Abstract
With an aging population, skeletal fractures are increasing in incidence, including the typical closed and the less common open fractures in normal bone, as well as fragility fractures in patients with osteoporosis. For the older age group, there is an urgent unmet need to induce predictable bone formation as well as improve implant fixation in situations such as hip joint replacement. Using a murine model of slow-healing fractures, we have previously shown that coverage of the fracture with muscle accelerated fracture healing and increased union strength. Here, we show that cells from muscle harvested after 3 d of exposure to an adjacent fracture differentiate into osteoblasts and form bone nodules in vitro. The osteogenic potential of these cells exceeds that of adipose and skin-derived stromal cells and is equivalent to bone marrow stromal cells. Supernatants from human fractured tibial bone fragments promote osteogenesis and migration of muscle-derived stromal cells (MDSC) in vitro. The main factor responsible for this is TNF-α, which promotes first MDSC migration, then osteogenic differentiation at low concentrations. However, TNF-α is inhibitory at high concentrations. In our murine model, addition of TNF-α at 1 ng/mL at the fracture site accelerated healing. These data indicate that manipulating the local inflammatory environment to recruit, then differentiate adjacent MDSC, may be a simple yet effective way to enhance bone formation and accelerate fracture repair. Our findings are based on a combination of human specimens and an in vivo murine model and may, therefore, translate to clinical care.
Fractures are a common clinical problem affecting 2% of the population per annum. High-energy open fractures are limb-threatening injuries, prone to delayed and nonunion with consequent pain, work loss, and disability (1–4). Moreover, with an aging population, fragility fractures in osteoporotic individuals, including those that are life-threatening, such as fractured neck of femur, are increasing dramatically. However, there has been little progress in treatment options for either fracture type in recent years.
Although addition of exogenous bone morphogenetic proteins (BMPs) results in improved healing in animal models, clinical trials of BMPs for tibial nonunion (5) and fracture healing (6) have failed to achieve the efficacy anticipated (7, 8). A single supraphysiological dose of BMP does not induce the complex pattern of growth factor and cytokine production required for optimal fracture repair. Ex vivo expansion of mesenchymal stromal cells transduced to express BMPs (8, 9) have been shown to promote fracture healing in experimental animal models (10). However, the clinical benefits of these complex and costly strategies are unclear. Safety concerns exist regarding ex vivo cell expansion, transgenic expression, and the use of viral vectors in humans (11, 12). Hence, there remains an urgent unmet need for simple yet effective therapeutic strategies to promote bone formation.
In the high-energy fracture environment, the supply of progenitor cells from bone is reduced because of the loss of periosteal tissue and marrow contents. Thus, contributions from adjacent skeletal muscle (9, 13, 14), fat (15), and skin (16) may be vital (17–19). We have previously compared the effect of muscle with fasciocutaneous tissue in exclusive contact with fractured, periosteally stripped, and endosteally reamed bone in a murine model of open fracture (20). A 50% increase in cortical bone content and threefold stronger union at 28 d was observed when fractured tibiae were in direct contact with muscle exclusively, as opposed to fasciocutaneous tissue. This result occurred despite a greater vascularity in the fasciocutaneous tissue at all time points (20, 21), suggesting that tissue factors influence the healing process.
There is increasing evidence that inflammation plays a vital role in early fracture repair (22, 23). In murine models, TNF-α, IL-1, and IL-6 are expressed at the fracture site within 24 h of injury (24, 25). The expression of TNF-α and IL-1 in fractures follows a biphasic pattern, with a peak during the initiation of fracture repair, followed by a second peak at the transition from chondrogenesis to osteogenesis during endochondral maturation (25, 26). A closed tibial fracture model using TNF-α receptor (p55−/−/p75−/−) knockout mice demonstrated delayed endochondral maturation (27), and IL-6 has been shown to stimulate osteoblast differentiation (28). A femoral fracture model using IL-6 knockout mice also demonstrated delayed callus remodeling and mineralization (29), and both TNF-α and IL-1β have been shown to recruit osteoblasts (30). Using human-fracture bone fragments and our murine model we identified the pivotal role of TNF-α in enhancing fracture healing.
Results
Osteogenic Differentiation Is Restricted to Muscle Cells Adjacent to Fractured Bone.
