Abstract
Sperm are the most diverse of all animal cell types, and much of the diversity in sperm design is thought to reflect adaptations to the highly variable conditions under which sperm function and compete to achieve fertilization. Recent work has shown that these conditions often evolve rapidly as a consequence of multiple mating, suggesting a role for sexual selection and sexual conflict in the evolution of sperm design. However, very little of the striking diversity in sperm design is understood functionally, particularly in internally fertilizing organisms. We use phylogenetic comparative analyses covering 16 species of the hermaphroditic flatworm genus Macrostomum to show that a complex sperm design is associated with reciprocal mating and that this complexity is lost secondarily when hypodermic insemination—sperm injection through the epidermis—evolves. Specifically, the complex sperm design, which includes stiff lateral bristles, is likely a male persistence trait associated with sexual conflicts over the fate of received ejaculates and linked to female resistance traits, namely an intriguing postcopulatory sucking behavior and a thickened epithelium of the sperm-receiving organ. Our results suggest that the interactions between sperm donor, sperm, and sperm recipient can change drastically when hypodermic insemination evolves, involving convergent evolution of a needle-like copulatory organ, a simpler sperm design, and a simpler female genital morphology. Our study documents that a shift in the mating behavior may alter fundamentally the conditions under which sperm compete and thereby lead to a drastic change in sperm design.
Keywords: Platyhelminthes, sexually antagonistic coevolution, simultaneous hermaphrodite, sperm morphology, traumatic insemination
Parker's (1–3) far-reaching extension of Darwin's (4) narrow focus on precopulatory mating interactions highlighted that sexual selection continues to operate after mating partners have agreed to mate and that considering postcopulatory sexual selection therefore is crucial to understand the evolution of many reproductive traits (5, 6). This insight has led to extensive research in evolutionary biology that focused on understanding the biology of sperm (7), the most diverse of all animal cell types (8, 9). From this research emerged an apparent consensus that the diversity in sperm design—the strikingly variable ways of constructing a sperm—reflects the highly variable physiological and morphological environments in which sperm have to survive, function, and compete for fertilization (5, 10–14). Moreover, recent studies have documented clearly that these environments can evolve rapidly, probably because of coevolutionary interactions linked to multiple mating and the resulting sexual selection and sexual conflicts (15–21). However, the bewildering diversity in sperm design is poorly understood at the functional level, particularly in internally fertilizing organisms (9, 12, 22). A recent review on the evolution of sperm morphological diversity concluded that we “currently have only a rudimentary understanding of the adaptive significance of [the awe-inspiring variation in sperm form]” and that we “know very little about sperm behavior, particularly within the female reproductive tract” (9). The reason for this lack of understanding is that the “goings-on” inside the female reproductive tract often are difficult to study (but see refs. 23–25).
In organisms with internal fertilization, multiple mating by females generally is expected to lead to sexual conflicts over the fate of received ejaculates (19). Females may mate multiply for different reasons: because it allows them to choose among sperm from different males based on compatibility (26) or male quality (including the competitive quality of their sperm) (27, 28), because they can obtain material benefits from the males (e.g., nuptial gifts or an adequate sperm supply) (26), or because, even though multiple mating may be costly to females, resisting male harassment is even costlier (18). After multiple mating, females may attempt to control the fate of the received ejaculates, either to exert choice or to lower direct costs [e.g., resulting from polyspermy (18, 29)], thereby removing some sperm from the fertilization set. In contrast, males always should prefer that their sperm, rather than that of a competitor, be used for fertilization (19), leading to sexual conflict. This definition of sexual conflict is a broad one (19, 30) and follows the reasoning of Parker (19) that “sexual conflict is present in all forms of female choice involving the rejection of some males, whether rejection occurs because they are not attractive enough or because of the costs they impose” (p. 236). In this scenario males therefore are expected to develop male persistence traits that allow them to overcome such female control, even if that persistence occurs at a cost to the females (19). This situation may lead to sexually antagonistic coevolution between the male persistence traits and the female resistance traits [as has been pointed out before (31), female resistance is equivalent to female preference in that both lead to a bias in the reproductive success toward persistent males] and is expected to affect the evolution of both the sperm and their environment.
