Abstract
Climate change is progressively increasing severe drought events in the Northern Hemisphere, causing regional tree die-off events and contributing to the global reduction of the carbon sink efficiency of forests. There is a critical lack of integrated community-wide assessments of drought-induced responses in forests at the macroecological scale, including defoliation, mortality, and food web responses. Here we report a generalized increase in crown defoliation in southern European forests occurring during 1987–2007. Forest tree species have consistently and significantly altered their crown leaf structures, with increased percentages of defoliation in the drier parts of their distributions in response to increased water deficit. We assessed the demographic responses of trees associated with increased defoliation in southern European forests, specifically in the Iberian Peninsula region. We found that defoliation trends are paralleled by significant increases in tree mortality rates in drier areas that are related to tree density and temperature effects. Furthermore, we show that severe drought impacts are associated with sudden changes in insect and fungal defoliation dynamics, creating long-term disruptive effects of drought on food webs. Our results reveal a complex geographical mosaic of species-specific responses to climate change–driven drought pressures on the Iberian Peninsula, with an overwhelmingly predominant trend toward increased drought damage.
Keywords: extreme events, earth system feedbacks, ecological networks, global change, Mediterranean biome
Global climate change is expected to cause progressively increased frequency and severity of drought events and heat waves in the Northern Hemisphere (1, 2). Globally, increased drought impacts have already been recorded over the last several decades, with anthropogenic forcing widely accepted as the most plausible cause (2–7). These drought impacts have presumably altered carbon cycling dynamics over extensive areas, possibly contributing to the progressive global reduction in the efficiency of terrestrial sinks (5, 7, 8). Major drought impacts on vegetation are to be expected in arid and semiarid biomes, which usually respond to increased water deficit with greater reductions in productivity, although drought-induced tree mortality occurs across a broad range of forest types and mean climate conditions (9). In semiarid and Mediterranean systems, several studies have recently reported increased plant mortality rates and die-off events, reduced seedling recruitment, long-term shifts in vegetation composition, reduced radial growth, and increased crown defoliation responses (9–13). Severe droughts also modify forest biogeochemical cycles by increasing nutrient loss through premature leaf fall without complete nutrient translocation (14). In addition, several studies have suggested the existence of important drought-induced cascading effects at higher trophic levels, affecting vertebrate, invertebrate, and fungal consumer populations; promoting insect outbreaks; and altering fundamental mutualistic processes, such as seed dispersal and pollination (10, 11, 15). Overall, the long-term effects of climate change–type droughts may alter forest physiological responses over extensive areas (10, 11, 15), potentially leading to extensive tree mortality and associated consequences for earth system processes (9, 16).
In the Mediterranean basin and meridional Europe, long-term climatic series and multiproxy studies have demonstrated an unprecedented and significant increase in heat waves and drought impacts over the last several decades (6, 12, 17–20). In line with these findings, the significant increase in the frequency of positive phases of the North Atlantic Oscillation during winter over the last several decades has promoted a northward shift of the Atlantic storm track and possibly triggered droughts and heat waves in southern Europe (21, 22). Comparisons of observational data over the last several decades and regional climate change simulations have identified the Mediterranean basin as a hot spot of hydrological cycle changes, and several regional and global models have consistently predicted increased drought impacts and heat waves in this area in the subsequent decades (23, 24). Droughts produce heterogeneous spatial and temporal impacts, however, and local studies have reported a wide variety of site-dependent and species-specific trends, including both positive and negative physiological responses in forest tree species (14). These differing findings preclude making generalizations based on available data at the local scale, and highlight the need for extensive community-wide assessments of the impacts of drought (11). We currently lack large-scale, integrative, community-wide assessments of drought-induced forest responses, such as tree crown defoliation, mortality, and food web responses.
European national crown condition inventories derived from the International Cooperative Program on Assessment and Monitoring of Air Pollution Effects on Forests (hereinafter the ICP Forest Inventory) provide yearly species-specific measures of the percentage of defoliation of tree crowns over a wide geographic area (25). During drought periods, a reduction in total leaf-transpiration area is a basic response of temperate and Mediterranean forests (26). Forests affected by drought reduce overall tree transpiration through adjustments in total leaf area, allowing improved tree water balance and restoring leaf-specific hydraulic conductivity (26). In the present study, we gathered crown defoliation data from the ICP Forest Inventories (25) to assess the macroecological impacts of drought on water-limited southern European forests over the 20-y period of 1987–2006.
