Abstract
The binary switch gene Sex-lethal (Sxl) controls sexual identity in Drosophila. When activated, Sxl imposes female identity, whereas male identity ensues by default when the gene is off. The decision to activate Sxl is controlled by an X chromosome counting system that regulates the Sxl establishment promoter, Sxl-Pe. The counting system depends upon the twofold difference in the gene dose of a series of X-linked transcription factors or numerators. Because of this difference in dose, early female embryos express twice the amount of these transcription factors, and the cumulative action of these transcription factors turns on Sxl-Pe. Here we show that the Drosophila Myc gene diminutive is an X-linked numerator.
Sexual identity in Drosophila is controlled by the binary switch gene Sex-lethal (Sxl) (1). When Sxl is on, it imposes female development, whereas male development proceeds by default when it is off. During most of the life cycle, on/off regulation is at the level of alternative splicing (2, 3). In males, Sxl transcripts are spliced in the default pattern that incorporates a translation terminating male-specific exon, exon 3. In females, exon 2 is spliced directly to exon 4, skipping exon 3. The resulting female mRNAs encode 36- to 40-kDa proteins that have two RNA recognition motif binding domains. These Sxl proteins direct female-specific splicing of Sxl premRNAs expressed from the Sxl maintenance promoter, Sxl-Pm, and this establishes a positive autoregulatory feedback loop that serves to maintain female identity (4). In addition to maintaining the determined state, Sxl orchestrates female development by promoting the female-specific splicing of transformer (tra) mRNA and turns off X chromosome dosage compensation by blocking male-specific lethal-2 (msl-2) mRNA translation (1). In males, where Sxl is off, default splicing of tra generates nonproductive mRNAs and male differentiation ensues, whereas expression of Msl-2 permits the assembly of a functional dosage compensation system. As would be expected from its binary activity, loss of Sxl has no phenotypic consequences in males, whereas it leads to sex transformation and lethality in females. Conversely, ectopic activation of Sxl in males induces female development and is lethal due to the lack of dosage compensation.
Whereas Sxl is regulated by alternative splicing during much of development, the initial decision of whether to activate it or not and, thus the choice of sexual identity is made at the level of transcription by regulating the Sxl establishment promoter, Sxl-Pe (5). Sxl-Pe is located in the first intron of the Sxl-Pm transcription unit and is the target of the system that measures the X chromosome-to-autosome “ratio” in precellular blastoderm embryos. The counting system consists of zygotically transcribed X-linked “numerators,” which function to activate Sxl-Pe and correspond to genes encoding transcription factors like scute (sc) and runt (6–8). On the autosomes, there is only one known zygotically transcribed “denominator,” deadpan (9). In addition there are a series of maternally derived transcription factors. Some like daughterless act as cofactors for the numerators (sc), whereas others, like groucho (gro), appear to function as autosomal denominators (10, 11). The activation of Sxl-Pe pivots on the difference in dose of the X-linked numerators in 2X female and 1X male embryos. This difference is sufficient to turn on Sxl-Pe in female precellular blastoderm embryos, but keep it off in males. Except for ~20 amino acids at the N terminus, the Sxl-Pe mRNAs encoded proteins are identical to the Sxl-Pm female mRNAs and they activate the positive autoregulatory feedback loop by directing female-specific splicing of the first Sxl-Pm transcripts, which appear as Sxl-Pe shuts down just before cellularization (5).
Sxl-Pe consists of an evolutionarily conserved 0.4-kb switch element that confers sex-specific transcription and an upstream augmentation element that generates high levels of expression (12, 13). Within these elements are multiple (typically noncanonical) binding sites for numerators like sc and runt and denominators like dpn. Here we report the identification of a previously unknown X-linked numerator, diminutive (dm), which encodes the Drosophila bHLH transcription factor dMyc.
Results
To identify genes important for Sxl regulation we screened for deficiencies that suppress or enhance the weak female-specific lethality induced by a dominant negative hsp83:N′–β-gal transgene. The chimeric Sxl–β-gal protein expressed by this transgene is assembled into Sxl splicing complexes and interferes with Sxl autoregulation (14). Whereas we expected to recover mostly splicing factors in this screen, mutations in known numerators such as sc also enhance female lethality. One of the X-linked deficiencies identified in our screen was Df(1)dm75e19, which uncovers the 3C-E region. When Df(1)dm75e19 females were crossed to N′–β-gal males, the viability of Df(1)dm75e19/+ females was 75% that of their +/+ sibs (n = 196). Female lethal interactions with the transgene are exacerbated by removing a copy of Sxl. Transgenic females trans-heterozygous for Df(1)dm75e19 and the Sxl deletion, Sxl7BO, are only half as viable as their transgenic Sxl7BO female sibs (n = 96).
dm Mutations Are Preferentially Female Lethal.
One gene uncovered by Df(1)dm75e19 is diminutive (dm). dm encodes the Drosophila Myc protein (15–17). In other organisms, Myc, together with its partner Max, is a transcriptional activator. Because dm is X linked and is transcribed in precellular blastoderm embryos, a plausible idea is that dm is a new numerator. If this is the case, it should be possible to duplicate the female lethal interactions between the N′–β-gal transgene and Df(1)dm75e19 using dm mutants. Consistent with this expectation, N′–β-gal females that are trans-heterozygous for Sxl7B0 and the dm1 allele are two-thirds as viable as their N′–β-gal Sxl7B0/+ sisters (n = 644). Although the female lethality induced by dm1 is less than that of Df(1)dm75e19, this could be due to the fact that dm1 is not a null.
