Abstract
The establishment of modern humans in the Late Pleistocene, subsequent to their emergence in eastern Africa, is likely to have involved substantial population increases, during their initial dispersal across southern Asia and their subsequent expansions throughout Africa and into more northern Eurasia. An assessment of younger (20–40 y) versus older (>40 y) adult mortality distributions for late archaic humans (principally Neandertals) and two samples of early modern humans (Middle Paleolithic and earlier Upper Paleolithic) provides little difference across the samples. All three Late Pleistocene samples have a dearth of older individuals compared with Holocene ethnographic/historical samples. They also lack older adults compared with Holocene paleodemographic profiles that have been critiqued for having too few older individuals for subsistence, social, and demographic viability. Although biased, probably through a combination of preservation, age assessment, and especially Pleistocene mobility requirements, these adult mortality distributions suggest low life expectancy and demographic instability across these Late Pleistocene human groups. They indicate only subtle and paleontologically invisible changes in human paleodemographics with the establishment of modern humans; they provide no support for a life history advantage among early modern humans.
Keywords: paleodemography, age-at-death, teeth, postcrania, mandible
The emergence of modern humans in the later Pleistocene involved an emergence of modern human morphology in equatorial eastern Africa in the late Middle Pleistocene (≥150 kyr B.P.), followed over the subsequent 100,000 y by an uneven expansion through the remainder of the Old World (1, 2). There was a geographical expansion approximately 100 kyr B.P. into southern Asia, evident paleontologically in southwestern and southeastern Asia. The subsequent approximately 50,000 y involved early modern humans in eastern Africa and portions of southern Asia with late archaic humans (western Eurasian Neandertals plus non-Neandertal archaic populations elsewhere) in southern and northwestern Africa, across more northern Eurasia, and reoccupying portions of southwestern Asia after approximately 75 kyr B.P.. The final period of modern human establishment took place between approximately 50 and 35 kyr B.P., during which process modern human biology became the dominant form of humanity. Whatever the extent to which the eventual replacement of late archaic human morphology involved admixture, absorption, and/or population displacement (2–6), the process was ultimately a demographic one.
There are several indirect indications of a demographic contrast between late archaic and early modern humans. The morphology of all the early modern humans is overwhelming the derived morphology of extant humans (cf. ref. 7), to whatever degree those early modern humans exhibit morphological characteristics of late archaic humans not present in the earliest modern human samples (3, 4, 6, 8–10). Their ancestry must therefore have been predominantly that of expanding populations of early modern humans rather than of late archaic humans.
Although the anatomical evidence suggests demographic expansion of modern human populations approximately 100 kyr B.P. and subsequently approximately 45 kyr B.P., it is principally after approximately 45 kyr B.P. that there are multiple indirect indications of such population increases, in at least some portions of the Old World. Cultural traditions in both technology and ornamentation become stable in some regions (11, 12), implying more demographic stability (13). Evidence from stable isotopes and faunal remains suggests that populations were increasingly needing to exploit small package food resources requiring greater investment of acquisition effort (14–17). The demise of at least one of the large Pleistocene carnivores, Ursus spelaeus, has been attributed to increased competition for space from expanding human populations, especially after approximately 50 kyr B.P. (18, 19). And although body decoration appears sporadically earlier (20, 21), there was a marked increase in the social modification of one's appearance, especially through beads and pigments (12, 22). The increase in this uniquely human behavior suggests population densities sufficiently large to warrant personal image modification for projection beyond the local social group. These changes appear in the initial and early Upper Paleolithic, especially in western Eurasia, but they become pronounced across the Old World during the post-35 kyr B.P. Mid Upper Paleolithic.
Despite these indirect indicators of demographic changes, especially with the final establishment of modern humans 45 to 35 kyr B.P., there has been little consideration of human paleodemographic indicators of such populational shifts. There have nonetheless been suggestions, based on the dearth of older Neandertals (23) and more rapid development among them hypothesized from dental histology (24), that there may have been life history contrasts across this Late Pleistocene transition (cf. ref. 25).
Although it is apparent that one cannot conduct a true paleodemographic analysis of Late Pleistocene human populations, given the small sample sizes from individual sites and the distribution of the diagnostic fossil remains across tens of millennia and thousands of square kilometers, it is nonetheless possible to assess the patterns of mortality for these groups of late archaic and early modern humans. This was done previously for the Neandertals (23), in which it was noted that their immature mortality pattern approximated those of normal recent human populations, but that their adult mortality distribution contrasted strongly with demographically viable recent human populations. To date, similar assessments have been done for immature early modern humans (26) but not for their adult mortality patterns.
