of transcription in G2 or M allow for easier silencing establishment in those phases (46).
Asymmetric Silencing Establishment.
In our previous work, mother and daughter cells usually established silencing synchronously within the same cell division. However, when mother and daughter cells established silencing asynchronously, the daughter cells were more likely to silence HML than their mothers. It was conceivable that the asymmetry observed in our earlier study was a consequence of the methods used rather than of the biology being studied. Because daughter cells must achieve a certain size threshold before budding, a mother cell divides before her daughter cell in time and hence moves past G1 (the time when silencing is evaluated using the previous assay) before the daughter cell does. Therefore, were the mother and daughter to silence at the exactly the same moment in time, the differences in their cell-cycle progression rate could give the illusion of asymmetry.
To determine whether the bias toward daughter cell silencing was real or an artifact of G1 length, we performed an analysis on pairs of mother–daughter cells as they established silencing. Our analysis confirmed by this independent method that mother and daughter cells do show a high degree of expression concordance. Nevertheless, 38% of cell divisions were asymmetric with one cell silencing before the other, and in those cases the daughter cell was significantly more likely to establish silencing than the mother. Thus, mother–daughter cell bias could not be explained by the shorter cell cycle of the mother cell.
The mechanism behind the daughter cell bias in silent chromatin establishment remains unexplained but would be compatible with three or more classes of explanation. Perhaps the easiest to imagine is that asymmetric gene expression causes the accumulation of either silencing-promoting proteins in the daughter cell or silencing-inhibitory proteins in the mother cell. Second, an asymmetric inheritance of proteins or cytoplasmic material between the two cells could result in a similar situation. In this regard, the Sir2-dependent segregation of oxidatively damaged proteins to mothers provides contextual support for such possibilities (47). Finally, the phenomenon could be due to a biased segregation of sister chromatids into the two cells if a chromatid with a silenced HML was more likely to segregate into the daughter. A priori, one would expect such segregation biases to operate at centromeres rather than at HML. Nevertheless, heterochromatin has a role in segregation and it is formally possible that HML may be an indicator of chromatin status at other regions of that same chromatid. At this point, all models of the mechanism of the daughter cell bias in silencing establishment remain speculative, but previous examples of asymmetric phenomena have proved to be a rich source of biological insight.
Materials and Methods
Strain Construction.
W303-derived yeast strains containing the hml::pURA3::GFP::PEST::NLS were generated from Yex730 (23). The original Yex730 strain was created by cloning the first 55 amino acids of the CLN2 PEST degradation tag onto the carboxy-terminal end of yEGFP (Clontech) (40, 48). This construct was transformed into a plasmid to produce the fusion gene URA3promoter::NLS::EGFP::PEST::URA3 terminator. The resulting construct was transformed into the HML locus, replacing the α1 and α2 genes with the GFP construct.
Gene deletions were generated using the one-step integration of knockout cassettes (49, 50). Strains containing DOT1 and dot1G401R overexpression plasmids (JRY9108–RY9114) were produced by transforming the plasmids pTCG, pFvL18, and pFvL43 (gifts from F. van Leeuwen, Netherlands Cancer Institute, Amsterdam, and D. Gottschling, Fred Hutchinson Cancer Research Center, Seattle) (30) into DOT1 and dot1Δ strains housing the GFP construct at HML (JRY9106 and JRY9107). A yeast strain lacking endogenous histone H3 and H4 alleles, but carrying HHT2::HHF2 on a plasmid (JRY9119), was incorporated with the destabilized GFP allele at the HML locus by a cross of JRY9104 to JRY7989 (51). To produce strains with different mutations of histone H3 (JRY9121–JRY9127), we introduced vectors containing mutant versions of HHT2 (pMP6, pHCL80, and pHCL81, gifts from S. Briggs, Purdue University, West Lafayette, IN) (37, 52) into the strain with the GFP at HML (JRY9119 and JRY9120), thereby replacing the HH2::HHF2 plasmid.
Flow Cytometry.