We subsequently found that dm1 and two other hypomorphic alleles, dmP0 and dmP1, exhibit partial female-specific lethality (Table 1). Of the three, dm1 is the strongest and only 6% of dm1/dm1 females survive compared with 85% of sibling dm1/Y males. dmP1 and dmP0, which have P-element insertions upstream of the transcription start site (17), also preferentially kill females. Homozygous dmP1 females are half as viable as their male dmP1 sibs, whereas dmP0 females are two-thirds as viable as their male dmP0 sibs. Arguing against background effects, female lethality is also observed when the mutants are tested over Df(1)dm75e19 (Table S1).
Table 1.
dm mutations are female lethal
| dm/dm, % (n) | dm/FM7 (n) | dm/Y, % (n) | FM7/Y (n) | (n) | |
| dm1/FM7 x dm1/Y | 6 (46) | (731) | 85 (624) | (456) | (1,857) |
| dmP0/FM7 x dmP0/Y | 46 (233) | (522) | 73 (380) | (343) | (1,388) |
| dmP1/FM7 x dmP1/Y | 23 (94) | (410) | 51 (209) | (374) | (1,087) |
Progeny of each type from crossing dm/Bal females to dm/Y males (as indicated in column 1) were counted. Percentage in second and fourth columns is calculated on the basis of number of dm/FM7 females.
Female Lethal Effects of dm Are Due to Sxl Misregulation.
We used two experimental approaches to confirm that dm functions in Sxl regulation. First, we examined the effects of dm on Sxl expression by probing embryos from wild-type or dm1/+ mothers mated to dm1/Y fathers with Sxl antibodies. We found that the reduction in dm activity in progeny of wild-type mothers had a small but obvious effect on Sxl. As expected, half of the embryos (46%, n = 124) expressed Sxl, whereas the other half (56%) did not. However, the dm1/+ female progeny from this cross express less Sxl than fully wild-type female embryos probed in parallel. Moreover, whereas Sxl is always expressed uniformly throughout the soma in +/+ females, about 40% of the dm1/+ females (20% of total population) had patchy Sxl expression. Even greater effects on Sxl were evident when dm1/+ mothers were crossed to dm1/Y fathers (Fig. S1). As before, half (48%, n = 223) of the embryos had no Sxl and these are presumed to be males. The remaining embryos fell into three classes: class 1, 13% were uniformly stained although not as strongly as wild-type females; class 2, 15% also had intermediate levels of staining but Sxl expression was uneven; and class 3, 24% had lower levels of Sxl and were patchy. On the basis of the frequency of the different classes (and the results with wild-type mothers crossed to dm fathers), we believe that embryos in classes 1 and 2 are dm1/+, whereas embryos in class 3 are dm1/dm1.
In the second approach, we recombined dmP0 with the gain-of-function allele SxlM4. SxlM4 has a transposon inserted into the male exon that causes constitutive female splicing of Sxl-Pm premRNAs irrespective of the signal from the X/A counting system. Eleven independent dmP0 SxlM4 recombinants were mated to dmP0/Y males. As expected, the SxlM4, dm P0/dmP0 females are fully viable (Table S2). Whereas constitutive Sxl expression rescues the female-specific lethality of dmP0, it does not rescue the dm bristle or female sterility phenotypes. As expected, the constitutive SxlM4 is male lethal and no dmP0 SxlM4 males are recovered from the cross, whereas balancer males are.
Sxl and Numerators Show Female Lethal Interactions with dm.
The misregulation of Sxl evident when dm activity is compromised supports the idea that it functions as a numerator. Mutations in numerators show dose-dependent female lethal interactions with Sxl and each other. On the basis of the relative severity of these lethal effects, sc and sis-a have been classified as primary numerators, whereas runt (run) and the JAK/STAT ligand, unpaired (upd) are secondary numerators (1). To provide further evidence that dm is a numerator and ascertain its relative importance, we first tested for female lethal interactions with Sxl. Males carrying dm mutations were mated to Sxl7B0/Bal females. Females compromised for only one copy of dm are fully viable as are Sxl7B0/+ females. However, females trans-heterozygous for Sxl7B0 and one of the dm alleles are only about two-thirds as viable as their dm/+ sibs (Table 2). The female lethal interactions between Sxl7B0 and the three dm alleles are not as strong as those observed for mutations in sc or sis-b, but are stronger than run. In the reciprocal experiment, we introduced dm− (or Df(1)dm75e19) from the mother (Table S3). As expected for a numerator, there is no indication of a significant maternal effect.
Table 2.