In such assessments, it needs to be kept in mind that these regional groups of humans, whether late archaic or early modern, persisted for tens of millennia. Local groups may well have gone extinct as a result of nonviable demographic profiles, but the sum total of their demographic parameters permitted them to persist under the adverse conditions of Late Pleistocene foraging populations.
Results
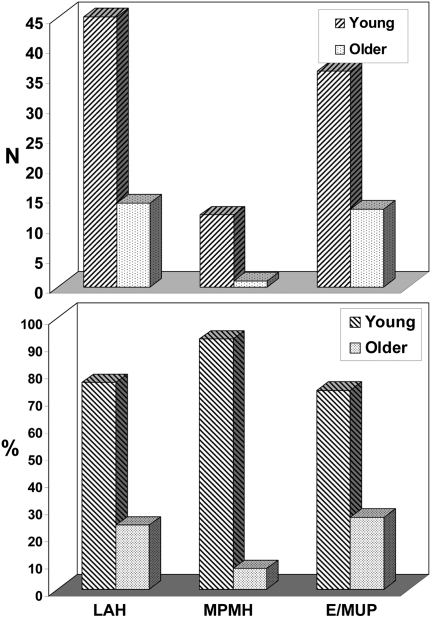
The distributions of younger versus older Late Pleistocene adults (Table 1, Fig. 1, and Table S1) confirm the previous finding (23) of a dearth of older adults in the late archaic human sample. However, the earlier Upper Paleolithic modern human sample and especially the Middle Paleolithic modern human sample similarly have far more prime age adults than older individuals. When the paleontological distributions are compared with the average percentages of older individuals for the archeological and ethnographic samples (39.3% and 65.0%, respectively; Table 1 and Table S2), the former are significantly different at P < 0.05 and the latter at P < 0.001, all after a multiple comparison correction.
Table 1.
Distributions of younger and older adults for Late Pleistocene samples
|
P value |
||||
| Sample | Younger adults | Older adults (%) | Vs. archeological mean | Vs. ethnographic mean |
| Late Archaic* | 45 | 14 (23.7) | 0.0049 | <0.0001 |
| MPMH† | 12 | 1 (7.7) | 0.0128 | <0.0001 |
| Upper Paleolithic‡ | 36 | 13 (26.5) | 0.0219 | <0.0001 |
Late Pleistocene samples are shown in Table S1. Binomial P values are for comparisons with the average of the percentage of older adults in the Holocene archeological (39.3%) and ethnographic (65.0%) demographic samples (Table S2).
*Eurasian late archaic humans.
†Late Pleistocene Middle Paleolithic modern humans from northeastern Africa and southern Asia.
‡Earlier upper Paleolithic modern humans from approximately 45 to 25 kyr B.P., normally included in Early and Mid Upper Paleolthic human samples (cf. ref. 3).
Fig. 1.
Distributions of younger adult (~20 to 40 y old) versus older adult (≥40 y old) for late archaic humans (LAH), Middle Paleolithic modern humans (MPMH), and earlier (Early and Mid) Upper Paleolithic modern humans (E/MUP) in raw counts (Upper) and as percentages of total number (Lower).
If the five late archaic and Upper Paleolithic specimens that may well be less than 40 y old (27, 28) are placed in the younger age samples, their differences with the archeological average older adult mortality increase (P = 0.0010 and P = 0.0025, respectively), making all the comparisons significant at P < 0.01. Similarly, if the southern and northwestern Middle Paleolithic African remains are added to the Middle Paleolithic samples, they are different from the archeological average at P < 0.01 (P = 0.0031 and P = 0.0080, respectively, for the late archaic and early modern human samples). All of them remain markedly different from the ethnographic average (P < 0.0001).
The three paleontological samples are significantly different (P = 0.0198), which is largely driven by the marked absence of older individuals in the Middle Paleolithic modern human sample. The late archaic and earlier Upper Paleolithic samples are not significantly different from each other (P = 0.1664).
Discussion
It is therefore apparent, given available Marine Isotope Stage (MIS) 5–3 human remains, that all of these samples have adult mortality profiles at variance with those of viable human populations. As noted (29, 30) for the less extreme recent human paleodemographic profile from the Libben site (31), which has a higher older adult percentage (30.5%) than any of the MIS 5–3 samples, all these fossil samples would have had high immature-adult ratios, elevated work loads for the adults, a dearth of grandparenting, an excess of orphans, and levels of fertility at least 20% higher than those expected by using model life tables. There are several possible, non–mutually exclusive, reasons for these biased distributions.
The young adult mortality may well reflect generally low adult life expectancy among these populations, in response to the multitude of stresses associated with a Late Pleistocene foraging existence. Paleopathological indicators of such stress, particularly in the form of dental enamel hypoplasias and healed traumatic lesions, are evident in all of these samples (32–37). Yet, there were decreases or stasis in the incidence of both forms of lesions among both Middle Paleolithic and earlier Upper Paleolithic modern humans relative to the late archaic humans (33, 35, 37, 38). This paleopathological shift is not likely to reflect differential survival, given the survival of individuals with serious developmental and degenerative abnormalities in both groups (6, 37, 39–45).