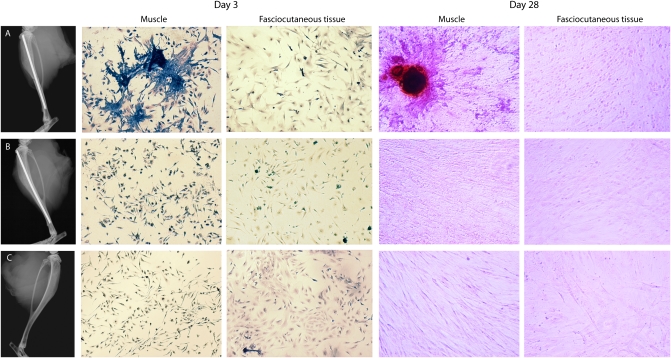
The influence of fractured bone on adjacent muscle was investigated using our murine model of a periosteally stripped (high-energy) open tibial fracture (20). At day 3, cells isolated from muscle adjacent to the fracture were harvested, cultured for 7 d, and then fixed and stained for alkaline phosphatase (ALP), a surrogate marker of early osteogenic differentiation. Muscle cells adjacent to the fracture site clustered and stained intensely for ALP. When these cells were cultured to day 28, they produced bone nodules (Fig. 1A). In contrast, cells isolated from the fasciocutaneous tissue adjacent to the tibial osteotomy neither clustered and expressed ALP nor led to bone nodule formation (Fig. 1B). Similarly, cells isolated from muscle and fasciocutaneous tissue adjacent to the sham (periosteally stripped, intact cortex) fracture did not exhibit ALP expression or form bone nodules. There was also no effect in the contralateral unfractured limbs (Fig. 1C).
Fig. 1.
Cells from muscle adjacent to fracture express ALP and exhibit clustering at day 7 and bone nodule formation at day 28. Cells isolated from muscle and fasciocutaneous tissue adjacent to the fractured, sham-fractured, and unfractured tibiae 3 d postsurgery were cultured. After 7 d, half were stained for ALP and the remainder cultured for a further 21 d and stained using Alizarin red. Representative radiographs and images of (A) fractured, (B) sham-fractured, and (C) unoperated (contralateral to A) tibiae are shown. All microscopic images 20× magnification; three animals in each group per time point.
Human Skeletal Muscle-Derived Cells Exhibit Stromal Cell Markers.
Results from rodent models are not always directly applicable to humans. Hence, an in vitro model using human cells was developed to seek bone-inducing factors. Samples of naive (not exposed to injury) skeletal muscle were isolated. Using flow cytometry, the muscle cell populations expressed CD73, CD90, CD105, and HLA-ABC but not CD14, CD31, CD34, CD45, CD106, CD117, CD146, and HLA-DR, confirming that they were of mesenchymal, and not hematopoietic or endothelial origin (Table 1). This profile of cell-surface markers is observed in numerous stromal populations and in agreement with our earlier work (31). The stem-like potential of this muscle-derived stromal cell (MDSC) population was confirmed by their ability to differentiate into bone, fat, and cartilage (Fig. S1).
Table 1.
Phenotypic analysis of the MDSC cell population
| Cell surface marker | % Positive cells | ±SEM |
| MHC 1 | 90.5 | 5.0 |
| CD14 | 0.1 | 0.1 |
| CD31 | 0.6 | 0.5 |
| CD34 | 0.5 | 0.6 |
| CD45 | 0.8 | 0.8 |
| CD73 | 85.6 | 3.1 |
| CD90 | 95.6 | 3.0 |
| CD105 | 64.9 | 10.3 |
| CD106 | 0.2 | 0.1 |
| CD117 | 0.4 | 0.5 |
| CD146 | 0.7 | 0.5 |
Values represent the percentage of cells positive for the surface expression of each marker ± SEM gated against the appropriate isotype control, with a background of less than 1% (n = 4, including two from one donor with 12 cell passages between samples).
MDSCs Make Bone in Vitro.
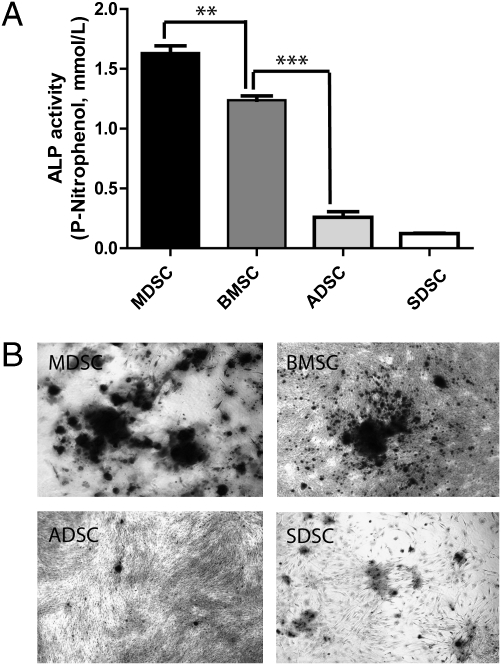
The osteogenic potential of MDSCs was compared with that of bone marrow-stromal cells (BMSCs), adipose-derived stromal cells (ADSCs) (15), and skin- (dermis) derived stromal cells (SDSCs) (16). ALP production by MDSCs and BMSCs was significantly in excess of that produced by ADSCs and SDSCs (P < 0.001) (Fig. 2A). This finding was consistent with bone nodule formation observed at day 28 (Fig. 2B). Hence, the ALP assay was used as a surrogate marker for osteogenic differentiation for subsequent experiments.