Female lethal interactions between dm and other sex determination genes
| Maternal | Paternal |
|||||
| dm1/Y | dmP0/Y | dmP1/Y | sis-a/Y | sc3-1/Y | runt3/Y | |
| Sxl7BO/FM7 | 60 (2,031) | 63 (312) | 68 (260) | 7 (32) | 9 (204) | 81 (671) |
| sis-a/SM1 | 76 (616) | 88 (279) | 84 (408) | ND | ND | 51 (565) |
| sc3-1/FM7 | 23.8 (2,385) | 90.3 (745) | 76.1 (1,200) | 14 (409) | ND | 60 (564) |
| runt3/FM7 | 100 (271) | 79 (516) | 89 (484) | 1 (281) | 38 (67) | ND |
For each cross, paternal genotype is indicated in the top row and maternal genotype in the first column. Percentage of surviving trans-heterozygous females from each cross were calculated relative to balancer females. Percentage of offspring indicated outside parenthesis. Total number of offspring indicated in parentheses. ND, not determined.
Trans-heterozygous combinations between dm and other numerators also enhance female lethality (Table 3). The strongest interactions were seen between sc and dm1 with female viability being reduced to less than one-fourth, whereas there were weaker interactions between sc and the two other dm alleles. Viability is only slightly reduced in the sis-a and dm trans-heterozygous combinations, with dmP1/sis-a being the strongest. run3 also interacts most strongly with dmP1, whereas it shows no interactions with dm1.
Table 3.
Effects of dm on Sxl-Pe3.0kb activity
| Maternal | Paternal | +++, % | ++, % | +, % | −, % | n |
| w1/w1 | w1/Y; 3.0 | 47 | 53 | 678 | ||
| dm1/+ | w1/Y; 3.0 | 25 | 22 | 53 | 623 | |
| dm1/+ | dm1/Y; 3.0 | 31 | 67 | 471 | ||
| w1/w1 | sc3-1/Y; 3.0 | 48 | 52 | 163 | ||
| dm1/+ | sc3-1/Y; 3.0 | 27 | 28 | 46 | 79 |
Sxl-Pe3.0kb activity in progeny from each of the indicated crosses. +++, high level of LacZ; ++, intermediate level; +, low level; −, none. n = number scored.
dm Is Required for Activation of Sxl-Pe in Females.
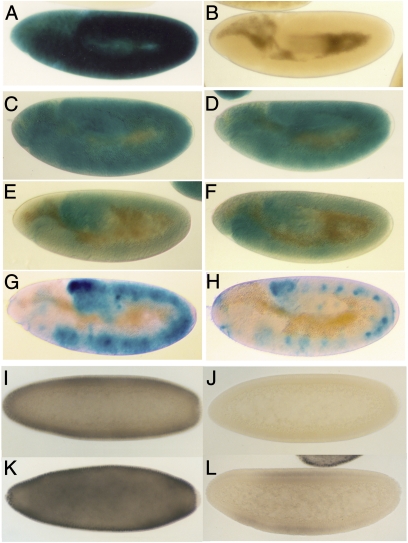
Numerators activate Sxl by turning on the establishment promoter Sxl-Pe. We used two different Sxl-Pe:LacZ reporters to determine whether dm regulates Sxl-Pe. The larger promoter, Sxl-Pe3.0kb, contains both the conserved sex-specific switch element and the upstream augmentation element, whereas the smaller reporter, Sxl-Pe0.4kb, only has the switch element. In wild type, a single copy of Sxl-Pe3.0kb drives a high level of LacZ expression throughout the soma of female embryos (Fig. 1A), whereas no LacZ is detected in males (Fig. 1B). We first crossed wild-type females carrying two copies of Sxl-Pe3.0kb to dm1 or, as a control, sc males. All female progeny have a single copy of Sxl-Pe3.0kb and are heterozygous for dm1 or sc. Like wild type, LacZ is expressed in ~50% of the embryos; however, as can be seen by comparing the dm1/+ embryo in Fig. 1C with wild type in Fig. 1B, the level of LacZ expression is reduced. In fact, the Sxl-Pe3.0kb reporter appears to be almost as sensitive to reduced dm activity as it is to the sc control (Fig. 1D).
Fig. 1.
dm regulates Sxl. (A–H) (LacZ expression) Sxl-Pe3.0kb (8) in wild-type female (A) and male (B) embryos, in sc/+ (C), or dm1/+ (D) female embryos, and (E and F) in dm1/sc1 female embryos. sc/+ and dm1/+ females were obtained by crossing w1 females to sc or dm1 males carrying two copies of Sxl-Pe3.0kb. The sc/dm1 embryos were from a cross between sc/FM7 females and dm1 males homozygous for Sxl-Pe3.0kb. Controls were obtained by mating w1 females to two-copy Sxl-Pe3.0kb males and processed in parallel with the experimentals. (G and H) Sxl-PeGOF (22, 23) wild-type and dm1/Y male embryos. Males carrying two copies of Sxl-PeGOF were mated to wild-type or dm+/FM7 females. (I–L) (Sxl expression) Four classes of embryos are generated by crossing females homozygous for UAS-dm to h-GAL4/TM3 males. (I) Class I. WT female (UAS-dm;TM3). (J) Class II, WT male (UAS-dm;TM3). (K) UAS-dm/h-GAL4 female. (L) UAS-dm/h-GAL4 male.