In species with separate sexes, females often may be able to evade some of these postcopulatory sexual conflicts by avoiding males—either altogether or via precopulatory female choice—whenever the costs of mating exceed the benefits. This outcome is reflected nicely in the classical (precopulatory) mating roles, namely, eager males and choosy females (32–34). In contrast, simultaneous hermaphrodites cannot resolve these conflicts so easily, because each individual is both male and female at the same time. Here mating should be attempted whenever an individual can gain a net benefit from mating, for example, when its male benefits minus its female costs are positive (35). This inequality will be fulfilled even if the costs of mating to an individual's female fitness are substantial, as long as they are compensated by sufficiently large benefits to its male fitness. Multiple mating in the female role therefore may occur not only for the reasons mentioned above but also as a consequence of behaviors that primarily serve the interests of an individual's male sex function, such as actively approaching mating partners and attempting to engage in sperm donation. Matings that are disadvantageous to the female function therefore are more likely to occur in simultaneous hermaphrodites, shifting sexual selection and sexual conflict from the pre- to the postcopulatory stage. This argument assumes that multiple mating in simultaneous hermaphrodites is primarily male-driven and that all individuals in a population therefore tend to show a general willingness to donate but not necessarily to receive sperm in most matings, leading to conflicting mating interests (36). The extent to which the biology of many simultaneous hermaphrodites supports this assumption is an important focus of empirical research and is the subject of an ongoing debate (37–39), but in the following discussion we will assume that it approximately holds.
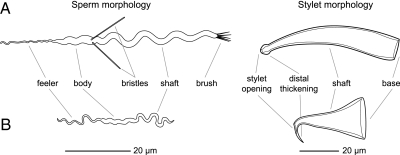
One solution to such conflicting mating interests is reciprocal mating in which mating partners simultaneously assume both the male and the female role, with individuals accepting an ejaculate from the partner in exchange for an opportunity to donate an ejaculate (36, 40). Although this behavior initially appears to be a cooperative solution, it offers ample opportunity for postcopulatory sexual selection and sexual conflicts. Specifically, we expect the evolution of female resistance traits that allow the fate of any unwanted ejaculates to be controlled (36, 40) [e.g., sperm digestion (41, 42)] and male persistence traits that defend against such control [e.g., love darts and chemical manipulation (42, 43)]. A suite of reproductive characters in the free-living flatworm Macrostomum lignano is highly suggestive of such a scenario (Fig. 1A). Based on detailed behavioral (44), in vivo light microscopic, and transmission electron microscopic observations (23), we here define the “reciprocal mating syndrome.” First, mating is reciprocal, with the stylet (the male intromittent genitalia) of each mate inserted into the partner via the female genital opening and sperm deposited into the female antrum (the sperm-receiving organ) of its partner (23). Moreover, mating often is followed directly by a postcopulatory sucking behavior (44) during which the worm bows down onto itself, places its pharynx over its own female genital opening, and appears to suck. After this behavior, sperm shafts often stick out of the female genital opening (Movie S1). We have hypothesized that this behavior is a female resistance trait involved in manipulating the fate of the received ejaculate (23, 44). Second, the aflagellate (45, 46) but highly motile sperm carries unusual characters with a number of proposed functions (Fig. 1A and Movie S2) (23). It has a frontal feeler, which allows the sperm to anchor itself in the epithelium of the female antrum (Movies S3 and S4), and two stiff lateral bristles, which, by interaction with the wall of the female genital opening, may prevent the sperm from being removed during the sucking behavior (23). Third, the female antrum, which is also involved in egg laying, has a thickened part of the epithelium, the cellular valve, in which the great majority of the sperm feelers are anchored (23).
Fig. 1.
Morphology of the sperm and stylet of two Macrostomum species. (A) M. lignano, a species that represents the reciprocal mating syndrome. (B) M. hystrix, a species that represents the hypodermic mating syndrome.
A very different solution to the above-mentioned conflicting mating interests is the uncooperative insistence of the mating partners on sperm donation while attempting to avoid sperm receipt, a scenario that is likely to lead to forced unilateral sperm donation, such as hypodermic insemination. Another species of the same genus, Macrostomum hystrix, exhibits a suite of reproductive characters that fits this scenario and which we here call the “hypodermic mating syndrome” (Fig. 1B). First, mating occurs via hypodermic insemination, during which sperm are injected through the epidermis into the parenchyma of the partner (Movie S5) using a needle-like stylet (i.e., a stylet with a rigid and pointed distal thickening and a subterminal stylet opening, which can puncture the partner's epidermis, presumably without clogging the stylet opening). Sucking behavior never has been observed in this species. Second, the aflagellate sperm also are highly motile (Movie S6) but are smaller, simpler, and carry no bristles, presumably facilitating their movement through tissue. Third, the female antrum is simple, without a thickened epithelium, and lacks a prominent cellular valve. The female antrum is involved only in egg laying.
Here we examine the evolution and coevolution of these morphological and behavioral characters in the free-living flatworm genus Macrostomum. Specifically, we are interested in understanding how stable and widespread the reciprocal and hypodermic mating syndromes are and how they are distributed phylogenetically. To this end, we collected and morphologically described 16 different Macrostomum species, established the molecular phylogeny of this taxonomic group, and used this information to perform phylogenetic comparative analyses, including character mapping, constraint analyses, ancestral state reconstructions, and analyses of correlated evolution. Our results show that the majority of species fall into one of the two syndromes and that the molecular phylogeny does not fully match this morphological dichotomy, with the hypodermic mating syndrome having re-evolved independently at least once from the reciprocal mating syndrome. Moreover, we find strong evidence for correlated evolution between the mating behavior and both male and female morphological characters, shedding light on the evolution of sperm design.