The prevalence of younger adults in the sample may indicate the frequent occurrence of local population crashes, from which the mortality profile (a catastrophic one) would more closely resemble the life pyramid than an attritional distribution (46). Although populational instability has been argued for Middle Paleolithic late archaic and early modern humans (13, 23), the archeological indicators listed above imply more populational stability in the Upper Paleolithic.
The adult age distributions may reflect socially motivated differential burial, in which preferential burial was given to prime-age adults. However, considering only the remains from definite or probable burials (Table S1) increases the percentages of older adults for all three fossil samples to 44.4% (n = 18), 9.1% (n = 11), and 33.3% (n = 27) for the late archaic, Middle Paleolithic modern, and earlier Upper Paleolithic modern human samples, respectively. Therefore, although there may have been an age bias in who was buried, the available remains indicate that it was not responsible for the dearth of older individuals tabulated here.
It is possible that the dearth of older adults is the product of differential preservation of younger versus older adult skeletal remains, with more fragile older adult skeletal material preserving less well (cf. ref. 47). The division here between young and old of approximately 40 y postnatal, however, should be before significant geriatric loss of skeletal matrix, especially in robust nonmechanized humans (48). Indeed, the older adults in the sample exhibit robust remains with little cortical bone loss (6, 39, 43, 49–51). Moreover, as principally teeth in maxillae or mandibles are included, nonrecognition of individuals represented paleontologically only by heavily worn teeth should not be a factor. The paleontological recognition of older isolated specimens, such as the Arcy-Hyène 8 and Artenac 1 maxillae and the Banyoles 1 and Paglicci 14, 15, and 24 mandibles, further indicates that this should not be an important bias.
There are indications that skeletal aging techniques frequently underestimate the ages of older adults (30, 52), especially those older than approximately 60 y. This applies particularly to degenerative skeletal reflections of age (e.g., pubic symphysis, auricular surface) but less so to dental attrition (28, 52). Although this effect may modestly inflate the number of younger adults in the paleontological samples, the individuals inaccurately assigned to the samples with age younger than 40 y would have to have had true ages at death close to 40 y given average biases in that age range of less than 10 y (52). Moreover, the same bias should also affect the Holocene archeological samples, all of which have higher older adult percentages than the fossil samples.
A more probable factor in the dearth of older individuals comes from the evident necessity for mobility among all of these Late Pleistocene humans. All of them have elevated lower limb diaphyseal robustness (6, 53). None of the individuals with preserved remains sustained and healed a lower limb injury or deformity that would have prevented locomotion; the oldest known such injury (54) is early Holocene in age. Even those individuals, who sustained serious developmental or traumatic deformities of the lower limbs (6, 37, 44, 45, 49) or developed advanced posttraumatic osteoarthritis of primary weight-bearing articulations (39, 40), continued to be mobile. Under these conditions, it is likely that older individuals with reduced mobility were left behind, to die and have their remains consumed by the ubiquitous carnivores on the landscape. They would not have entered the paleontological record, and hence mobility may account for some of the scarcity of older individuals.
Whatever the ultimate causes of these depressed adult mortality profiles, and in whatever proportions they were responsible for producing the adult mortality distributions in these samples of MIS 5–3 humans, they do not support the substantial increase in human population size inferred from multiple lines of evidence, especially with the emergence of the early and then Mid Upper Paleolithic after approximately 45 kyr B.P. (as detailed earlier). If this stasis in adult mortality patterns through MIS 5–3 reflects the expected longevity for those individuals reaching maturity, any differential in population growth must have been in terms of greater fertility and/or more rapid reproductive maturation among early modern humans, especially during the final establishment of those modern humans.
Given the generally poor archeological preservation of immature skeletal remains (23, 30, 47), combined with a proportional bias against the intentional burial of preadolescent individuals during the earlier Upper Paleolithic (55, 56), it is not possible to currently assess differential fertility paleodemographically. The apparently lower level of developmental and degenerative stress indicators among early modern humans (as detailed earlier) may imply reduced immature mortality, which could allow demographic increases. Yet, reduced juvenile mortality is associated with slower development among extant subsistence-level societies (57). Any such shift in developmental survival cannot be shown to occur in the context of augmented overall longevity, given the dearth of older adults across these Late Pleistocene samples.