In these experiments, all female embryos are heterozygous for either dm1 or sc and all showed a reduction in LacZ expression compared with wild type processed in parallel. In the reciprocal experiment, we mated reporter males to dm1 or sc females. In this case, half the female embryos will be wild type, whereas the other half will be dm/+ or sc/+. For the sc control, females from this cross fall into two equal classes. In one class, which corresponds to +/+ females, LacZ expression is the same as in female progeny of wild-type mothers. In the other class, which corresponds to sc/+ females, LacZ expression is reduced like that when the father is sc (12). As would be expected if dm functions as a numerator, the female progeny of dm/+ mothers also fall into two nearly equal classes (Table 3).
Although these experiments show that Sxl-Pe3.0kb is sensitive to the dose of dm (or sc), most if not all female embryos (i.e., 50% of the population) still express LacZ. This would be expected because dm/+ (or sc/+) females are fully viable. On the other hand, under conditions in which female viability is compromised, the percentage of embryos expressing LacZ might be expected to drop below half. This is the case. When dm1/+ females are mated to dm1/Y males, half of the females (25% of total) will be dm1/dm1. In this cross, Sxl-Pe3.0kb is active in only about one-third of the progeny instead of 50%, indicating that it is not turned on in a significant fraction of dm1 females (Table 3). We also tested trans combinations of dm1 and sc. Whereas Sxl-Pe3.0kb is still active in ~50% of the progeny, half of these (which are expected to be dm1/sc) have only a low level of LacZ (Fig. 1 E and F and Table 3), whereas the remaining embryos resemble sc/+ or dm/+ females. (Note: As would be predicted from these experiments, Fig. S1 shows that SxlM4 rescues dmP0 female lethality by bypassing, rather than correcting, the defect in Sxl-Pe activation.)
We next determined whether dm exerts its effects on Sxl-Pe through the conserved Sxl-Pe0.4kb switch element. As indicated in Table S2, Sxl-Pe0.4kb also depends upon dm for full activity. When dm/+1 or dmP0/+ mothers are crossed to wild-type fathers, LacZ expression is reduced in half of the female progeny. When dm1/+ mothers are crossed to dm1/Y fathers, a significant fraction of the female embryos fail to activate Sxl-Pe0.4kb (Table S4). Similar reductions in LacZ expression are evident when either dm1 or dmP1 are combined with a mutation in another numerator (Table S4). For dm/sc or dm/sis-a combinations, the promoter is active in less than 50% of the embryos and in many, there is only little LacZ. Similarly, although nearly 50% of the progeny express LacZ when dm is crossed with upd, about half the females (presumably upd/dm1) have only a very low level of expression.
Excess dm Activates Sxl in Males.
If dm promotes Sxl-Pe activity, it should be possible to inappropriately turn it on in males by overexpressing Dm. For this purpose we used a hairy (h)-GAL4 driver to activate a UAS-dm transgene. h exhibits a dynamic expression pattern in blastoderm stage embryos. It is initially active in a broad domain and then resolves into stripes spanning the central two-thirds of the embryo. Females homozygous for UAS-dm were mated to h-GAL4/TM3 males. If Dm functions to activate Sxl-Pe, then the pattern of Sxl expression should differ from wild type in that there should be four distinct (equal in number) classes instead of just two. This prediction is correct. Embryos in the first and second class resemble wild-type males (no expression) and wild-type females (uniformly high expression) (Fig. 1 I and J). These embryos are expected to carry UAS-dm but not the h-GAL4 driver. Embryos in the third class express higher levels of Sxl than wild-type females (Fig. 1K) and are expected to be females that have both UAS-dm and the h driver. Finally, the fourth class has a low level of Sxl in the central region of the embryo (Fig. 1L). From the level and pattern of expression, these embryos must be males that have UAS-dm and h-GAL4.
dm Is Required to Activate the Sxl-PeGOF Promoter in Males.
To provide additional evidence that Sxl-Pe is sensitive to dm levels in males, we took advantage of a “gain-of-function” promoter, Sxl-PeGOF, which is active in both sexes. It has four copies of a 72-bp sequence from the switch element appended to Sxl-Pe0.4kb. The multimer shifts the balance between negative (denominator) and positive (numerator) cis-acting target sequences and the GOF promoter is turned on in 1X/2A embryos in a characteristic spatially restricted pattern (18, 19). Males carrying Sxl-PeGOF were mated to dm1/+ females. As would be predicted if dm affects the activity of the Sxl-PeGOF in both sexes, the embryos could be divided into approximately four equal classes on the basis of the pattern and level of LacZ expression. One class resembled wild-type Sxl-PeGOF females, whereas the second resembled wild-type Sxl-PeGOF males (Fig. 1G). In the third class, the spatial pattern of LacZ expression is like that of Sxl-PeGOF in wild-type females, but the level of expression is reduced. This class is expected to correspond to dm1/+ females. Similarly, in the fourth class (Fig. 1H), the spatial pattern of LacZ resembles that of wild-type Sxl-PeGOF males; however, there is much less LacZ. This class is expected to be dm1/Y males.
dm Responsive Elements in Sxl-Pe.