Results
Molecular Phylogeny, Character Mapping, and Constraint Analysis.
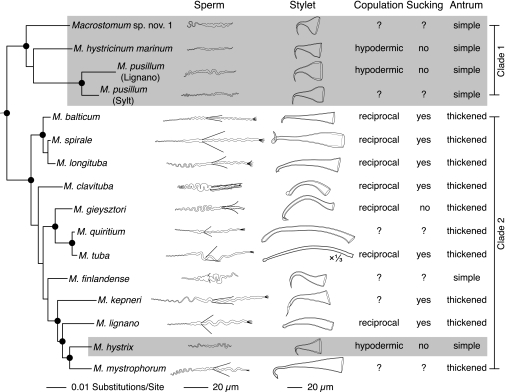
To study the phylogenetic distribution of the above-mentioned reproductive character states, we assembled a molecular phylogeny comprising 16 species of the free-living flatworm genus Macrostomum. By mapping the character states of the collected species (Table S1) onto this phylogeny, we can identify a first clade (clade 1 in Fig. 2), all members of which share the hypodermic mating syndrome. In addition, we can identify a second clade (clade 2 in Fig. 2), most members of which exhibit the reciprocal mating syndrome. However, clade 2 also includes one species, M. hystrix, with the hypodermic mating syndrome (second species from the bottom in Fig. 2) (for a detailed tree with outgroups and exact nodal support values see Fig. S1). A Shimodaira–Hasegawa test strongly rejects a constrained tree with a single origin of the hypodermic mating syndrome (i.e., forcing M. hystrix within clade 1) as an alternative a priori hypothesis to the current placement of this species (P < 0.001) (Fig. S2). The split between the two clades and the nodes at their base are well supported (Fig. S1), suggesting that the hypodermic mating syndrome has evolved independently twice, representing a clear case of convergent evolution. Indeed, the species exhibiting the hypodermic mating syndrome show striking similarities in the morphology of sperm, stylet, and female antrum, whereas species with the reciprocal mating syndrome exhibit considerable interspecific variation in these structures (Fig. 2). Additionally, Macrostomum finlandense, whose mating behavior currently is unknown, appears to be in transition between the two syndromes. Its sperm bristles are short but externally visible, and although the stylet is pointed and fairly rigid, it lacks a pointed distal thickening and has an oblique rather than subterminal stylet opening.
Fig. 2.
Phylogenetic mapping of reproductive character states. Variation in sperm and stylet morphology, mating behavior, and female antrum morphology among 16 species in the genus Macrostomum. (Details on character states are given in Table S1.) The character states are mapped on the maximum-likelihood tree of the genus (complete ssrDNA and partial lsrDNA), and nodes marked with a circle have Bayesian posterior probability >0.95 and maximum-likelihood bootstrap support of >70%. (A detailed tree with outgroups and exact nodal support values is given in Fig. S1.) Note that the stylet of Macrostomum tuba is depicted at one-third of the original size.
Ancestral State Reconstruction.
To understand further the origins and evolution of the studied reproductive characters, we performed ancestral state reconstructions to assess the most probable character states at important nodes in the molecular phylogeny. Although the results of these analyses do not allow conclusions to be drawn about the ancestral states of any of the investigated characters at the base of the genus Macrostomum, they clearly support the presence of the reciprocal mating syndrome at the base of clade 2 (for trees with the estimated ancestral character states, see Fig. S3). This result further supports the independent origin of the hypodermic mating syndrome in M. hystrix.
Correlated Evolution.
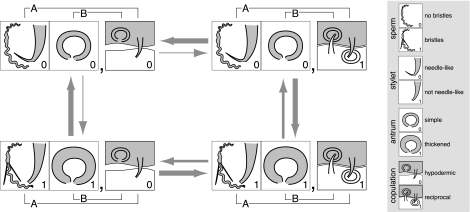
To assess statistically the degree to which there is coevolution between the morphological and behavioral characters, we performed two analyses of correlated evolution (analysis A: male morphology vs. copulation behavior; analysis B: female morphology vs. copulation behavior). The likelihoods resulting from these analyses indicate strong evidence for correlated evolution, both for analysis A between the sperm design (and the stylet morphology) and copulation behavior (logHdep = −9.82, logHindep = −13.01, test statistic = 6.38), and for analysis B between the female antrum morphology and copulation behavior (logHdep = −11.77, logHindep = −14.61, test statistic = 5.68). Moreover, the posterior distributions for the rate parameters resulting from both analyses clearly confirm that the data do not fit an independent model of character evolution (Fig. 3 and Fig. S4). Because our ancestral state reconstructions leave the character states at the base of the genus Macrostomum open, the analyses further suggest that the hypodermic and reciprocal mating syndromes act as evolutionary attractors (i.e., species evolve away from the transitional states toward the syndromes; Fig. 3, thick arrows) and that it is somewhat more likely that a species evolves a transition from the reciprocal to the hypodermic mating syndrome than vice versa (Fig. 3, medium arrows vs. thin arrows).