Results
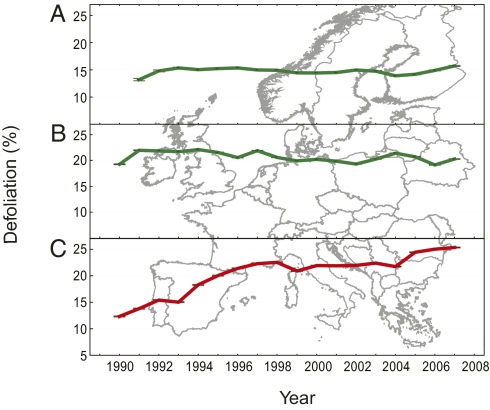
We first contrasted the defoliation patterns of southern water-limited forests relative to central and northern European forests. According to Fluxnet studies, the gross primary production and terrestrial ecosystemic respiration of European forests is limited by water deficit at latitudes below 52°N, whereas temperature effects predominate at latitudes above 52°N (27). Our analysis of temporal defoliation dynamics across latitudinal bands demonstrated a significant increase in crown defoliation rates over the last two decades only in southern European forests, in contrast to the stable and moderate to high defoliation levels seen in northern and central European forests (Fig. 1). From the mid-2000s to 2007, the highest defoliation levels were found in southern Europe.
Fig. 1.
A comparison of crown defoliation trends in northern, central, and southern European forests during 1990–2007. Annual trends in averaged defoliation per plot (for all species grouped) are plotted for three latitudinal bands: (A) northern European forests (>58°N of latitude); (B) central European forests (46°N < latitude < 58°N); and (C) southern European forests (<46°N of latitude).
To quantitatively assess the factors associated with this recent doubling of crown defoliation rates in southern European forests, we modeled crown defoliation and drought impacts in the Iberian Peninsula during 1987–2006. To study drought dynamics, we applied geographic information system–based interpolation techniques to obtain a monthly sequence of climatic maps for temperature, rainfall, and water deficit during 1951–2006. We then modeled defoliation responses using a battery of modeling approaches (Materials and Methods). We assessed the relative effect on defoliation of (i) climatic and topographic variables (i.e., temperature, rainfall, Emberger water deficit index, solar global radiation, and altitude); (ii) biological interactions (i.e., levels of vertebrate and insect herbivory, and fungal damage); (iii) soil structure (i.e., soil type and humus layer depth); (iv) forest management and fire damage; and (v) interactions between all of the independent variables explored (SI Appendix, Tables S1–S6). We also assessed the existence of drought-induced demographic responses by gathering tree mortality data from the Spanish National Forest Inventory (Materials and Methods).
We observed a significant tendency for increased mean annual temperatures and decreased annual rainfall (P < 0.0001) in 1951–2006, coinciding with recently published meteorological studies of the study area (28). We studied drought dynamics in the Iberian Peninsula during 1951–2006 and used time series analysis to identify trends (SI Appendix, Figs. S1 and S2). We found that severe droughts occurred in 2005–2006 and during a long period of drought from 1990 to 1995 that coincided with an anomalous general circulation situation (4, 22). Coinciding with this long drought in 1990–1995, we found a strong and generalized crown defoliation response in all of the tree species examined (Fig. 2). The increase in crown defoliation during 1987–2006 was statistically significant for all tree species examined (ordinary least squares fits, P < 0.001) (SI Appendix, Fig. S3).
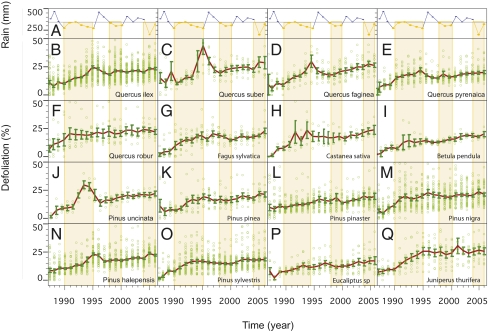
Fig. 2.