The 0.4-kb Sxl-Pe switch element contains two copies of a “D box” (CACGCG) in a conserved 23-bp sequence block ~100 bp upstream of the transcription start site. These D-box sequences are bound by the transcriptional repressor Dpn in vitro, and when they are mutated in the context of a Sxl-Pe1.4kb reporter (which like Sxl-Pe3.0kb has the switch and augmentation elements), the promoter is inappropriately activated in male embryos (13, 20). Although these findings demonstrate that the two D-box motifs function as target sites for negative regulation by dpn (and probably also a maternal bHLH protein), mutations of the two D boxes in the context of the Sxl-Pe0.4kb reporter had paradoxical properties (21). Unlike the Sxl-Pe1.4kb D-box double mutant, the Sxl-Pe0.4kb double mutant was not activated in males, whereas in females it was less active than the wild-type Sxl-Pe0.4kb. The mutant promoter was also more sensitive to a reduction in the dose of dpn than wild-type Sxl-Pe0.4kb.
One explanation for the unusual properties of the Sxl-Pe0.4kb mutant is that these two D-box sequences are a regulatory target not only for Dpn but also for some unknown numerator. This numerator and its target sequences would have a more critical function in the context of Sxl-Pe0.4kb than they do in the context of Sxl-Pe1.4 kb. As the D-box corresponds to one of the noncanonical motifs recognized by Myc:Max complexes (22, 23) a plausible candidate for the mystery numerator would be dm.
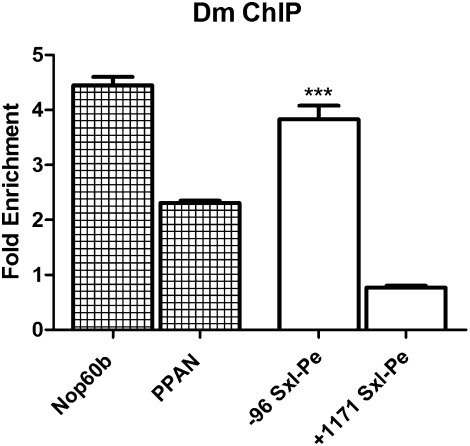
To test this model, we first asked whether Dm associates with the switch element:D-box region in Sxl-Pe during the period when the promoter is being activated by the X/A counting system. For this purpose we immunoprecipitated cross-linked chromatin from 1.0- to 3.5-h embryos with Dm preimmune and immune serum. We used two known dm targets, Nop60b and peterpan (ppan) (24), as positive controls, and a sequence 1 kb downstream of the Sxl-Pe start site as a negative control. Although there is no enrichment of the Sxl downstream region, enrichment of sequences spanning the paired Sxl-Pe D boxes is close to that of Nop60b and almost twice that of ppan (Fig. 2).
Fig. 2.
Dm binds to Sxl-Pe in vivo. ChIP on 1- to 3.5-h Ore R embryos using anti-Dm or control preimmune serum. Fold enrichment is of ChIP immune versus preimmune serum. Nop60b and PPAN are positive controls previously reported as Dm targets. The −96 Sxl-Pe is upstream of the Sxl-Pe start site and spans the D-box region; the negative control +1,171 is downstream within the coding region. Unpaired t test using GraphPad to analyze signal difference at −96 versus +1,171 gives significance at ***P < 0.0001.
As a further test of the model, we appended a multimerized 34-bp sequence spanning the paired D boxes to the minimal Sxl-Pe0.4kb reporter (Sxl-Pe5xDbox). This Sxl-Pe5xDbox reporter is expected to behave differently depending upon whether the 34-bp multimer has regulatory targets for just dpn (and the postulated maternal bHLH protein, ref. 13) or for both dpn and dm. If the multimer is only a target for dpn, Sxl-Pe5xDbox should be strongly repressed in females, induced to the same extent as Sxl-Pe0.4kb in the absence of dpn and largely unresponsive to dm. On the other hand, if the multimer has regulatory targets for both dpn and dm, the Sxl-Pe5xDbox reporter should still be on in females, whereas it should respond in an unusual fashion to changes in the relative levels of dpn and dm.
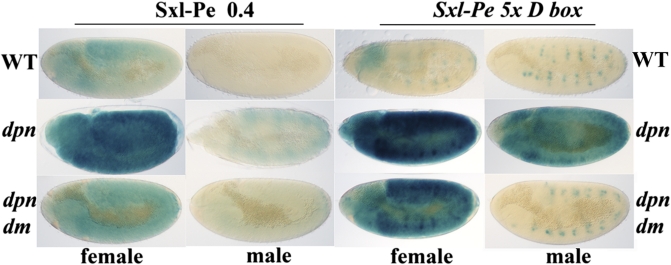
The properties of the Sxl-Pe5xDbox reporter argue that the multimer contains targets not only for dpn but also for dm. Instead of being completely silenced, it is still active in close to 50% of the embryos. However, the balance is clearly shifted in favor of repression as it drives much less LacZ expression than the parental Sxl-Pe0.4kb (Fig. 3). The multimer promoter also seems to be somewhat less sensitive to a twofold reduction in dpn than Sxl-Pe0.4kb; there is only a small increase in activity in dpn7/+ females (Fig. S3) and no activation in dpn7/+ males. A quite different result is seen for dpn7/dpn7. Fig. 3 shows that the parental Sxl-Pe0.4kb is up-regulated in dpn7/dpn7 females and weakly activated in males. By contrast, there is a dramatic increase in Sxl-Pe5xDbox activity in dpn7/dpn7 embryos. Although Sxl-Pe5xDbox is only weakly active in wild-type females, it drives a higher level of LacZ expression in dpn7/dpn7 females than Sxl-Pe0.4kb. Moreover, the multimer is also strongly activated in dpn7/dpn7 males where the level of expression is even higher than Sxl-Pe0.4kb in wild-type females (Fig. 3). The substantial activation of Sxl-Pe5xDbox in the absence of dpn indicates that the multimer must contain sequences for at least one numerator. Additionally, it would appear that the activity of this numerator(s) is antagonized by Dpn.