Fig. 3.
Correlated evolution of reproductive character states. Schematic diagram showing the most probable evolutionary routes for transitions between the four possible combinations of character states of two binary variables. For analysis A, the first variable represents the sperm morphology (0, bristles absent; 1, bristles present) or the stylet morphology (0, needle-like; 1, not needle-like) (SI Materials and Methods). For analysis B, the first variable represents female antrum morphology (0, simple; 1, thickened). For both analyses A and B, the second variable represents the copulation behavior (0, hypodermic; 1, reciprocal). Because the results of the two analyses are qualitatively similar, we show only one summary graph. The different arrows indicate three classes of transition rate parameters: transition rates with a high probablitiy (>55%, thin arrows), intermediate probability (30–40%, medium arrows), and low probability (<15%, thick arrows) of being zero. (The actual distributions of the transition rate parameters are given in Fig. S4.) Thus, thicker arrows represent more likely transitions. Under the null hypothesis of independent evolution, certain pairs of transition parameters must be equal. (The rationale is discussed in SI Materials and Methods.) The results clearly reject this null hypothesis, suggesting strong evidence for correlated evolution between the analyzed character states. Specifically, the estimated transition rate parameters tend to favor correlated evolution toward either the hypodermic mating or reciprocal mating syndrome.
Discussion
Our study reveals an evolutionary link between mating behavior, male and female genital morphology, and sperm design. Specifically, our results suggest that the character states defining the reciprocal mating and the hypodermic mating syndromes in the genus Macrostomum are the result of concerted evolutionary changes and show that the majority of the studied species clearly exhibit one of the two syndromes. The reciprocal mating syndrome probably is driven by sexual conflict over the fate of received ejaculates; this conflict occurs as a result of reciprocal mating, leading to elaborate and highly variable female resistance and male persistence traits. In contrast, the hypodermic mating syndrome probably results from selection to by-pass female resistance traits (36, 40, 47, 48), leading to striking convergent evolution with little morphological variation between species.
Hypodermic insemination is expected to impose novel selection pressures related to this type of sperm transfer and simultaneously to relax selection on the character states defining the reciprocal mating syndrome. For example, sperm may be selected for efficient movement through tissue, perhaps favoring the loss of the bristles. As hypodermic insemination spreads through a population, the way sperm of different donors compete within the body of the recipient should resemble more closely a fair-raffle sperm competition (2, 48), potentially favoring smaller and more numerous sperm. Furthermore, we expect selection on the stylet for efficient hypodermic delivery of sperm and that the evolution of the stylet no longer will be affected by the female antrum. Similarly, selection on the female antrum will be solely on the function of egg laying, because the antrum no longer interacts with the stylet or the sperm.
A more detailed look at the stylet and female antrum morphologies associated with the reciprocal mating syndrome may help identify possible starting points for the evolution of hypodermic insemination, because these morphologies suggest that wounding might occur during reciprocal mating. In several species the stylets carry blunt distal thickenings, indicative of selection on the male function against wounding of the female antrum. Furthermore, at least two species of Macrostomum have sclerotized regions in the female antrum (49, 50) that might represent female adaptations against wounding. However, when the costs of wounding are small for the sperm donor, accidental wounding during copulation could lead eventually to the evolution of hypodermic insemination.
The evolutionary origin of the sperm bristles currently is unclear (51). A study of sperm ultrastructure suggests a homology between the bristles in Macrostomum and lateral ledges in the sperm of Bradynectes sterreri (one of our outgroup species) and Psammomacrostomum turbanelloides (51) (a likely relative of our Gen. nov. 1, sp. nov. 1). These morphological structures do not protrude outside the sperm, however, and therefore cannot have an anchoring function. Moreover, it has been shown that Macrostomum pusillum (a member of clade 1 in Fig. 2) has rudimentary bristles that are visible only at the ultrastructural level (52). Together, these findings suggest that the morphological structures that gave rise to the bristles were already present at the base of the genus Macrostomum. The externally visible bristles thus may have arisen at the base of the genus and been lost twice, or they may have arisen at the base of clade 2 and been lost once. Future studies on a wider range of taxa may arbitrate between these two scenarios and may uncover more cases of gains and losses in this structure.