At the same time, recent attempts to assess rates of dental development, and by extension ages of sexual maturity, have suggested that early modern humans from the Middle Paleolithic onward may have been developing more slowly than the Neandertals (24, 58; but see refs. 25, 59–61 for assessments finding little difference in dental developmental rates). Yet, it is unclear how any difference in dental calcification rates would translate into overall rates of somatic and reproductive maturation (25), especially given the substantial variation in growth rates and times of reproductive maturity among extant subsistence-level societies living under varying environmental conditions (57). Moreover, if hypotheses of faster maturation among late archaic humans are substantiated, any reduction in developmental rates, and hence presumed delays in sexual maturity, should have reduced the ability of the early modern human populations to compensate for elevated prime-age adult mortality levels. Contrary to published reports (24), modestly delayed maturation among Late Pleistocene modern humans is not likely to have given them an advantage.
Conclusion
A series of indirect sources of evidence suggest that there were increases in early modern human population size and potential growth relative to those of late archaic humans, to some extent during the time of the MIS 5–4 Middle Paleolithic but especially in the mid-MIS 3 earlier Upper Paleolithic. An assessment of the available Late Pleistocene adult skeletal remains—those that can be assigned ages at death during the prime reproductive decades between approximately 20 and 40 y or to the postprime age period after approximately 40 y postnatal—shows no change in younger versus older adult mortality patterns through this time period. All the samples have a dearth of older individuals, which should reflect a complex combination of low life expectancy for adults, demographic instability, and the demands of mobility, possibly compounded by preservation and aging assessments.
If indeed there was a demographic advantage for early modern humans, at least during transitional phases of Late Pleistocene human evolution, it must have been the result of increased fertility and/or reduced immature mortality. Neither adult longevity nor proposed modest shifts in developmental rates are likely to have played a role in this demographic transition.
Materials and Methods
Distributions of young (~20–40 y postnatal) versus older (at least ~40 y postnatal) adults for the Late Pleistocene (MIS 5–3) samples are based on the maximum number of mature individuals for which these gross categories of age at death can be reasonably assessed. The specimens counted minimally include maxillae and/or mandibles with postcanine teeth in situ and/or associated postcrania with reliable age indicators; isolated teeth, individually or in sets, are not included so as to minimize the effect of nonrecognition of older individuals with heavily worn teeth.
The ages of the majority of the specimens were based on assessments of occlusal wear, in which all of the postcanine teeth of the individuals in the younger age category preserved the majority of their occlusal enamel [Smith (62) categories 1–5]. When possible, these assessments were combined with skeletal indicators, including pubic symphysis and auricular surface metamorphosis, femoral diaphyseal histomorphology, costal cartilage ossification, proximal femoral trabecular changes, and sacral body ventral ossification, but not suture closure. In specimens of late archaic and early modern humans, for which it has been possible to assess multiple age-at-death indicators by using recent human standards (28, 39, 40, 43, 49, 63–66), there is consistent agreement across the age indicators within the two categories used here. The few specimens which are likely younger but could belong in either group (La Chapelle-aux-Saints 1, Dolní Věstonice 3, La Ferrassie 2, Pavlov 1, Předmostí 3) have been conservatively assigned to the older age group; making them younger than 40 y would increase the differences between the fossil and recent human mortality distributions.
Even though individuals normally achieve reproductive maturity by the second half of the second decade of life (57), it is not often possible to assign adolescent skeletal remains to the first or second half of that developmental period. Adolescents, including specimens normally included among Late Pleistocene adults (e.g., Dolní Věstonice 14, Mladeč 1, Oase 2), are therefore not included in the distributions. Including them would accentuate any differences between younger and older adult percentages for those samples (all the Pleistocene ones) with more young than old adults.
It is currently debated whether the MIS 5–3 Middle Paleolithic/Middle Stone Age human remains from southern and northwestern Africa should be assigned to early modern versus late archaic humans (e.g., ref. 67 versus ref. 1). They have an abundance of archaic features and few if any derived characteristics of modern humans (68–73); they are nonetheless treated as morphologically ambiguous and added post hoc to the Eurasian late archaic sample and then to the Middle Paleolithic modern human sample. All but one of the five specimens from those samples providing age indicators (entirely dental attrition) are in the young adult category.
The distributions of younger and older adults in the fossil samples are compared with the averaged proportions of these mortality categories for nine Holocene archeological samples (31, 74–80) and for six 18th- to 20th-century historical and ethnographic samples (81–86) (Table S2). The former samples should contain the same sets of biases that might arise from skeletal aging techniques and especially preservation. The latter samples should provide more accurate assessments of mortality in recent human populations without medical care, albeit in a modern epidemiological context. Because the fossil samples are necessarily pooled across many populations, it is appropriate to compare them to the averages of these recent human demographic profiles with respect to younger versus older adult mortality.
The Late Pleistocene distributions are compared with the archeological and ethnographic mean values by using a binomial test and a sequentially reductive multiple comparison correction.