Fig. 3.
Regulation of Sxl-Pe5xDbox by dpn and dm. (Top) LacZ in wild-type embryos carrying a single copy of Sxl-Pe0.4kb of Sxl-Pe5xDbox as indicated. For both promoters, embryos fall into two equal classes (corresponding to female and male) on the basis of LacZ pattern: representative embryos are shown. The LacZ positive spots in Sxl-Pe5xDbox embryos is due to a sex nonspecific activation in the peripheral nervous system. (Middle) LacZ in dpn− embryos as indicated. Sxl-Pe0.4kb: +/+; dpn7/Cyo ftz:LacZ X +/Y: dpn7 Sxl-Pe0.4kb/Cyo ftz:LacZ. Sxl-Pe5xDbox: +/+; dpn7/Cyo ftz:LacZ X +/Y: dpn7 /Cyo ftz:LacZ; Sxl-Pe5xDbox/Sxl-Pe5xDbox. dpn− Sxl-Pe reporter embryos lack the ftz:LacZ stripes and fall into two equal classes (female and male) that differ in level of LacZ. We used Sxl/LacZ antibody staining to confirm the sexual identify of dpn embryos. (Bottom) LacZ in dpn−/dm− embryos as indicated. Sxl-Pe0.4kb: dm1/+; dpn7/Cyo ftz:LacZ X dm1/Y: dpn7 Sxl-Pe0.4kb/Cyo ftz:LacZ. Sxl-Pe5xDbox: dm1/+; dpn7/Cyo ftz:LacZ X dm1/Y: dpn7/Cyo ftz:LacZ; Sxl-Pe5xDbox/Sxl-Pe5xDbox. dpn− Sxl-Pe reporter embryos lack the ftz:Lac stripes and fall into four equal classes (dm/+ or dm/dm female and +/Y or dm/Y male) that differ in level of LacZ. A control cross using males wild type for dm was done in parallel to help distinguish dm/+ and +/Y from dm/dm and dm/Y embryos, respectively. dm/+ females have an intermediate (between dm; dpn and dpn females) level of LacZ, whereas +/Y; dpn males have less LacZ than dm; dpn females. We used Sxl/LacZ antibody staining to confirm the existence of four classes of dpn embryos from this cross and the sexual identity of each class.
To determine whether dm might be one of these numerators, we examined the effects of altering the relative dose of dm and dpn. An extra copy of dm in an otherwise wild-type background has even less effect on Sxl-Pe5xDbox than a twofold reduction in dpn. However, the multimer promoter is strongly activated when females carrying three copies of dm are also heterozygous for dpn7 (Fig. 3). Further supporting a multimer-dependent numerator function are the effects of dm1 in homozygous dpn7 embryos. Fig. 3 shows that activation of Sxl-Pe5xDbox in dpn7/dpn7 females is reduced in dm1/dm1 females, whereas it appears to be completely eliminated in dm1 males. In this respect, Sxl-Pe5xDbox also differs from the parental Sxl-Pe0.4kb, which is still weakly active in dpn−; dm1 males. The loss of promoter activity in males indicates that dm plays a pivotal role in activating Sxl-Pe5xDbox. On the other hand, the fact that LacZ expression in dpn−/dm− Sxl-Pe5xDbox females remains substantially above that in wild type (Fig. 3) indicates that the multimer promoter is still activated by a numerator(s) in the dm1 mutant. Whereas two copies of the hypomorphic dm1 would be expected to provide females with more numerator activity than males, it is not clear that this would be sufficient to account for the amount of LacZ that is expressed by the Sxl-Pe5xDbox in dpn−/dm− females.
Discussion
The choice of sexual identity pivots on differences in the dose of X-linked numerators that function to activate Sxl-Pe. The most important numerators are sis-a and sc (1). They are expressed throughout the embryo, and mutations in either gene can have quite pronounced effects on Sxl activation. The two other previously described numerators, runt and upd, are spatially restricted in their pattern of expression and have more modest effects on Sxl. In the studies reported here, we have identified a previously unknown numerator, dm, which encodes the fly dMyc protein.
Several lines of evidence demonstrate that dm is a numerator. First, three independent hypomorphic alleles exhibit preferential female lethality. Second, these dm mutations show synergistic female lethal interactions with mutations in other known numerators. Moreover, as required for a numerator element, the female lethal effects of dm are dependent upon zygotic activity. Third, dm activity is required for Sxl protein expression in female embryos. Fourth, it is possible to rescue the female-specific lethal effects of dm mutant females with a gain-of-function Sxl allele that activates the Sxl autoregulatory feed loop independently of the X/A ratio. Fifth, as observed for other X chromosome counting elements, Sxl protein expression can be induced in males by excess Dm. Sixth, dm acts as a dose-dependent regulator of Sxl-Pe and the activation of the promoter in female embryos is compromised by mutations in dm. Seventh, as expected for a numerator, Dm is found associated with Sxl-Pe in blastoderm-stage embryos. On the basis of the effects of dm mutations on female viability, Sxl protein expression and Sxl-Pe activity, the role of dm in X chromosome counting would appear to be more similar to that of the “secondary” numerators runt and upd than to the “primary” numerators sc and sis-a. However, the one caveat is that the three dm alleles we have studied are hypomorphs rather than nulls.