It has been suggested that sexual selection and sexual conflicts can generate rapid evolutionary diversification in reproductive traits, and this notion is reflected by the use of these traits for taxonomy in many groups of organisms (5, 9, 11, 53, 54). Our results suggest that the degree to which this morphological diversification occurs may depend on the nature of the mating behavior. The striking convergent evolution between the species exhibiting the hypodermic mating syndrome (i.e., M. hystrix and the members of clade 1) helps explain why these species are difficult to distinguish based on reproductive morphology alone and has considerable impact on a number of pressing taxonomical questions in this genus (SI Materials and Methods). In contrast, the reciprocal mating syndrome appears to generate extensive diversification in reproductive traits, thus providing ample morphological information to distinguish species. It is interesting to speculate that the greater diversification within the reciprocal mating syndrome may make it less evolutionarily stable than the hypodermic mating syndrome, as suggested by the correlated evolution analyses (Fig. 3). If so, we would expect that expanding the taxon sampling will reveal additional departures from the reciprocal mating syndrome in clade 2. Alternatively, because clade 1 currently contains few studied species, it also is possible that wider taxon sampling will reveal departures from the hypodermic mating syndrome.
Most studies that have investigated variation in sperm morphology in internally fertilizing organisms have aimed at explaining quantitative variations such as in sperm length within one particular sperm design and have identified sperm competition (9, 12, 55) or the interactions between the sperm and the female genital tract (11, 17, 21, 56) as the main evolutionary forces. Our results suggest that a change in the mating behavior may lead to strikingly different sperm designs within a single genus, and we thus have identified conditions under which drastic shifts in sperm design may arise rapidly and repeatedly. Shifts in mating behavior from normal copulation to hypodermic or traumatic insemination have occurred many times in both hermaphroditic [e.g., acoel flatworms (57), polyclad flatworms (58), and sea slugs (59)] and separate-sexed organisms [e.g., bed bugs (47), plant bugs (60), and drosophilids (61)]. Therefore it will be interesting to investigate further the consequences of these shifts for the evolution of sperm design, morphology, and function. The striking diversity in sperm and genital morphology in the genus Macrostomum, combined with the transparent nature of these worms, offers a veritable ‘window of opportunity’ to begin to understand the evolution of sperm morphological diversity.
Materials and Methods
Specimens.
We collected specimens in a range of countries, habitats, and water bodies, using a range of extraction techniques (i.e., decantation with MgCl2, oxygen deterioration, direct extraction). For each species we give information (SI Materials and Methods) on specimens, collection sites, species identification, taxonomic notes, and accession numbers to digital reference material deposited on the online Macrostomorpha Taxonomy and Phylogeny database, an EDIT scratchpad (62) available at http://macrostomorpha.info. Given the highly variable quality of taxonomic descriptions of the >100 species in the genus, only data on Macrostomum species that we have collected and observed ourselves were included in our data set, but data for the outgroups were taken in part from the literature.
Digital Documentation and DNA Samples.
We observed live specimens in squeeze preparations using a Diaplan or a DM2500 compound microscope (Leica Microsystems) with bright-field, differential interference contrast, or phase-contrast illumination and at magnifications of 40×–1000× and documented their morphology with spatially calibrated micrographs and video, using a digital videocamera [DFW-X700 (SONY) or DFK 41BF02 (Imaging Source)] and image capture software (BTV Pro 5.4.1 or 6.0b1, available at http://www.bensoftware.com/). Because these worms are transparent, we can observe and document internal structures in detail without histological sections (23, 63). Stylets were scored as “needle-like” if they had a rigid and pointed distal thickening and a subterminal stylet opening, and the female antrum was scored as “thickened” if the epithelium was clearly visible (Movie S3) and/or if there was a clear cellular valve (23) (Table S1). To document sperm morphology, we recovered worms from squeeze preparations, amputated the tail plate (64), and ruptured it by placing it on a microscope slide in 1–2 μL of liquid and covering it with a coverslip. Fully formed sperm emerged from the ruptured seminal vesicle and were documented (65). Because sperm are sensitive to low osmotic pressure, we added small amounts of diluted seawater for freshwater species to prevent osmotic damage to the sperm (artifacts thereof sometimes are reported as bona fide sperm morphology). Sperm were scored as “bristles present” if they had externally visible bristles (Table S1). To prepare a DNA sample, we placed the frontal part of the worm into absolute ethanol and stored it at 4 °C. We documented the mating behavior with our established technique (44) for a total of 6–52 h of observation of 4–29 individuals per field-collected species (in pairs and larger groups) and analyzed the videos by frame-by-frame observation. However, not all field-collected species mated under these conditions, leading to some missing data in these character states (Table S1). Moreover, extensive observational data were derived from observations of the laboratory-cultured species (i.e., M. pusillum (Lignano), M. spirale, M. lignano, and M. hystrix).
PCR, Sequencing, and Phylogenetic Analysis.