Cells were grown to 0.1 OD600 in SC medium (53) over two sequential nights of growth at 30 °C, diluting back the culture each day. Cells were harvested by centrifugation, fixed in a 4% paraformaldehyde/3.4% sucrose solution for 15 min at room temperature, and then washed and stored in a 1.2-M sorbitol, 0.1-M KPO4 solution, pH 7.5. Cells were stored at 4 °C for a maximum of 24 h. GFP expression data were collected for each sample using the FC-500 (Beckman-Coulter) flow cytometer. A total of 100,000 cells were measured per run and gated to identify those that were within a specific size and granularity (∼40,000 cells/experiment). We used Flow-Jo 7.5 analysis software (TreeStar) and the Bioconductor flowCore package for R (54, 55) for data analysis and display. For histograms and density plots generated in these programs, the Logicle scale is used. This is a scaled transformation that optimizes the display of low signals (56). Violin plots were generated using the Vioplot package for R (54, 57). These plots illustrate a kernel density plot overlaid with a box plot. The height for the density estimator was set to h = 100 in all plots to standardize the images. For samples grown in nicotinamide, cells were grown in SC medium containing 5 mM nicotinamide over 2 consecutive night's growth at 30 °C with a dilution between each night. For silencing establishment time-course assays, cells were grown in 5 mM nicotinamide in SC medium over 2 nights at 30 °C and then collected by centrifugation, washing, and resuspension in SC medium. Time points were collected during washing (0 min) and at 30, 60, 120, 240, and 360 min postwashing.
Microscopy.
Fluorescence microscope images were obtained using a computer-controlled fluorescence microscope system (DeltaVision; Applied Precision) based on an inverted fluorescence microscope (IX70; Olympus) with an oil immersion Plan-Apochromat 60× NA 1.4 lens (Olympus) for imaging of live cells. This system was used in a temperature-controlled room set to 30 °C (58, 59). For time-course assays, cells were grown overnight to a maximum of 0.1 OD in 5 mM nicotinamide + SC medium (53) at 30 °C. Live cells were placed on a 35-mm glass-bottom culture dish (MatTek) coated with Con A (from a stock of 0.2% Con A, 0.1 M NaCl, 20 mM Tris, 2 mM CaCl2, 0.5 mM MnCl2, pH 6.8), washed with SC medium, and overlaid with a 25-μL 2% low-melt temperature agarose (SC medium) pad. Images were acquired using SoftWoRx software (Applied Precision) provided as part of the DeltaVision system. A stack of 12 images, separated in the z axis by 0.5-μm increments and centered about the focal plane, was recorded every 10 min over a 6-h time course (37 time points). Image stacks were summed using the fast projection protocol in the SoftWoRx software. Images were exported to VCell-ID 0.4 (Molecular Sciences Institute) for cell identification, tracking, and quantification (61). VCell-ID data were analyzed using the RCell package for R (54, 61, 62). Cells were called accurately by the VCell-ID program ∼93–95% of the time and were manually curated to remove false-positive calls.
Degradation Time.
To determine the degradation rate of GFP in our strains, we grew cells containing the hml::GFP::PEST::NLS reporter in the sir3Δ background (JRY9103) in SC medium overnight at 30 °C to a maximum 0.1 OD. Cells were mounted onto glass-bottom culture dishes as described above and incubated with either SC medium or 2.5 μg/mL cycloheximide + SC medium. Quantitative microscopy time-course experiments were performed and analyzed as described above.
Protein Immunoblot.
Protein immunoblots were performed using previously published methods and using the AbCam AB2886 anti-H3 K79 me3 antibody, the AbCam AB1791 H3 antibody, the Invitrogen A6457 anti-PGK1 antibody, and a Dot1 antibody generously provided by F. van Leeuwen (Netherlands Cancer Institute, Amsterdam).