Because dm mutations affect the activity not only of the full-length Sxl-Pe, but also of the minimal Sxl-Pe0.4kb, there must be target sequences for dm in the highly conserved region that functions as the sex-specific switch. Supporting this possibility, ChIP experiments show that Dm associates with the Sxl-Pe switch element region in early embryos. As the switch element does not contain canonical high-affinity dMyc (Max) binding sites (CACGTG) dm must exert its regulatory effects through noncanonical sites. Probably the best candidates for noncanonical Dm sites in the switch element are a pair of closely spaced D-box motifs. These two D-box motifs have unusual properties. Consistent with in vitro studies showing that the D-box motifs are binding sites for Dpn, mutations in the two D-box motifs in the context of a full-length Sxl-Pe activate the promoter (11). On the other hand, mutations in the context of the minimal switch element Sxl-Pe0.4kb promoter decrease its activity instead of increasing it as expected (21). This contradictory context-dependent behavior of the D-box mutants argues that the D boxes (or sequences overlapping them) are targets not only for Dpn, but also for a numerator such as dm. We tested this idea by multimerizing a short sequence spanning the two D-box motifs and appending the multimer to Sxl-Pe0.4kb. As would be expected if the multimer has Dpn targets, Sxl-Pe5xDbox is less active than the parental Sxl-Pe0.4kb in wild-type females. However, the multimer must also contain targets for numerators as Sxl-Pe5xDbox is much more strongly activated than Sxl-Pe0.4 kb in dpn−, turning on at very high levels not only in females but also in males. Importantly, ectopic activation in males requires dm activity as it is eliminated when the dpn− males are hemizygous for the hypomorphic dm1. Although this finding argues that dm plays a critical role in activating the multimer, we cannot exclude the possibility that the multimer contains target sites for at least one other numerator.
If Dm binds to Sxl-Pe via the D boxes in the multimer, it would presumably exert at least a part of its regulatory effects by preventing Dpn (and the maternal repressor) from binding to Sxl-Pe. This would be the first instance of a numerator competing with a denominator(s) for the same or overlapping cis-acting elements in Sxl-Pe. However, even if Dm functions to prevent Dpn binding, this cannot be its only activity as it must also be able to promote transcription in the absence of dpn activity. In this context, it is interesting to note that mammalian Myc functions by recruiting the transcription elongation factor P-TEFb to paused polymerases (25). A postinitiation function for Dm would be attractive as it would help explain the effects of one of the global germ cell transcriptional quiescence factors, nanos (nos), on Sxl-Pe (26). In the absence of nos, Sxl-Pe is inappropriately turned on in the soma of male embryos and in the germ cells of both sexes by a mechanism involving Pol II carboxy terminal domain (CTD) phosphorylation that is independent of the chromosome counting system. Because it was assumed that numerators and denominators regulated Sxl-Pe by controlling Pol II recruitment, it was hard to understand how Sxl-Pe could ever be activated in nos male embryos by a general up-regulation of CTD phosphorylation. However, if Pol II is localized to Sxl-Pe in both sexes, and the numerators/denominators control a subsequent step, such as recruitment of P-TEFb, the bypass of the counting system in nos embryos is more readily explained.
Experimental Procedures
Sxl-Pe5xDbox was generated by PCR amplifying a 34-bp sequence spanning the two D-box motifs using DMYCBAM5′-CAATTCGCGGGATCCTAGGTAGC and DMYCBGL3′-GCCAGGTAGAAGATCTAAGGAGG. The resulting fragment was ligated, cloned into the BamHI site of pBSK, and a recombinant containing five tandem copies isolated. The tandem copies were excised with EcoRI and XbaI, placed upstream of Sxl-Pe0.4kb, and then inserted into the EcoEI site of pCasp. Nine independent Sxl-Pe5xDbox lines were isolated. All had expression patterns similar to the examples presented here. Two representative lines, 63 and 142, were used for most of the genetic experiments.
Supplementary Material
Acknowledgments
We thank T. Cline, P. Gergen, D. Stein, and the Bloomington Stock Center for flies, antibodies, and reagents. We also thank members of the laboratory for reagents, advice, and helpful discussion. Support from National Institutes of Health GM043432 (to P.S.) and GM085165 (to J.I.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017006108/-/DCSupplemental.