For each species we sequenced (in one to three specimens) the complete small subunit ribosomal DNA (ssrDNA) and part (D1–D3) of the large subunit ribosomal DNA (lsrDNA) resulting in a total of ∼2,850 base pairs. (Details on primers, PCR, sequencing, and deposition of sequences are given in Tables S2 and S3 and SI Materials and Methods.) The resulting sequences were used to generate a phylogenetic hypothesis for the interrelationships between the studied taxa using both Bayesian inference and maximum likelihood analyses. (Details on alignments and parameters used in analyses are given in SI Materials and Methods.)
Constraint Analysis, Ancestral State Reconstruction, and Analysis of Correlated Evolution.
We performed a constraint analysis (66) that tested whether the data support the a priori hypothesis of a single origin of the hypodermic mating syndrome. (Details are given in Fig. S2 and SI Materials and Methods.) Next we performed ancestral state reconstructions (67) to infer the character states at the base of the genus Macrostomum and the base of clade 2, both nodes that are well supported in the maximum likelihood and Bayesian inference analyses. (Details are given in Table S1, Fig. S3, and SI Materials and Methods.) Finally, we tested for correlated evolution between pairs of discrete binary character states (i.e., male morphology vs. mating behavior and female morphology vs. mating behavior) using BayesDiscrete implemented in BayesTraits (68). The analyses were performed on a reduced taxon set containing only the genus Macrostomum, the main focus of our study. (Details are given in Fig. 3, Fig. S4, and SI Materials and Methods.)
Supplementary Material
Acknowledgments
We are indebted to the late Reinhard Rieger for introducing us to the taxonomy of the Macrostomorpha, and thank Seth Tyler and colleagues for maintaining the Turbellarian Taxonomic Database (http://turbellaria.umaine.edu/). We also thank many volunteers and colleagues who helped us with field collections, especially Gregor Schulte, Peter Ladurner, Floriano Papi, and Werner Armonies. We are grateful to Göran Arnqvist, Dieter Ebert, Tim Janicke, Joris Koene, Nico Michiels, Markus Pfenninger, Steve Ramm, Gunde Rieger, and Walter Salzburger for discussion and/or comments on earlier versions of the manuscript.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. FJ715315–FJ715305).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013892108/-/DCSupplemental.
References
- 1.Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol Rev Camb Philos Soc. 1970;45:525–567. [Google Scholar]
- 2.Parker GA. Sperm competition and the evolution of ejaculates: Towards a theory base. In: Birkhead TR, Møller AP, editors. Sperm Competition and Sexual Selection. London, England: Academic; 1998. pp. 3–54. [Google Scholar]
- 3.Parker GA. Sexual selection and sexual conflict. In: Blum MS, Blum NA, editors. Sexual Selection and Reproductive Competition in Insects. London: Academic; 1979. pp. 123–166. [Google Scholar]
- 4.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 5.Eberhard WG. Postcopulatory sexual selection: Darwin's omission and its consequences. Proc Natl Acad Sci USA. 2009;106(Suppl 1):10025–10032. doi: 10.1073/pnas.0901217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birkhead TR. How stupid not to have thought of that: Post-copulatory sexual selection. J Zool (Lond) 2010;281:78–93. [Google Scholar]
- 7.Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Burlington, MA: Academic; 2009. [Google Scholar]
- 8.Sivinski J. Sperm in competition. In: Smith RL, editor. Sperm Competition and the Evolution of Animal Mating Systems. Orlando, FL: Academic; 1984. pp. 85–115. [Google Scholar]
- 9.Pitnick S, Hosken DJ, Birkhead TR. Sperm morphological diversity. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Burlington, MA: Academic; 2009. pp. 69–149. [Google Scholar]
- 10.Franzén Å. On spermiogenesis, morphology of the spermatozoon, and biology of fertilization among invertebrates. Zoologiska Bidrag från Uppsala. 1956;33:1–28. [Google Scholar]
- 11.Pitnick S, Wolfner MF, Suarez SS. Ejaculate-female and sperm-female interaction. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Burlington, MA: Academic; 2009. pp. 247–304. [Google Scholar]
- 12.Pizzari T, Parker GA. Sperm competition and the sperm phenotype. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Burlington, MA: Academic; 2009. pp. 207–245. [Google Scholar]
