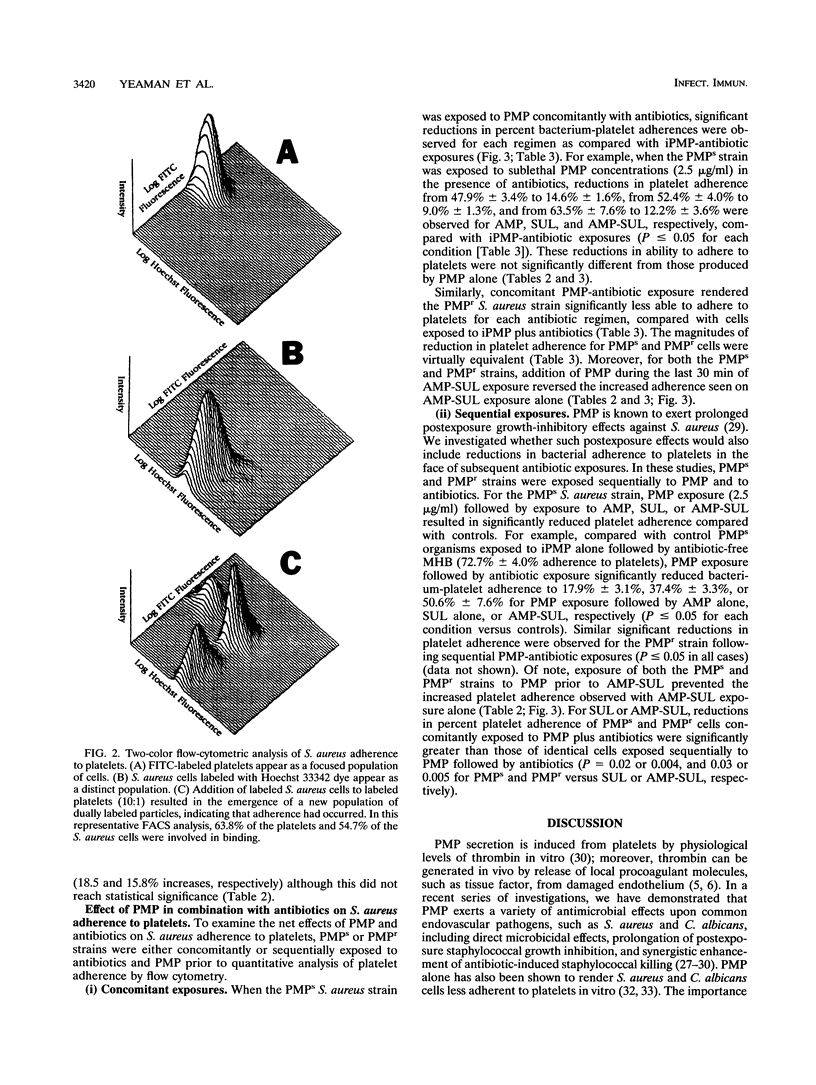
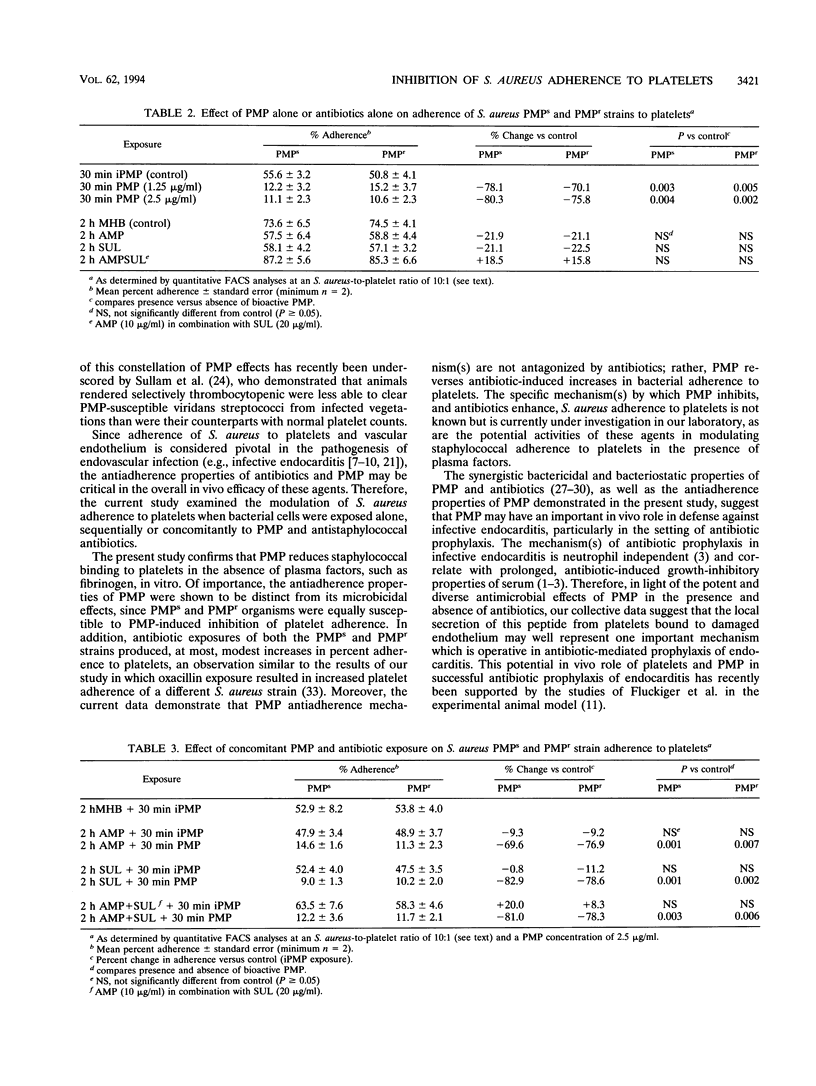
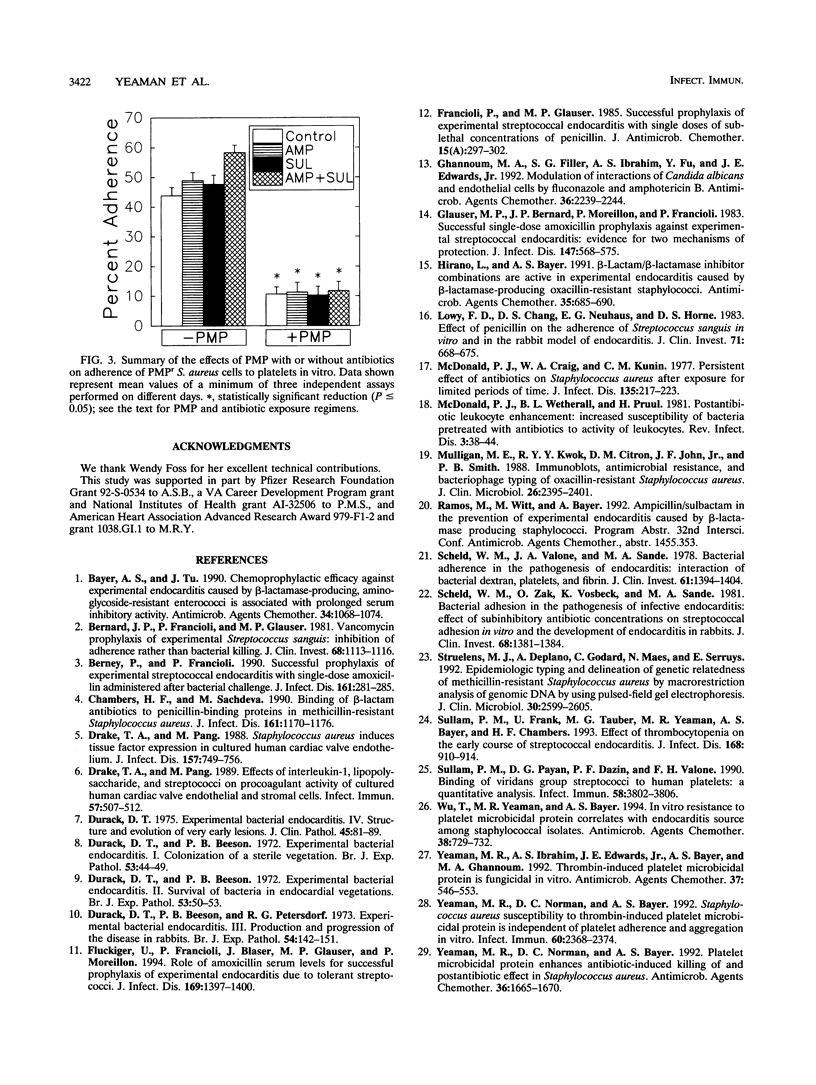
Abstract
Bacterial adherence to platelets on the cardiac valve surface is believed to be critical in the induction of infective endocarditis. Recent studies have confirmed that thrombin-activated platelets secrete platelet microbicidal protein (PMP), which can both kill and exert nonlethal antiadherence effects against endovascular pathogens. In the present study, we quantified the influence of antibiotic and/or PMP exposures on in vitro platelet adherence of two Staphylococcus aureus strains, identical by DNA restriction and cell wall protein profiles, that differed in their susceptibility to PMP-induced killing (PMPs or PMPr, respectively). Adherence assays were performed by flow cytometry in the presence of sublethal PMP concentrations (1 to 2.5 micrograms/ml) alone or in combination with ampicillin (AMP) alone, sulbactam (SUL) alone, or AMP plus SUL (AMP-SUL), at levels achievable in serum. Exposure of the PMPs and PMPr S. aureus strains to antibiotics (for 2 h at 37 degrees C) prior to flow cytometry resulted in no substantive changes in the percent adherence to platelets compared with that for S. aureus cells not exposed to antibiotics, except for modestly increased adherence of both PMPs and PMPr cells exposed to AMP-SUL (18.5 and 15.8% increases, respectively). Addition of PMP to antibiotic-S. aureus mixtures (final 30 min) caused a significant decrease in S. aureus adherence to platelets, for both the PMPs and PMPr S. aureus strains, compared with antibiotic exposure alone (e.g., reduction in platelet adherence from 57.9 +/- 8.2% to 12.2 +/- 3.6% for PMPs cells exposed to AMP-SUL and PMP [P = 0.01]). Moreover, addition of PMP following exposure of the PMPs and PMPr strains to AMP-SUL reversed the enhanced bacterium-platelet adherence observed with such antibiotic exposures alone (P < or = 0.005). These data demonstrate that PMP exerts a potent antiplatelet adherence effect which is independent of its microbicidal capacity, rendering S. aureus cells less adherent to platelets in the presence or absence of