Trends in crown defoliation for tree species in the Iberian Peninsula. (Upper) Spring–summer rainfall trends during 1987–2006. Orange bands indicate drought periods with spring–summer rainfall of <400 mm (1990–1995, 1999–2000, and 2005–2006). (B–Q) Crown defoliation trends for 16 main forest tree species (labelled in each panel).
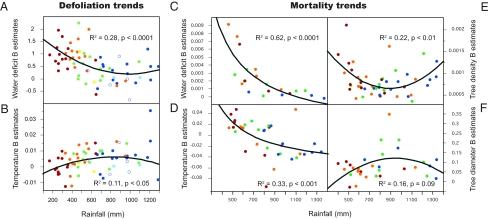
The models indicated that drought damage was consistently the most important factor associated with the generalized increase in defoliation occurring during 1987–2006 (SI Appendix, Table S4). Associations with drought-related variables were significant and strongest in species distributed in more xeric areas (Fig. 3A). To more precisely assess how drought constrained the heterogeneity of tree physiological responses along climatic gradients, we divided the dataset for each species into quartiles of annual rainfall and independently modeled the defoliation and mortality responses to water deficit and temperature in each quartile (SI Appendix, Materials and Methods). We found close associations between water deficit and defoliation in the drier parts of the species’ ranges (Fig. 3A), although each species demonstrated an idiosyncratic response pattern (SI Appendix, Fig. S4). The trends for increasing defoliation were consistent with mortality responses (Fig. 3 C–F). We found a significant and generalized increase of tree mortality rates between 1989–1996 and 1997–2007 by comparing the Second and Third Spanish National Forest Inventories (SI Appendix, Figs. S5 and S6). In turn, this increase in mortality was significantly associated with increased tree density and temperature effects in the 1997–2007 survey (SI Appendix, Fig. S7).
Fig. 3.
Geographical variation in the effects of water deficit and temperature on crown defoliation and mortality. Defoliation is modeled as a function of Emberger water deficit and temperature in generalized linear mixed first-order autocorrelative models for each species and each rainfall quartile. Similarly, mortality is modeled as a function of temperature, water deficit, tree density, and tree diameter using generalized linear models for each species and quartile. Significant β estimates for all tree species are plotted. (A) Changes in Emberger water deficit β coefficient values with increased rainfall for defoliation models. (B) Changes in temperature β coefficient values with increased rainfall for defoliation models. (C) Changes in Emberger water deficit β coefficient values with increased rainfall for mortality models. Note that the water deficit variable was square-transformed to account for hump-shaped responses detected in exploratory graphical analyses. (D) Changes in temperature β coefficient values with increased rainfall for mortality models. (E) Changes in plot tree density β coefficient values with increased rainfall for mortality models. (F) Changes in tree diameter β coefficient values with increased rainfall for mortality models. The red dots represent 0–25 quantiles; orange dots, 25–50 quantiles; yellow dots, 0–50 quantiles; green dots, 50–75 quantiles; dark-blue dots, 75–100 quantiles; light-blue dots, 50–100 quantiles; white dots, species of restricted geographical distribution.
These results illustrate a complex geographical mosaic of species-specific responses to increased water deficit pressures. Our results show that most of the species studied experienced only partial recovery of crown condition after the 1990–1995 drought (Fig. 2), suggesting long-lasting chronic effects of drought on crown structure. This reduced capacity for recovery after drought is possibly due to a combination of limited investment in leaf production due to chronic stress and the presence of defoliated or dead modules in the crown that remain as nonfunctional units for several years (26).