- 13.Jamieson BGM, Rouse GW. The spermatozoa of the polychaeta (Annelida): An ultrastructural review. Biol Rev Camb Philos Soc. 1989;64:93–157. doi: 10.1111/j.1469-185x.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Burns JR, Weitzman SH, Grier HJ, Menezes NA. Internal fertilization, testis and sperm morphology in glandulocaudinae fishes (Teleostei: Characidae: Glandulocaudinae) J Morphol. 1995;224:131–145. doi: 10.1002/jmor.1052240203. [DOI] [PubMed] [Google Scholar]
- 15.Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc Natl Acad Sci USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 17.Snook RR. Sperm in competition: Not playing by the numbers. Trends Ecol Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Arnqvist G, Rowe L. Sexual Conflict. Princeton: Princeton Univ Press; 2005. [Google Scholar]
- 19.Parker GA. Sexual conflict over mating and fertilization: An overview. Philos Trans R Soc Lond B Biol Sci. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koene JM, Schulenburg H. Shooting darts: Co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol Biol. 2005;5:25. doi: 10.1186/1471-2148-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beese K, Armbruster GFJ, Beier K, Baur B. Evolution of female sperm-storage organs in the carrefour of stylommatophoran gastropods. J Zool Syst Evol Res. 2009;47:49–60. [Google Scholar]
- 22.Martin OY, Demont M. Reproductive traits: Evidence for sexually selected sperm. Curr Biol. 2008;18:R79–R81. doi: 10.1016/j.cub.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Vizoso DB, Rieger G, Schärer L. Goings-on inside a worm: Functional hypotheses derived from sexual conflict thinking. Biol J Linn Soc Lond. 2010;99:370–383. [Google Scholar]
- 24.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 25.LaMunyon CW, Ward S. Larger sperm outcompete smaller sperm in the nematode Caenorhabditis elegans. Proc R Soc London Ser B. 1998;265:1997–2002. doi: 10.1098/rspb.1998.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeh JA, Zeh DW. Toward a new sexual selection paradigm: Polyandry, conflict and incompatibility. Ethology. 2003;109:929–950. [Google Scholar]
- 27.Keller L, Reeve HK. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv Stud Behav. 1995;24:291–315. [Google Scholar]
- 28.Evans JP, Simmons LW. The genetic basis of traits regulating sperm competition and polyandry: Can selection favour the evolution of good- and sexy-sperm? Genetica. 2008;134:5–19. doi: 10.1007/s10709-007-9162-5. [DOI] [PubMed] [Google Scholar]
- 29.Birkhead TR, Møller AP, Sutherland WJ. Why do females make it so difficult for males to fertilize their eggs? J Theor Biol. 1993;161:51–60. [Google Scholar]
- 30.Eberhard WG, Cordero C. Sexual conflict and female choice. Trends Ecol Evol. 2003;18:438–439. doi: 10.1016/s0169-5347(00)89205-8. [DOI] [PubMed] [Google Scholar]
- 31.Rowe L, Cameron E, Day T. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am Nat. 2005;165(Suppl 5):S5–S18. doi: 10.1086/429395. [DOI] [PubMed] [Google Scholar]
- 32.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 33.Dewsbury DA. The Darwin-Bateman paradigm in historical context. Integr Comp Biol. 2005;45:831–837. doi: 10.1093/icb/45.5.831. [DOI] [PubMed] [Google Scholar]
- 34.Snyder BF, Gowaty PA. A reappraisal of Bateman's classic study of intrasexual selection. Evolution. 2007;61:2457–2468. doi: 10.1111/j.1558-5646.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 35.Michiels NK, Koene JM. Sexual selection favors harmful mating in hermaphrodites more than in gonochorists. Integr Comp Biol. 2006;46:473–480. doi: 10.1093/icb/icj043. [DOI] [PubMed] [Google Scholar]
- 36.Charnov EL. Simultaneous hermaphroditism and sexual selection. Proc Natl Acad Sci USA. 1979;76:2480–2484. doi: 10.1073/pnas.76.5.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard JL. Sexual selection: Lessons from hermaphrodite mating systems. Integr Comp Biol. 2006;46:349–367. doi: 10.1093/icb/icj041. [DOI] [PubMed] [Google Scholar]
- 38.Janicke T, Schärer L. Sex allocation predicts mating rate in a simultaneous hermaphrodite. Proc R Soc London Ser B. 2009;276:4247–4253. doi: 10.1098/rspb.2009.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthes N, et al. Quantifying sexual selection through Bateman gradients: A re-examination of methods for hermaphroditic organisms. Am Nat. 2010;173:249–263. [Google Scholar]
- 40.Michiels NK. Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead TR, Møller AP, editors. Sperm Competition and Sexual Selection. London: Academic; 1998. pp. 219–254. [Google Scholar]
- 41.Sluys R. Sperm resorption in triclads (Platyhelminthes, Tricladida) Inv Rep Dev. 1989;15:89–95. [Google Scholar]
- 42.Koene JM. Tales of two snails: Sexual selection and sexual conflict in Lymnaea stagnalis and Helix aspersa. Integr Comp Biol. 2006;46:419–429. doi: 10.1093/icb/icj040. [DOI] [PubMed] [Google Scholar]