Supplementary Material
Acknowledgments
This project built upon the pioneering research of Eugenia Xu, Karl Zawadzki, and James Broach who created the original hml::GFP::PEST yeast strains. We thank Fred Van Leeuwen, Dan Gottschling, and Scott Briggs for antibodies and plasmids; David Drubin and Voytek Okreglak for help with microscopy; and Hector Candela for help with the Flow Cytometer. Da-Qiao Ding, Yuji Chikashige, Hiromi Maekawa, and especially Haruhiko Asakawa provided instruction in microscopy, quantification, imaging technology, and general life skills in Japan. The Rine laboratory and especially Oliver Zill, Meru Sadhu, and Bilge Ozaydin provided helpful discussion and feedback. Marc Nishimura provided commentary and feedback. This research was supported by a National Science Foundation Predoctoral Research Fellowship (to E.A.O.), a National Science Foundation- and Japan Society for the Promotion of Science-sponsored East Asian Pacific Summer Institute Fellowship (to E.A.O.), and National Institutes of Health Research Grant GM31105 (to J.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018742108/-/DCSupplemental.
References
- 1.Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herskowitz I. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol Rev. 1988;52:536–553. doi: 10.1128/mr.52.4.536-553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusche LN, Kirchmaier AL, Rine J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem. 2003;72:481–516. doi: 10.1146/annurev.biochem.72.121801.161547. [DOI] [PubMed] [Google Scholar]
- 5.Miller AM, Nasmyth KA. Role of DNA replication in the repression of silent mating type loci in yeast. Nature. 1984;312:247–251. doi: 10.1038/312247a0. [DOI] [PubMed] [Google Scholar]
- 6.Rivier DH, Rine J. Silencing: The establishment and inheritance of stable, repressed transcription states. Curr Opin Genet Dev. 1992;2:286–292. doi: 10.1016/s0959-437x(05)80286-2. [DOI] [PubMed] [Google Scholar]
- 7.Kirchmaier AL, Rine J. Cell cycle requirements in assembling silent chromatin in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:852–862. doi: 10.1128/MCB.26.3.852-862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katan-Khaykovich Y, Struhl K. Heterochromatin formation involves changes in histone modifications over multiple cell generations. EMBO J. 2005;24:2138–2149. doi: 10.1038/sj.emboj.7600692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martino F, et al. Reconstitution of yeast silent chromatin: Multiple contact sites and O-AADPR binding load SIR complexes onto nucleosomes in vitro. Mol Cell. 2009;33:323–334. doi: 10.1016/j.molcel.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Hoppe GJ, et al. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol. 2002;22:4167–4180. doi: 10.1128/MCB.22.12.4167-4180.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LM, Kayne PS, Kahn ES, Grunstein M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1990;87:6286–6290. doi: 10.1073/pnas.87.16.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 13.Xu F, Zhang Q, Zhang K, Xie W, Grunstein M. Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol Cell. 2007;27:890–900. doi: 10.1016/j.molcel.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Miller A, Kirchmaier AL. HST3/HST4-dependent deacetylation of lysine 56 of histone H3 in silent chromatin. Mol Biol Cell. 2008;19:4993–5005. doi: 10.1091/mbc.E08-05-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carmen AA, Milne L, Grunstein M. Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J Biol Chem. 2002;277:4778–4781. doi: 10.1074/jbc.M110532200. [DOI] [PubMed] [Google Scholar]
- 16.Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D. Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell. 2005;121:515–527. doi: 10.1016/j.cell.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Kirchmaier AL, Rine J. DNA replication-independent silencing in S. cerevisiae. Science. 2001;291:646–650. doi: 10.1126/science.291.5504.646. [DOI] [PubMed] [Google Scholar]
- 18.Li YC, Cheng TH, Gartenberg MR. Establishment of transcriptional silencing in the absence of DNA replication. Science. 2001;291:650–653. doi: 10.1126/science.291.5504.650. [DOI] [PubMed] [Google Scholar]