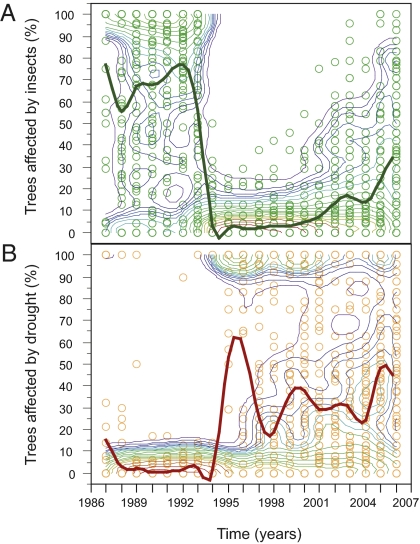
The trophic cascade impacts of climate change–related droughts at the macroecological scale remain largely unexplored, although several previous studies have suggested the existence of important drought-induced cascading effects at higher trophic levels (10, 11, 15, 29–31). Forest drought usually results in reduced shoot growth, reduced nitrogen and water foliar concentrations, and increased allocation to secondary defensive metabolites, such as tannins. The combined effect of these factors can severely increase the mortality rates of insect herbivores during severe drought periods (29) and even truncate multiyear insect outbreak dynamics (32). Similarly, drought can significantly reduce the rate of fungal infection and sporulation capacity (33). We explored the existence of higher trophic-level effects by mapping insect- and fungal-related defoliation trends for each tree species, and compared the temporal dynamics of drought-induced defoliation, fungal damage, and insect herbivory damage. In the most widespread tree species, Quercus ilex, we found a sudden decrease in the impact of insect and fungal defoliation coincident with the abrupt increase of drought effects on trees in 1994–1995 (Fig. 4). We noted similar significant trends in insect defoliation in the less common, closely related species Q. suber (SI Appendix, Figs. S8 and S9). For all other tree species, fungal and insect defoliation patterns were unrelated or only weakly associated with drought dynamics. These results suggest the existence of species-specific drought-induced cascading effects at broad scales in the Iberian Peninsula.
Fig. 4.
Shift in insect herbivore dynamics associated with drought impacts in Q. ilex. (A) Temporal trends in the percentage of trees affected by insect defoliation in the Iberian Peninsula. (B) Temporal trends in the percentage of trees affected by drought. Dots represent sampled plots. A smooth surface showing the density of sampled plots is provided. Red contour lines indicate maximum point density. Spline fits describing the temporal variation in the percentage of trees affected by insect damage and drought are shown.
Discussion
All of the forest tree species that we examined in the Iberian Peninsula have experienced a significant increase in crown defoliation over the last two decades, attributable mainly to the impacts of drought. The observed defoliation trends are consistent with increased tree mortality rates in drier areas and with sudden dynamic changes at higher trophic levels. Our results show that Iberian forests are experiencing long-term chronic effects due to severe climate change–related droughts, and that these effects are progressively more pronounced in more xeric localities.
The reported trends toward increasing defoliation and mortality in southern European forests may have positive and negative effects on the climate system through diverse paths that remain to be quantified more precisely (34, 35). For instance, increased crown defoliation in more xeric forested areas might elevate the albedo of defoliated forests and increase sensible heat flux to the atmosphere (34, 36). Widespread crown decline also might reduce the effects of forest evaporative cooling (34), thereby possibly contributing to the reported declining trend of global land evapotranspiration (37). Notably, the increase in crown defoliation might reduce the evaporative cooling capacity of forests during hot periods and thus have a positive effect on extreme summer heat waves and long-lasting summer drought events (38). Moreover, widespread crown condition declines over large areas potentially could alter local or regional convective uplift dynamics and surface roughness effects (35, 36), as well as the production of volatile organic compounds and derived aerosols by forests, thereby possibly affecting the solar radiation balance and cloud formation processes (39).
In terms of chemical cycling dynamics, the trend of increasing defoliation (Fig. 2) suggests that the effects of drought are likely reducing the carbon sink efficiency of southern European forests, thereby contributing to the global reduction in carbon sink efficiency observed in the Northern Hemisphere and at the global scale (5, 8, 40). These results are in line with the recently reported global reduction in terrestrial net primary production over the last decade (7) and suggest that recurrent severe droughts may directly translate into generalized changes in carbon and nutrient cycling dynamics at the macroecological scale in more xeric Mediterranean areas. Indeed, previous empirical studies assert that severe defoliation events are also associated with increased nutrient cycling through leaf fall losses (14). Similarly, water availability has recently been described as a major determinant of terrestrial gross carbon dioxide uptake in Mediterranean and temperate regions (41). In line with this assertion, European carbon flux anomalies are correlated with water deficit anomalies (42), terrestrial ecosystems seem to respond to droughts with increased carbon flux to the atmosphere (27), and dendrochronological studies at the local scale suggest that important geographic areas in the Mediterranean basin are already experiencing chronic drought-induced effects on tree radial growth, growth variability, and crown condition (12, 19). In the same vein, several empirical studies have reported significant associations between crown condition decline and fine root mortality, reduced radial growth, and tree mortality (43–45).