- 43.Chase R, Blanchard KC. The snail's love-dart delivers mucus to increase paternity. Proc R Soc London Ser B. 2006;273:1471–1475. doi: 10.1098/rspb.2006.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schärer L, Joss G, Sandner P. Mating behaviour of the marine turbellarian Macrostomum sp.: These worms suck. Mar Biol. 2004;145:373–380. [Google Scholar]
- 45.Bedini C, Papi F. Peculiar patterns of microtubular organisation in spermatozoa of lower Turbellaria. In: Baccetti B, editor. Comparative Spermatology. New York: Academic; 1970. pp. 363–368. [Google Scholar]
- 46.Morrow EH. How the sperm lost its tail: The evolution of aflagellate sperm. Biol Rev Camb Philos Soc. 2004;79:795–814. doi: 10.1017/s1464793104006451. [DOI] [PubMed] [Google Scholar]
- 47.Stutt AD, Siva-Jothy MT. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc Natl Acad Sci USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schärer L, Janicke T. Sex allocation and sexual conflict in simultaneously hermaphroditic animals. Biol Lett. 2009;5:705–708. doi: 10.1098/rsbl.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt P, Sopott-Ehlers B. Interstitielle Fauna von Galapagos XV. Macrostomum O. Schmidt, 1948 und Siccomacrostomum triviale nov. gen. nov. spec. (Turbellaria, Macrostomidae) Mikrofauna Meeresboden. 1976;57:1–45. [Google Scholar]
- 50.Ax P. Zur Systematik, Ökologie und Tiergeographie der Turbellarienfauna in den ponto-kaspischen Brackwassermeeren. Zool Jb Syst. 1959;87:43–187. [Google Scholar]
- 51.Willems M, et al. Ontogeny of the complex sperm in the macrostomid flatworm Macrostomum lignano (Macrostomorpha, Rhabditophora) J Morphol. 2009;270:162–174. doi: 10.1002/jmor.10675. [DOI] [PubMed] [Google Scholar]
- 52.Rohde K, Faubel A. Spermatogenesis in Macrostomum pusillum (Platyhelminthes, Macrostomida) Inv Rep Dev. 1997;32:209–215. [Google Scholar]
- 53.Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends Ecol Evol. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Eberhard WG. Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard Univ Press; 1985. [Google Scholar]
- 55.Fitzpatrick JL, et al. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc Natl Acad Sci USA. 2009;106:1128–1132. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dybas LK, Dybas HS. Coadaptation and taxonomic differentiation of sperm and spermathecae in featherwing beetles. Evolution. 1981;35:168–174. doi: 10.1111/j.1558-5646.1981.tb04869.x. [DOI] [PubMed] [Google Scholar]
- 57.Apelt G. Fortpflanzungsbiologie, Entwicklungszyklen und vergleichende Frühentwicklung acoeler Turbellarien. Mar Biol. 1969;4:267–325. [Google Scholar]
- 58.Michiels NK, Newman LJ. Sex and violence in hermaphrodites. Nature. 1998;391:647. [Google Scholar]
- 59.Anthes N, Michiels NK. Precopulatory stabbing, hypodermic injections and unilateral copulations in a hermaphroditic sea slug. Biol Lett. 2007;3:121–124. doi: 10.1098/rsbl.2006.0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tatarnic NJ, Cassis G, Hochuli DF. Traumatic insemination in the plant bug genus Coridromius Signoret (Heteroptera: Miridae) Biol Lett. 2006;2:58–61. doi: 10.1098/rsbl.2005.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamimura Y. Twin intromittent organs of Drosophila for traumatic insemination. Biol Lett. 2007;3:401–404. doi: 10.1098/rsbl.2007.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith VS, Rycroft SD, Harman KT, Scott B, Roberts D. Scratchpads: A data-publishing framework to build, share and manage information on the diversity of life. BMC Bioinformatics. 2009;10(Suppl 14):S6. doi: 10.1186/1471-2105-10-S14-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ladurner P, Schärer L, Salvenmoser W, Rieger RM. A new model organism among the lower Bilateria and the use of digital microscopy in taxonomy of meiobenthic Platyhelminthes: Macrostomum lignano, n. sp. (Rhabditophora, Macrostomorpha) J Zool Syst Evol Res. 2005;43:114–126. [Google Scholar]
- 64.Schärer L, Vizoso DB. Phenotypic plasticity in sperm production rate: There's more to it than testis size. Evol Ecol. 2007;21:295–306. [Google Scholar]
- 65.Janicke T, Schärer L. Sperm competition affects sex allocation but not sperm morphology in a flatworm. Behav Ecol Sociobiol. 2010;64:1367–1375. [Google Scholar]
- 66.Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16:1114–1116. [Google Scholar]
- 67.Maddison WP, Maddison DR. Mesquite: A modular system for evolutionary analysis. 2008 Version 2.5. http://mesquiteproject.org. Accessed August 15, 2010. [Google Scholar]
- 68.Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov Chain Monte Carlo. Am Nat. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.