antibiotics. Reduction of microbial adherence to platelets by PMP alone or with antibiotics provides further insight into the mechanism(s) that may be involved in host defense and antibiotic prophylaxis of infective endocarditis and other endovascular infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer A. S., Tu J. Chemoprophylactic efficacy against experimental endocarditis caused by beta-lactamase-producing, aminoglycoside-resistant enterococci is associated with prolonged serum inhibitory activity. Antimicrob Agents Chemother. 1990 Jun;34(6):1068–1074. doi: 10.1128/aac.34.6.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J. P., Francioli P., Glauser M. P. Vancomycin prophylaxis of experimental Streptococcus sanguis. Inhibition of bacterial adherence rather than bacterial killing. J Clin Invest. 1981 Oct;68(4):1113–1116. doi: 10.1172/JCI110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berney P., Francioli P. Successful prophylaxis of experimental streptococcal endocarditis with single-dose amoxicillin administered after bacterial challenge. J Infect Dis. 1990 Feb;161(2):281–285. doi: 10.1093/infdis/161.2.281. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Sachdeva M. Binding of beta-lactam antibiotics to penicillin-binding proteins in methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990 Jun;161(6):1170–1176. doi: 10.1093/infdis/161.6.1170. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989 Feb;57(2):507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988 Apr;157(4):749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. II. Survival of a bacteria in endocardial vegetations. Br J Exp Pathol. 1972 Feb;53(1):50–53. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Fluckiger U., Francioli P., Blaser J., Glauser M. P., Moreillon P. Role of amoxicillin serum levels for successful prophylaxis of experimental endocarditis due to tolerant streptococci. J Infect Dis. 1994 Jun;169(6):1397–1400. doi: 10.1093/infdis/169.6.1397. [DOI] [PubMed] [Google Scholar]