Our present findings add to the increasing number of reports of drought-induced tree mortality responses, regional forest die-offs, and vegetation shifts around the globe (9). All of this empirical evidence highlights the need for improved long-term networks devoted to monitoring the impacts of climate change on forest health, functional trait variation, genetic variation, and forest demography (9). Critically, the diverse physiological mechanisms implicated in the reported defoliation and mortality responses also remain to be elucidated. These may include long-distance phloem transport effects, carbon reserve dynamics, metabolic unbalances, and/or hydraulic failure processes (46).
Finally, our results demonstrate that extreme droughts can substantially disrupt insect and fungi communities across extensive areas and induce long-term changes in community structure. These findings are consistent with previous studies that have reported 10-fold reductions in arthropod richness and abundance after long-lasting severe droughts and have identified foliage quantity and quality as important drivers of community structure (30, 31). Severe persistent droughts produce parallel disruptions in different groups, affecting ecto-mycorrizal fungi (15), defoliating fungi, herbivore and predator canopy insects, and parasitoids (30, 31). Bottom-up effects on vertebrate trophic chains have been poorly quantified but might occur, given the structural importance of insect resource channels in vertebrate networks in the Mediterranean basin (47, 48). Whether large-scale food web disruptions produced by drought can influence the extinction risk of vulnerable insect species and secondary consumers is an open question that warrants further research. This topic may emerge as a relevant concern related to the conservation of currently endangered biotic communities in the Mediterranean basin (49).
Materials and Methods
Data.
Defoliation data 1987–2007 were gathered from the ICP Forests program (25), mortality data were provided by the Second and Third Spanish National Inventory (50), and climatic data were derived from records of the Spanish National Institute of Meteorology (SI Appendix, Materials and Methods).
Climatic and Crown Defoliation Maps.
Interpolated climatic and crown defoliation maps were derived by applying mixed spatial interpolation methods that combine global and local interpolations (SI Appendix, Materials and Methods).
Statistical Analyses.
For defoliation analyses, we contrasted a battery of modeling approaches including ordinary least squares, generalized linear models, spatial simultaneous autoregressive models, generalized estimating equations, and generalized linear mixed models. First-order autocorrelative terms were introduced to account for temporal autocorrelation in the models, using the CorAR1 function in the R package. Spatial autocorrelation was assessed by applying Moran's I correlograms and plotting spatial maps of the distributions of residuals. Mortality models were based on generalized linear models with a binomial error distribution (SI Appendix, Materials and Methods). Times series analyses were applied to assess the significance of temperature and rainfall trends during 1950–2006 (SI Appendix, Materials and Methods).
Supplementary Material
Acknowledgments
We thank Martin Lorenz, Volker Mues, Georg Becher, and Oliver Granke (ICP Forests) and the SPCAN team (Julio Martínez-Saavedra, Belén Torres, Miguel Prieto, Eudaldo González, Gema Revenga, Jesús Dieste, and Paloma García) for their technical assistance and data management; Gorka Muñoa and Jordi Vayreda for their help with the geographic information systems analyses and data management; and Carsten Dormann, Phillip van Mantgem, and Roger Bivand for advice on the statistical analyses. We also acknowledge the insightful improvements to the manuscript suggested by Craig D. Allen, the two reviewers, and the editor. J.C. was funded by Spanish Ministry of Education and Science/Fulbright Research Grant 2008-0200. This study was supported by Spanish Government Grants CGL2006-04025/BOS, CGL2006-01293/BOS, and CGL2010-17172/BOS; Consolider-Ingenio Montes Grant CSD2008-00040; CSIC Grant PIF08-006-3; and Catalan Government Grants SGR 2009-458 and SGR 2009-1511.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010070108/-/DCSupplemental.