- 19.Gartenberg MR, Neumann FR, Laroche T, Blaszczyk M, Gasser SM. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell. 2004;119:955–967. doi: 10.1016/j.cell.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Lau A, Blitzblau H, Bell SP. Cell-cycle control of the establishment of mating-type silencing in S. cerevisiae. Genes Dev. 2002;16:2935–2945. doi: 10.1101/gad.764102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Wang CL, Sternglanz R. Promoter strength influences the S phase requirement for establishment of silencing at the Saccharomyces cerevisiae silent mating type loci. Genetics. 2010;186:551–560. doi: 10.1534/genetics.110.120592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne EA, Dudoit S, Rine J. The establishment of gene silencing at single-cell resolution. Nat Genet. 2009;41:800–806. doi: 10.1038/ng.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu EY, Zawadzki KA, Broach JR. Single-cell observations reveal intermediate transcriptional silencing states. Mol Cell. 2006;23:219–229. doi: 10.1016/j.molcel.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Kurdistani SK, Tavazoie S, Grunstein M. Mapping global histone acetylation patterns to gene expression. Cell. 2004;117:721–733. doi: 10.1016/j.cell.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Liu CL, et al. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 2005;3:e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer MS, et al. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics. 1998;150:613–632. doi: 10.1093/genetics/150.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Leeuwen F, Gafken PR, Gottschling DE. Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell. 2002;109:745–756. doi: 10.1016/s0092-8674(02)00759-6. [DOI] [PubMed] [Google Scholar]
- 28.Ng HH, et al. Lysine methylation within the globular domain of histone H3 by Dot1 is important for telomeric silencing and Sir protein association. Genes Dev. 2002;16:1518–1527. doi: 10.1101/gad.1001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng HH, Ciccone DN, Morshead KB, Oettinger MA, Struhl K. Lysine-79 of histone H3 is hypomethylated at silenced loci in yeast and mammalian cells: A potential mechanism for position-effect variegation. Proc Natl Acad Sci USA. 2003;100:1820–1825. doi: 10.1073/pnas.0437846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frederiks F, et al. Nonprocessive methylation by Dot1 leads to functional redundancy of histone H3K79 methylation states. Nat Struct Mol Biol. 2008;15:550–557. doi: 10.1038/nsmb.1432. [DOI] [PubMed] [Google Scholar]
- 31.Schulze JM, et al. Linking cell cycle to histone modifications: SBF and H2B monoubiquitination machinery and cell-cycle regulation of H3K79 dimethylation. Mol Cell. 2009;35:626–641. doi: 10.1016/j.molcel.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris A, Bianchet MA, Boeke JD. Compensatory interactions between Sir3p and the nucleosomal LRS surface imply their direct interaction. PLoS Genet. 2008;4:e1000301. doi: 10.1371/journal.pgen.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onishi M, Liou GG, Buchberger JR, Walz T, Moazed D. Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol Cell. 2007;28:1015–1028. doi: 10.1016/j.molcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Sampath V, et al. Mutational analysis of the Sir3 BAH domain reveals multiple points of interaction with nucleosomes. Mol Cell Biol. 2009;29:2532–2545. doi: 10.1128/MCB.01682-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Cosgrove MS, Youngman E, Wolberger C, Boeke JD. A core nucleosome surface crucial for transcriptional silencing. Nat Genet. 2002;32:273–279. doi: 10.1038/ng982. [DOI] [PubMed] [Google Scholar]
- 36.van Welsem T, et al. Synthetic lethal screens identify gene silencing processes in yeast and implicate the acetylated amino terminus of Sir3 in recognition of the nucleosome core. Mol Cell Biol. 2008;28:3861–3872. doi: 10.1128/MCB.02050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fingerman IM, Li HC, Briggs SD. A charge-based interaction between histone H4 and Dot1 is required for H3K79 methylation and telomere silencing: Identification of a new trans-histone pathway. Genes Dev. 2007;21:2018–2029. doi: 10.1101/gad.1560607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altaf M, et al. Interplay of chromatin modifiers on a short basic patch of histone H4 tail defines the boundary of telomeric heterochromatin. Mol Cell. 2007;28:1002–1014. doi: 10.1016/j.molcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salama SR, Hendricks KB, Thorner J. G1 cyclin degradation: The PEST motif of yeast Cln2 is necessary, but not sufficient, for rapid protein turnover. Mol Cell Biol. 1994;14:7953–7966. doi: 10.1128/mcb.14.12.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mateus C, Avery SV. Destabilized green fluorescent protein for monitoring dynamic changes in yeast gene expression with flow cytometry. Yeast. 2000;16:1313–1323. doi: 10.1002/1097-0061(200010)16:14<1313::AID-YEA626>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Schneider BL, et al. Yeast G1 cyclins are unstable in G1 phase. Nature. 1998;395:86–89. doi: 10.1038/25774. [DOI] [PubMed] [Google Scholar]