Supplementary Material
Acknowledgments
D. Guatelli-Steinberg, T. W. Holliday, and J. Zilhão provided helpful comments. This work was supported by the National Science Foundation and the Wenner-Gren and Leakey Foundations.
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018700108/-/DCSupplemental.
References
- 1.Trinkaus E. Early modern humans. Annu Rev Anthropol. 2005;34:207–230. [Google Scholar]
- 2.Liu W, et al. Human remains from Zhirendong, South China, and modern human emergence in East Asia. Proc Natl Acad Sci USA. 2010;107:19201–19206. doi: 10.1073/pnas.1014386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trinkaus E. European early modern humans and the fate of the Neandertals. Proc Natl Acad Sci USA. 2007;104:7367–7372. doi: 10.1073/pnas.0702214104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith FH. In: A Companion to Biological Anthropology. Larsen CS, editor. New York: Wiley-Blackwell; 2009. pp. 357–378. [Google Scholar]
- 5.Green RE, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang H, Trinkaus E. The Early Modern Human from Tianyuan Cave, China. College Station: Texas A&M Univ Press; 2010. [Google Scholar]
- 7.Trinkaus E. Modern human versus Neandertal evolutionary distinctiveness. Curr Anthropol. 2006;47:597–620. [Google Scholar]
- 8.Henry-Gambier D, Sacchi D. La Crouzade V-VI (Aude, France): Un des plus anciens fossiles d'anatomie moderne en Europe occidentale. Bull Mem Soc Anthropol Paris. 2008;20:79–104. [Google Scholar]
- 9.Frayer DW, Jelínek J, Oliva M, Wolpoff MH. In: Early Modern Humans at the Moravian Gate: The Mladeč Caves and their Remains. Teschler-Nicola M, editor. Vienna: Springer; 2006. pp. 185–272. [Google Scholar]
- 10.Bailey SE, Weaver TD, Hublin JJ. Who made the Aurignacian and other early Upper Paleolithic industries? J Hum Evol. 2009;57:11–26. doi: 10.1016/j.jhevol.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Bar-Yosef O, Zilhão J, editors. Towards a definition of the Aurignacian. Proceedings of the symposium held in Lisbon, Portugal, June 25-30, 2002. Trabalhos de Arqueologia 45. Lisbon: Instituto Português de Arqueologia; 2006. [Google Scholar]
- 12.Vanhaeren M, d'Errico F. Aurignacian ethno-linguistic geography of Europe revealed by personal ornaments. J Archaeol Sci. 2006;33:1105–1128. [Google Scholar]
- 13.Hovers E, Belfer-Cohen A. Now you see it, now you don't: modern human behavior in the Middle Paleolithic. In: Hovers E, Kuhn SL, editors. Transitions Before the Transition. New York: Springer; 2006. pp. 295–304. [Google Scholar]
- 14.Hu Y, et al. Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc Natl Acad Sci USA. 2009;106:10971–10974. doi: 10.1073/pnas.0904826106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards MP, Trinkaus E. Isotopic evidence for the diets of European Neandertals and early modern humans. Proc Natl Acad Sci USA. 2009;106:16034–16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiner MC, Munro ND, Surovell TA. The tortoise and the hare. Small-game use, the Broad-Spectrum Revolution, and Paleolithic demography. Curr Anthropol. 2000;41:39–79. [PubMed] [Google Scholar]
- 17.Kuhn SL, et al. The early Upper Paleolithic occupation at Üçağizli Cave (Hatay, Turkey) J Archaeol Sci. 2009;56:87–113. doi: 10.1016/j.jhevol.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Grayson DK, Delpech F. Ungulates and the Middle-to-Upper Paleolithic transition at Grotte XVI (Dordogne, France) J Archaeol Sci. 2003;30:1633–1648. [Google Scholar]
- 19.Stiller M, et al. Withering away—25,000 years of genetic decline preceded cave bear extinction. Mol Biol Evol. 2010;27:975–978. doi: 10.1093/molbev/msq083. [DOI] [PubMed] [Google Scholar]
- 20.Trinkaus E. Human Evolution: Neandertal gene speaks out. Curr Biol. 2007;17:R917–R919. doi: 10.1016/j.cub.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Zilhão J, et al. Symbolic use of marine shells and mineral pigments by Iberian Neandertals. Proc Natl Acad Sci USA. 2010;107:1023–1028. doi: 10.1073/pnas.0914088107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norton CJ, Jin JJH. The evolution of modern human behavior in east Asia: Current perspectives. Evol Anthropol. 2009;18:247–260. [Google Scholar]