References
- 1.Meehl GA, Tebaldi C. More intense, more frequent, and longer-lasting heat waves in the 21st century. Science. 2004;305:994–997. doi: 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- 2.Intergovernmental Panel on Climate Change . The Physical Science Basis: Contribution of Working Group I. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 3.Hoerling MK, Kumar A. The perfect ocean for drought. Science. 2003;299:691–694. doi: 10.1126/science.1079053. [DOI] [PubMed] [Google Scholar]
- 4.Yeh SW, et al. El Niño in a changing climate. Nature. 2009;461:511–514. doi: 10.1038/nature08316. [DOI] [PubMed] [Google Scholar]
- 5.Zeng N, Qian H. Impact of 1998–2002 midlatitude drought and warming on terrestrial ecosystems and the global carbon cycle. Geophys Res Lett. 2005;32:L22709. [Google Scholar]
- 6.Della-Marta P, Haylock MR, Luterbacher J, Wanner H. Doubled length of western European summer heat waves since 1880. J Geophys Res. 2007;112:D15103. [Google Scholar]
- 7.Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
- 8.Canadell JG, et al. Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks. Proc Natl Acad Sci USA. 2007;104:18866–18870. doi: 10.1073/pnas.0702737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag. 2010;259:660–684. [Google Scholar]
- 10.Mueller RC, et al. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J Ecol. 2005;93:1085–1093. [Google Scholar]
- 11.Breshears DD, et al. Regional vegetation die-off in response to global-change–type drought. Proc Natl Acad Sci USA. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreu L, et al. Climate increases regional tree-growth variability in Iberian pine forests. Glob Change Biol. 2007;13:804–815. [Google Scholar]
- 13.van Mantgem PJ, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- 14.Martínez-Alonso C, et al. Influence of intradecadal climate variability on the uncoupling of canopy dynamics, secondary growth and cone production in an old-growth Scots pine forest under Mediterranean conditions. For Ecol Manag. 2007;253:19–29. [Google Scholar]
- 15.Swaty RL, Deckert RJ, Whitham TG, Gehring CA. Ectomycorrhizal abundance and community composition shifts with drought: Predictions from tree rings. Ecology. 2004;85:1072–1084. [Google Scholar]
- 16.Adams HD, et al. Forest mortality feedbacks to the earth system under global climate change. Eos. 2010;91:153–154. [Google Scholar]
- 17.Luterbacher J, Dietrich D, Xoplaki E, Grosjean M, Wanner H. European seasonal and annual temperature variability, trends, and extremes since 1500. Science. 2004;303:1499–1503. doi: 10.1126/science.1093877. [DOI] [PubMed] [Google Scholar]
- 18.Luterbacher J, et al. The Mediterranean Climate: An Overview of the Main Characteristics and Issues. Amsterdam: Elsevier; 2006. [Google Scholar]
- 19.Sarris D, Christodoulakis D, Körner C. Recent decline in precipitation and tree growth in the eastern Mediterranean. Glob Change Biol. 2007;13:1187–1200. [Google Scholar]
- 20.Briffa KR, van der Schrier G, Jones PD. Wet and dry summers in Europe since 1750: Evidence for increasing drought. Int J Climatol. 2009;29:1894–1905. [Google Scholar]
- 21.López-Moreno JI, Vicente-Serrano SM. Positive and negative phases of the wintertime north Atlantic oscillation and drought occurrence over Europe: A multitemporal-scale approach. J Clim. 2008;21:1220–1243. [Google Scholar]
- 22.Rodriguez-Puebla C, Nieto S. Trends of precipitation over the Iberian Peninsula and the North Atlantic Oscillation under climate change conditions. Int J Climatol. 2010;30:1807–1815. [Google Scholar]
- 23.Giorgi F, Lionello P. Climate change projections for the Mediterranean region. Global Planet Change. 2008;63:90–104. [Google Scholar]
- 24.Mariotti A. Recent changes in the Mediterranean water cycle: A pathway toward long-term regional hydroclimatic change? J Clim. 2010;23:1513–1525. [Google Scholar]
- 25.International Cooperative Programme on Assessment and Monitoring of Air Pollution Effects on Forests . Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests. Hamburg, Germany: Federal Research Center for Forestry and Forest Products; 2006. [Google Scholar]