- 42.Lynch PJ, Rusche LN. A silencer promotes the assembly of silenced chromatin independently of recruitment. Mol Cell Biol. 2009;29:43–56. doi: 10.1128/MCB.00983-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu X, et al. The effect of H3K79 dimethylation and H4K20 trimethylation on nucleosome and chromatin structure. Nat Struct Mol Biol. 2008;15:1122–1124. doi: 10.1038/nsmb.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris A, Boeke JD. Silent information regulator 3: The Goldilocks of the silencing complex. Genes Dev. 2010;24:115–122. doi: 10.1101/gad.1865510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halter M, Tona A, Bhadriraju K, Plant AL, Elliott JT. Automated live cell imaging of green fluorescent protein degradation in individual fibroblasts. Cytometry A. 2007;71:827–834. doi: 10.1002/cyto.a.20461. [DOI] [PubMed] [Google Scholar]
- 46.Aparicio OM, Gottschling DE. Overcoming telomeric silencing: A trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. doi: 10.1101/gad.8.10.1133. [DOI] [PubMed] [Google Scholar]
- 47.Aguilaniu H, Gustafsson L, Rigoulet M, Nyström T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 48.Bailis JM, Bernard P, Antonelli R, Allshire RC, Forsburg SL. Hsk1-Dfp1 is required for heterochromatin-mediated cohesion at centromeres. Nat Cell Biol. 2003;5:1111–1116. doi: 10.1038/ncb1069. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 50.Longtine MS, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 51.Babiarz JE, Halley JE, Rine J. Telomeric heterochromatin boundaries require NuA4-dependent acetylation of histone variant H2A.Z in Saccharomyces cerevisiae. Genes Dev. 2006;20:700–710. doi: 10.1101/gad.1386306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly TJ, Qin S, Gottschling DE, Parthun MR. Type B histone acetyltransferase Hat1p participates in telomeric silencing. Mol Cell Biol. 2000;20:7051–7058. doi: 10.1128/mcb.20.19.7051-7058.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao W. Yeast Protocols. Totowa, NJ: Humana Press; 2006. p. 392. [Google Scholar]
- 54.R Development Core Team R: A language and environment for statistical computing. 2009. Available at http://www.r-project.org. Accessed January 2011.
- 55.Ellis B, Haaland P, Hahne F, Le Meur N. flowCore: Basic structures for flow cytometry data. 2008. Available at http://www.bioconductor.org/packages/2.2/bioc/html/flowCore.html. Accessed January 2011.
- 56.Parks DR, Roederer M, Moore WA. A new “Logicle” display method avoids deceptive effects of logarithmic scaling for low signals and compensated data. Cytometry A. 2006;69:541–551. doi: 10.1002/cyto.a.20258. [DOI] [PubMed] [Google Scholar]
- 57.Adler D. Vioplot: Violin plot. 2005. Available at http://cran.r-project.org/web/packages/vioplot/index.html. Accessed January 2011.
- 58.Haraguchi T, et al. Multiple-color fluorescence imaging of chromosomes and microtubules in living cells. Cell Struct Funct. 1999;24:291–298. doi: 10.1247/csf.24.291. [DOI] [PubMed] [Google Scholar]
- 59.Chikashige Y, et al. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J Cell Biol. 2009;187:413–427. doi: 10.1083/jcb.200902122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon A, et al. Single-cell quantification of molecules and rates using open-source microscope-based cytometry. Nat Methods. 2007;4:175–181. doi: 10.1038/nmeth1008. [DOI] [PubMed] [Google Scholar]
- 61.Bush A, Chernomoretz A. Rcell: Cell id data analysis. 2008. Available at http://lbms.df.uba.ar. Accessed January 2011.
- 62.Chernomoretz A, Bush A, Yu R, Gordon A, Colman-Lerner A. Using cell-ID 1.4 with R for microscope-based cytometry. Curr Protoc Mol Biol. 2008;Chap 14 doi: 10.1002/0471142727.mb1418s84. Unit 14.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.