- 23.Trinkaus E. Neanderthal mortality patterns. J Archaeol Sci. 1995;22:121–142. [Google Scholar]
- 24.Smith TM, et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc Natl Acad Sci USA. 2010;107:20923–20928. doi: 10.1073/pnas.1010906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guatelli-Steinberg D. Recent studies of dental development in Neandertals: Implications for Neandertal life histories. Evol Anthropol. 2009;18:9–20. [Google Scholar]
- 26.Zilhão J, Trinkaus E. Social implications. Trabalhos Arqueol. 2002;22:519–541. [Google Scholar]
- 27.Dawson JE, Trinkaus E. Vertebral osteoarthritis of the La Chapelle-aux-Saints 1 Neandertal. J Archaeol Sci. 1997;24:1015–1021. [Google Scholar]
- 28.Hillson SW, Franciscus RG, Holliday TW, Trinkaus E. The ages at death. In: Trinkaus E, Svoboda JA, editors. Early Modern Human Evolution in Central Europe: The People of Dolní Vĕstonice and Pavlov. New York: Oxford Univ Press; 2006. pp. 31–45. [Google Scholar]
- 29.Howell N. Village composition implied by paleodemographic life table: The Libben site. Am J Phys Anthropol. 1982;59:263–269. [Google Scholar]
- 30.Paine RR, Harpending HC. Effect of sample bias on paleodemographic fertility estimates. Am J Phys Anthropol. 1998;105:231–240. doi: 10.1002/(SICI)1096-8644(199802)105:2<231::AID-AJPA9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy CO, et al. Paleodemography of the Libben site, Ottawa county, Ohio. Science. 1977;198:291–293. doi: 10.1126/science.198.4314.291. [DOI] [PubMed] [Google Scholar]
- 32.Ogilvie MD, Curran BK, Trinkaus E. Incidence and patterning of dental enamel hypoplasia among the Neandertals. Am J Phys Anthropol. 1989;79:25–41. doi: 10.1002/ajpa.1330790104. [DOI] [PubMed] [Google Scholar]
- 33.Brennan MU. New York: New York University; 1991. Health and disease in the Middle and Upper Paleolithic of southwestern France: A bioarcheological study. PhD thesis. [Google Scholar]
- 34.Berger TD, Trinkaus E. Patterns of trauma among the Neandertals. J Archaeol Sci. 1995;22:841–852. [Google Scholar]
- 35.Tillier AM, Duday H, Arensburg B, Vandermeersch B. Dental pathology, stressful events and disease in Levantine early anatomically modern humans: Evidence from Qafzeh. In: Goren-Inbar N, Speth JD, editors. Human Paleoecology in the Levantine Corridor. Oxford, UK: Oxbow; 2004. pp. 135–148. [Google Scholar]
- 36.Guatelli-Steinberg D, Larsen CS, Hutchinson DL. Prevalence and the duration of linear enamel hypoplasia: A comparative study of Neandertals and Inuit foragers. J Hum Evol. 2004;47:65–84. doi: 10.1016/j.jhevol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Trinkaus E, Hillson SW, Franciscus RG, Holliday TW. Skeletal and dental paleopathology. In: Trinkaus E, Svoboda JA, editors. Early Modern Human Evolution in Central Europe: The People of Dolní Vĕstonice and Pavlov. New York: Oxford Univ Press; 2006. pp. 419–458. [Google Scholar]
- 38.Teschler-Nicola M, Czerny C, Oliva M, Schmall D, Schultz M. Pathological alterations and traumas in the human skeleton ramains from Mladeč. In: Teschler-Nicola M, editor. Early Modern Humans at the Moravian Gate: The Mladeč Caves and Their Remains. Vienna: Springer; 2006. pp. 473–487. [Google Scholar]
- 39.Trinkaus E. The Shanidar Neandertals. New York: Academic Press; 1983. [Google Scholar]
- 40.Trinkaus E. Pathology and the posture of the La Chapelle-aux-Saints Neandertal. Am J Phys Anthropol. 1985;67:19–41. doi: 10.1002/ajpa.1330670105. [DOI] [PubMed] [Google Scholar]
- 41.Churchill SE, Formicola V. A case of marked bilateral asymmetry in the upper limbs of an Upper Palaeolithic male from Barma Grande (Liguria), Italy. Int J Osteoarchaeol. 1997;7:18–38. [Google Scholar]
- 42.Tillier AM. Les Enfants Moustériens de Qafzeh. Paris: Centre National de la Recherche Scientifique; 1999. [Google Scholar]