- Francioli P., Glauser M. P. Successful prophylaxis of experimental streptococcal endocarditis with single doses of sublethal concentrations of penicillin. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):297–302. doi: 10.1093/jac/15.suppl_a.297. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Filler S. G., Ibrahim A. S., Fu Y., Edwards J. E., Jr Modulation of interactions of Candida albicans and endothelial cells by fluconazole and amphotericin B. Antimicrob Agents Chemother. 1992 Oct;36(10):2239–2244. doi: 10.1128/aac.36.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser M. P., Bernard J. P., Moreillon P., Francioli P. Successful single-dose amoxicillin prophylaxis against experimental streptococcal endocarditis: evidence for two mechanisms of protection. J Infect Dis. 1983 Mar;147(3):568–575. doi: 10.1093/infdis/147.3.568. [DOI] [PubMed] [Google Scholar]
- Hirano L., Bayer A. S. Beta-Lactam-beta-lactamase-inhibitor combinations are active in experimental endocarditis caused by beta-lactamase-producing oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1991 Apr;35(4):685–690. doi: 10.1128/aac.35.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy F. D., Chang D. S., Neuhaus E. G., Horne D. S., Tomasz A., Steigbigel N. H. Effect of penicillin on the adherence of Streptococcus sanguis in vitro and in the rabbit model of endocarditis. J Clin Invest. 1983 Mar;71(3):668–675. doi: 10.1172/JCI110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald P. J., Craig W. A., Kunin C. M. Persistent effect of antibiotics on Staphylococcus aureus after exposure for limited periods of time. J Infect Dis. 1977 Feb;135(2):217–223. doi: 10.1093/infdis/135.2.217. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Wetherall B. L., Pruul H. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev Infect Dis. 1981 Jan-Feb;3(1):38–44. doi: 10.1093/clinids/3.1.38. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Kwok R. Y., Citron D. M., John J. F., Jr, Smith P. B. Immunoblots, antimicrobial resistance, and bacteriophage typing of oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1988 Nov;26(11):2395–2401. doi: 10.1128/jcm.26.11.2395-2401.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Zak O., Vosbeck K., Sande M. A. Bacterial adhesion in the pathogenesis of infective endocarditis. Effect of subinhibitory antibiotic concentrations on streptococcal adhesion in vitro and the development of endocarditis in rabbits. J Clin Invest. 