References
- 1.Cline TW, Meyer BJ. Vive la différence: Males vs females in flies vs worms. Annu Rev Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 2.Bell LR, Maine EM, Schedl P, Cline TW. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55:1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- 3.Bell LR, Horabin JI, Schedl P, Cline TW. Positive autoregulation of sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell. 1991;65:229–239. doi: 10.1016/0092-8674(91)90157-t. [DOI] [PubMed] [Google Scholar]
- 4.Cline TW. Autoregulatory functioning of a Drosophila gene product that establish es and maintains the sexually determined state. Genetics. 1984;107:231–277. doi: 10.1093/genetics/107.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyes LN, Cline TW, Schedl P. The primary sex determination signal of Drosophila acts at the level of transcription. Cell. 1992;68:933–943. doi: 10.1016/0092-8674(92)90036-c. [DOI] [PubMed] [Google Scholar]
- 6.Cline TW. Evidence that sisterless-a and sisterless-b are two of several discrete “numerator elements” of the X/A sex determination signal in Drosophila that switch Sxl between two alternative stable expression states. Genetics. 1988;119:829–862. doi: 10.1093/genetics/119.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres M, Sánchez L. The sisterless-b function of the Drosophila gene scute is restricted to the stage when the X:A ratio determines the activity of Sex-lethal. Development. 1991;113:715–722. doi: 10.1242/dev.113.2.715. [DOI] [PubMed] [Google Scholar]
- 8.Duffy JB, Gergen JP. The Drosophila segmentation gene runt acts as a position-specific numerator element necessary for the uniform expression of the sex-determining gene Sex-lethal. Genes Dev. 1991;5(12A):2176–2187. doi: 10.1101/gad.5.12a.2176. [DOI] [PubMed] [Google Scholar]
- 9.Younger-Shepherd S, Vaessin H, Bier E, Jan LY, Jan YN. deadpan, an essential pan-neural gene encoding an HLH protein, acts as a denominator in Drosophila sex determination. Cell. 1992;70:911–922. doi: 10.1016/0092-8674(92)90242-5. [DOI] [PubMed] [Google Scholar]
- 10.Cronmiller C, Schedl P, Cline TW. Molecular characterization of daughterless, a Drosophila sex determination gene with multiple roles in development. Genes Dev. 1988;2(12A):1666–1676. doi: 10.1101/gad.2.12a.1666. [DOI] [PubMed] [Google Scholar]
- 11.Lu H, et al. Maternal Groucho and bHLH repressors amplify the dose-sensitive X chromosome signal in Drosophila sex determination. Dev Biol. 2008;323:248–260. doi: 10.1016/j.ydbio.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes PA, Keyes LN, Schedl P. Multiple response elements in the Sex-lethal early promoter ensure its female-specific expression pattern. Mol Cell Biol. 1995;15:904–917. doi: 10.1128/mcb.15.2.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, et al. Interpretation of X chromosome dose at Sex-lethal requires non-E-box sites for the basic helix-loop-helix proteins SISB and daughterless. Mol Cell Biol. 2001;21:1581–1592. doi: 10.1128/MCB.21.5.1581-1592.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande G, Calhoun G, Schedl PD. The N-terminal domain of Sxl protein disrupts Sxl autoregulation in females and promotes female-specific splicing of tra in males. Development. 1999;126:2841–2853. doi: 10.1242/dev.126.13.2841. [DOI] [PubMed] [Google Scholar]
- 15.Gallant P, Shiio Y, Cheng PF, Parkhurst SM, Eisenman RN. Myc and Max homologs in Drosophila. Science. 1996;274:1523–1527. doi: 10.1126/science.274.5292.1523. [DOI] [PubMed] [Google Scholar]
- 16.Schreiber-Agus N, et al. Drosophila Myc is oncogenic in mammalian cells and plays a role in the diminutive phenotype. Proc Natl Acad Sci USA. 1997;94:1235–1240. doi: 10.1073/pnas.94.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston LA, Prober DA, Edgar BA, Eisenman RN, Gallant P. Drosophila myc regulates cellular growth during development. Cell. 1999;98:779–790. doi: 10.1016/s0092-8674(00)81512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer SG, Jinks TM, Schedl P, Gergen JP. Direct activation of Sex-lethal transcription by the Drosophila runt protein. Development. 1999;126:191–200. doi: 10.1242/dev.126.1.191. [DOI] [PubMed] [Google Scholar]
- 19.Jinks TM, Polydorides AD, Calhoun G, Schedl P. The JAK/STAT signaling pathway is required for the initial choice of sexual identity in Drosophila melanogaster. Mol Cell. 2000;5:581–587. doi: 10.1016/s1097-2765(00)80451-7. [DOI] [PubMed] [Google Scholar]
- 20.Hoshijima K, et al. Transcriptional regulation of the Sex-lethal gene by helix-loop-helix proteins. Nucleic Acids Res. 1995;23:3441–3448. doi: 10.1093/nar/23.17.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jinks TM, Calhoun G, Schedl P. Functional conservation of the sex-lethal sex determining promoter, Sxl-Pe, in Drosophila virilis. Dev Genes Evol. 2003;213:155–165. doi: 10.1007/s00427-003-0304-1. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell TK, et al. Binding of myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993;13:5216–5224. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JW, et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teleman AA, Hietakangas V, Sayadian AC, Cohen SM. Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab. 2008;7:21–32. doi: 10.1016/j.cmet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Rahl PB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deshpande G, Calhoun G, Jinks TM, Polydorides AD, Schedl P. Nanos downregulates transcription and modulates CTD phosphorylation in the soma of early Drosophila embryos. Mech Dev. 2005;122:645–657. doi: 10.1016/j.mod.2004.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.