- 43.Smith FH, Smith MO, Schmitz RW. Human skeletal remains from the 1997 and 2000 excavations of cave deposits derived from Kleine Feldhofer Grotte in the Neander Valley, Germany. In: Schmitz RW, editor. Neanderthal 1856-2006. Mainz, Germany: Philipp von Zabern; 2006. pp. 187–246. [Google Scholar]
- 44.Formicola V, Buzhilova AP. Double child burial from Sunghir (Russia): pathology and inferences for upper Paleolithic funerary practices. Am J Phys Anthropol. 2004;124:189–198. doi: 10.1002/ajpa.10273. [DOI] [PubMed] [Google Scholar]
- 45.Crevecoeur I. Étude Anthropologique du Squelette du Paléolithique Supérieur de Nazlet Khater 2 (Égypte) Leuven, The Netherlands: Leuven Univ Press; 2008. [Google Scholar]
- 46.Klein RG, Cruz-Uribe K. The Analysis of Animal Bones from Archeological Sites. Chicago: Univ Chicago Press; 1984. [Google Scholar]
- 47.Walker PL, Johnson JR, Lambert PM. Age and sex biases in the preservation of human skeletal remains. Am J Phys Anthropol. 1988;76:183–188. doi: 10.1002/ajpa.1330760206. [DOI] [PubMed] [Google Scholar]
- 48.Ruff CB, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae—a biomechanical investigation: II. Sex, age, side differences. Am J Phys Anthropol. 1983;60:383–400. doi: 10.1002/ajpa.1330600309. [DOI] [PubMed] [Google Scholar]
- 49.Heim JL. Les hommes fossiles de La Ferrassie II. Arch Inst Paléontol Hum. 1982;38:1–272. [Google Scholar]
- 50.Trinkaus E. The lower limb remains. In: Trinkaus E, Svoboda JA, editors. Early Modern Human Evolution in Central Europe: The People of Dolní Vĕstonice and Pavlov. New York: Oxford Univ Press; 2006. pp. 380–418. [Google Scholar]
- 51.Mednikova M, Trinkaus E. Femoral midshaft diaphyseal cross-sectional geometry of the Sunghir 1 and 4 Gravettian human remains. Anthropologie (Brno) 2001;39:135–141. [Google Scholar]
- 52.Lovejoy CO, Meindl RS, Mensforth RP, Barton TJ. Multifactorial determination of skeletal age at death: A method and blind tests of its accuracy. Am J Phys Anthropol. 1985;68:1–14. doi: 10.1002/ajpa.1330680102. [DOI] [PubMed] [Google Scholar]
- 53.Holt BM. Mobility in Upper Paleolithic and Mesolithic Europe: Evidence from the lower limb. Am J Phys Anthropol. 2003;122:200–215. doi: 10.1002/ajpa.10256. [DOI] [PubMed] [Google Scholar]
- 54.Pittard E, Sauter MR. Un squelette magdalénien provenant de la Station des Grenouilles (Veyrier, Haute-Savoie) Arch Suisses Anthropol Gén. 1946;11:149–200. [Google Scholar]
- 55.Zilhão J. Burial evidence for social differentiation of age classes in the Early Upper Paleolithic. Etud Res Archéol Univ Liège. 2005;111:231–241. [Google Scholar]
- 56.Formicola V. From the Sunghir children to the Romito dwarf. Aspects of the Upper Paleolithic funerary landscape. Curr Anthropol. 2007;48:446–453. [Google Scholar]
- 57.Walker R, et al. Growth rates and life histories in twenty-two small-scale societies. Am J Hum Biol. 2006;18:295–311. doi: 10.1002/ajhb.20510. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez Rozzi FV, Bermúdez De Castro JM. Surprisingly rapid growth in Neanderthals. Nature. 2004;428:936–939. doi: 10.1038/nature02428. [DOI] [PubMed] [Google Scholar]
- 59.Tompkins RL. Relative dental development of Upper Pleistocene hominids compared to human population variation. Am J Phys Anthropol. 1996;99:103–118. doi: 10.1002/(SICI)1096-8644(199601)99:1<103::AID-AJPA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Macchiarelli R, et al. How Neanderthal molar teeth grew. Nature. 2006;444:748–751. doi: 10.1038/nature05314. [DOI] [PubMed] [Google Scholar]
- 61.Guatelli-Steinberg D, Reid DJ, Bishop TA, Larsen CS. Anterior tooth growth periods in Neandertals were comparable to those of modern humans. Proc Natl Acad Sci USA. 2005;102:14197–14202. doi: 10.1073/pnas.0503108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith BH. Patterns of molar wear in hunger-gatherers and agriculturalists. Am J Phys Anthropol. 1984;63:39–56. doi: 10.1002/ajpa.1330630107. [DOI] [PubMed] [Google Scholar]