1981 Nov;68(5):1381–1384. doi: 10.1172/JCI110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens M. J., Deplano A., Godard C., Maes N., Serruys E. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J Clin Microbiol. 1992 Oct;30(10):2599–2605. doi: 10.1128/jcm.30.10.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullam P. M., Frank U., Yeaman M. R., Täuber M. G., Bayer A. S., Chambers H. F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993 Oct;168(4):910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Payan D. G., Dazin P. F., Valone F. H. Binding of viridans group streptococci to human platelets: a quantitative analysis. Infect Immun. 1990 Nov;58(11):3802–3806. doi: 10.1128/iai.58.11.3802-3806.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Yeaman M. R., Bayer A. S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother. 1994 Apr;38(4):729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Ibrahim A. S., Edwards J. E., Jr, Bayer A. S., Ghannoum M. A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993 Mar;37(3):546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Norman D. C., Bayer A. S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Aug;36(8):1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Norman D. C., Bayer A. S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992 Jun;60(6):2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Puentes S. M., Norman D. C., Bayer A. S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992 Mar;60(3):1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Sullam P. M., Dazin P. F., Ghannoum M. A., Edwards J. E., Jr, Bayer A. S. Fluconazole and platelet microbicidal protein inhibit Candida adherence to platelets in vitro. Antimicrob Agents Chemother. 1994 Jul;38(7):1460–1465. doi: 10.1128/aac.38.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman M. R., Sullam P. M., Dazin P. F., Norman D. C., Bayer A. S. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992 Jul;166(1):65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]