- 26.Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term responses. Ann Sci. 2006;63:625–644. [Google Scholar]
- 27.Reichstein M, et al. Determinants of terrestrial ecosystem carbon balance inferred from European eddy covariance flux sites. Geophys Res Lett. 2007;34:L01402. [Google Scholar]
- 28.De Luis M, González-Hidalgo JC, Longares LA, Štepánek P. Seasonal precipitation trends in the Mediterranean Iberian Peninsula in second half of the XX century. J Clim. 2009;29:1312–1323. [Google Scholar]
- 29.Shure DJ, Mooreside PD, Ogle SM. Rainfall effects on plant–herbivore processes in an upland oak forest. Ecology. 1998;79:604–617. [Google Scholar]
- 30.Trotter RT, Cobb NS, Whitham TG. Arthropod community and trophic structure: A comparison between extremes of plant stress. Ecol Entomol. 2010;33:1–11. [Google Scholar]
- 31.Stone AC, Gehring CA, Whitham TG. Drought negatively affects communities on a foundation tree: Growth rings predict diversity. Oecologia. 2010;164:751–761. doi: 10.1007/s00442-010-1684-3. [DOI] [PubMed] [Google Scholar]
- 32.Esper J, Büntgen U, Frank DC, Nievergelt D, Liebhold A. 1200 years of regular outbreaks in alpine insects. Proc Biol Sci. 2007;274:671–679. doi: 10.1098/rspb.2006.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 34.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 35.Chapin FS, et al. Changing feedbacks in the climate–biosphere system. Front Ecol Environ. 2008;6:313–320. [Google Scholar]
- 36.Rotenberg E, Yakir D. Contribution of semi-arid forests to the climate system. Science. 2010;327:451–454. doi: 10.1126/science.1179998. [DOI] [PubMed] [Google Scholar]
- 37.Jung M, et al. Recent decline in the global land evapotranspiration trend due to limited moisture supply. Nature. 2010;467:951–954. doi: 10.1038/nature09396. [DOI] [PubMed] [Google Scholar]
- 38.Teuling AJ, et al. Contrasting response of European forest and grassland energy exchange to heat waves. Nat Geosci. 2010;3:722–727. [Google Scholar]
- 39.Peñuelas J, Staudt M. BVOCs and global change. Trends Plant Sci. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Ciais PM, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- 41.Beer C, et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science. 2010;329:834–838. doi: 10.1126/science.1184984. [DOI] [PubMed] [Google Scholar]
- 42.Reichstein M, et al. Reduction of ecosystem productivity and respiration during the European summer 2003 climate anomaly: A joint flux tower, remote sensing and modeling analysis. Glob Change Biol. 2006;12:1–18. [Google Scholar]
- 43.Dobbertin M, Brang P. Crown defoliation improves tree mortality models. For Ecol Manag. 2001;141:271–284. [Google Scholar]
- 44.Drobyshev I, Linderson H, Sonesson K. Relationship between crown condition and tree diameter growth in southern Swedish oaks. Environ Monit Assess. 2007;128:61–73. doi: 10.1007/s10661-006-9415-2. [DOI] [PubMed] [Google Scholar]
- 45.Eckmuller O, Sterba H. Crown condition, needle mass, and sapwood area relationships of Norway spruce (Picea abies) Can J Res. 2000;30:1646–1654. [Google Scholar]
- 46.Sala A, Piper F, Hoch G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010;186:274–281. doi: 10.1111/j.1469-8137.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- 47.Carnicer J, Jordano P, Melián CJ. The temporal dynamics of resource use by frugivorous birds: A network approach. Ecology. 2009;90:1958–1970. doi: 10.1890/07-1939.1. [DOI] [PubMed] [Google Scholar]
- 48.Carnicer J, Abrams PA, Jordano P. Switching behavior, coexistence and diversification: Comparing empirical community-wide evidence with theoretical predictions. Ecol Lett. 2008;11:802–808. doi: 10.1111/j.1461-0248.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 49.Stefanescu C, Carnicer J, Peñuelas J. Determinants of species richness in generalist and specialist Mediterranean butterflies: The negative synergistic forces of climate and habitat change. Ecography. 2010 10.1111/j.1600-0587.2010.06264.x. [Google Scholar]
- 50.Dirección General de Conservación de la Naturaleza . Tercer Inventario Forestal Nacional, 1997–2006. Ministerio de Medio Ambiente, Madrid; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.