- 63.Trinkaus E, Thompson DD. Femoral diaphyseal histomorphometric age determinations for the Shanidar 3, 4, 5, and 6 Neandertals and Neandertal longevity. Am J Phys Anthropol. 1987;72:123–129. doi: 10.1002/ajpa.1330720115. [DOI] [PubMed] [Google Scholar]
- 64.Heim JL. Les hommes fossiles de La Ferrassie I. Arch Inst Paléontol Hum. 1976;35:1–331. [Google Scholar]
- 65.Abbott S, Trinkaus E, Burr DB. Dynamic bone remodeling in later Pleistocene fossil hominids. Am J Phys Anthropol. 1996;99:585–601. doi: 10.1002/(SICI)1096-8644(199604)99:4<585::AID-AJPA5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 66.Buzhilova AP, Kozlovskaya MV, Mednikova MB. Sex and age estimation from the skeletal human remains of the Sunghir site: Sunghir 1 (Translated from Russian) In: Alexeeva TI, et al., editors. Homo Sungirensis. Moscow: Scientific World; 2000. pp. 54–56. 62. [Google Scholar]
- 67.Bräuer G. The origin of modern anatomy: By speciation or intraspecific evolution? Evol Anthropol. 2008;17:22–37. [Google Scholar]
- 68.Vallois HV, Roche J. La mandibule acheuléenne de Témara, Maroc. C R Acad Sci Paris. 1958;246:3113–3116. [PubMed] [Google Scholar]
- 69.Lam YM, Pearson OM, Smith CM. Chin morphology and sexual dimorphism in the fossil hominid mandible sample from Klasies River Mouth. Am J Phys Anthropol. 1996;100:545–557. doi: 10.1002/(SICI)1096-8644(199608)100:4<545::AID-AJPA8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 70.Churchill SE, et al. Morphological affinities of the proximal ulna from Klasies River Mouth Main Site: Archaic or modern? J Hum Evol. 1996;31:213–237. [Google Scholar]
- 71.Ferembach D. Les restes humains de la grotte de Dar-es-Soltane 2 (Maroc) Campagne 1975. Bull Mém Soc Anthropol Paris Série XIII. 1976;3:183–193. [Google Scholar]
- 72.Ferembach D. Le crâne Atérien de Témara (Maroc Atlantique) Bull Archéol Marocaine. 1998;18:19–66. [Google Scholar]
- 73.Schwartz JH, Tattersall I. The Human Fossil Record 2. New York: Wiley-Liss; 2002. [Google Scholar]
- 74.Palkovich AM. Demography and disease patterns in a protohistoric plains group: A study of the Mobridge site (39WW1) Plains Anthropol. 1981;26:71–84. doi: 10.1080/2052546.1981.11909054. [DOI] [PubMed] [Google Scholar]
- 75.Owsley DW, Bass WM. A demographic analysis of skeletons from the Larson site (39WW2), Walworth County, South Dakota: Vital statistics. Am J Phys Anthropol. 1979;51:145–154. [Google Scholar]
- 76.Ubelaker DH. Human skeletal remains from site OGSE-80. A preceramic site on the Sta. Elena Peninsula, coastal Ecuador. J Wash Acad Sci. 1980;70:3–24. [Google Scholar]
- 77.Ubelaker DH. The Ayalán Cemetery. A Late Integration Period Burial Site on the South Coast of Ecuador. Washington, DC: Smithsonian Institution Press; 1981. [Google Scholar]
- 78.Rose JC, Santeford LG. Burial descriptions. Arkansas Archeol Survey Res Ser. 1985;25:39–129. [Google Scholar]
- 79.Heinrich W, Teschler-Nicola M. Zur Anthropologie des Gräberfeldes F von Gemeinlebarn, Niederösterreich. Röm German Forschung. 1991;49:222–262. [Google Scholar]
- 80.Storey R. Life & Death in the Ancient City of Teotihuacan. Tuscaloosa: Univ Alabama Press; 1992. [Google Scholar]
- 81.Howell N. Demography of the Dobe !Kung. New York: Academic Press; 1979. [Google Scholar]
- 82.Blurton Jones NG, Smith LC, O'Connell JF, Hawkes K, Kamuzora CL. Demography of the Hadza, an increasing and high density population of Savanna foragers. Am J Phys Anthropol. 1992;89:159–181. doi: 10.1002/ajpa.1330890204. [DOI] [PubMed] [Google Scholar]
- 83.Hill K, Hurtado AM. Aché Life History. New York: Aldine de Gruyter; 1996. [Google Scholar]
- 84.Neel JV, Weiss KM. The genetic structure of a tribal population, the Yanomama Indians. XII. Biodemographic studies. Am J Phys Anthropol. 1975;42:25–51. doi: 10.1002/ajpa.1330420105. [DOI] [PubMed] [Google Scholar]
- 85.Early JD, Peters JF. The Population Dynamics of the Mucajai Yanomama. San Diego: Academic Press; 1990. [Google Scholar]
- 86.Molleson T, Cox M. The Spitalfields Project 2: The Anthropology. London: Council for British Archaeology; 1993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.