Fig. 2.
MDSCs and BMSCs exhibit osteogenic potential which exceeds that of ADSCs or SDSCs as assessed by (A) ALP production and (B) bone nodule formation. Each experiment was performed three times using cells from three donors (**P < 0.01, ***P < 0.001). One-way ANOVA with Bonferroni's multiple comparison test.
TNF-α Promotes Fracture Supernatant-Mediated Osteogenic Differentiation of MDSCs.
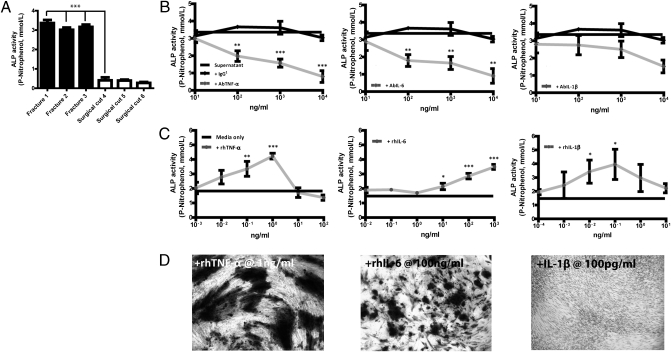
An in vitro model of the fracture environment was developed using supernatants of fractured or surgically cut tibial fragments in serum-free DMEM. Specimens were obtained during surgical debridement of high-energy open tibial fractures. The total protein content of the fracture versus surgically cut supernatants was 1.35 ± 0.6 mg/mL vs. 1.04 ± 0.51 mg/mL, 1 SD (P = N/S). Supernatants of fractured but not surgically cut bone stimulated expression of ALP by MDSCs at day 7 (Fig. 3A). ELISAs specific for BMP-2, BMP-4, BMP-7 (OP-1), and TGF-β demonstrated their presence in both fracture and surgically cut bone supernatants (Table S1). MDSCs were cultured in fracture supernatant with the addition of antibodies to BMP-2, -4, -7, and TGF-β. The efficacy of the antibodies was confirmed by neutralizing recombinant BMPs (Fig. S2A). Antibody inhibition of BMPs -2, -4, -7, and TGF-β in dose-response did not diminish the osteogenic effect of supernatant on MDSCs, indicating that these BMPs were not responsible for the osteogenic effect of fracture supernatants (Fig. S2B). Proinflammatory cytokines implicated in fracture repair include TNF-α, IL-1β, and IL-6 (22, 23, 25–27). Supernatants were evaluated for the presence of these cytokines using the Luminex xMAP system. Both TNF-α and IL-1β were below the limits of the assay (<10 pg/mL) (Table S2). However, as concentrations expected physiologically are of this magnitude (32), we tested for their presence and biological activity using antibody neutralization assays. Hence, MDSCs were cultured in fracture supernatant with the addition of neutralizing antibodies to TNF-α, IL-6, IL-1β, or isotype controls. ALP quantification revealed that AbTNF-α and AbIL-6 inhibited the osteogenic stimulus of fracture supernatant in a dose-dependent manner, but AbIL-1β did not (Fig. 3B). To confirm that TNF-α and IL-6 promoted osteogenic differentiation of MDSC, the cells were cultured in human serum-containing medium (HSM) supplemented with either rhTNF-α, rhIL-6, or rhIL-1β. TNF-α induced maximal production of ALP by MDSC at a concentration of 1 ng/mL. ALP production dropped sharply as the TNF-α concentration increased and was below the control level when TNF-α levels exceeded 100 ng/mL. In contrast, IL-6 continued to augment ALP production with increasing concentration. The ALP response to IL-1β was variable (Fig. 3C). MDSCs cultured in HSM with rhTNF-α, rhIL-6, or rhIL-1β at the optimal concentrations (Fig. 3C) confirmed that ALP production was followed by bone nodule formation. Nodule formation with TNF-α exceeded IL-6. IL-1β did not stimulate nodule formation (Fig. 3D).
Fig. 3.
TNF-α and IL-6 in fracture supernatant promotes ALP expression and bone nodule formation by MDSCs. (A) MDSCs were cultured in fracture-derived or surgically cut bone supernatants for 7 d and ALP quantified. ALP production by MDSCs in (B) fracture supernatant ± antibody neutralization of TNF-α, IL-6, and IL-1β (isotype control IgG1), and (C) in HSM with recombinant TNF-α, IL-6, and IL-1β. (D) Bone nodule formation by MDSCs in HSM plus recombinant cytokine at the optimal dose indicated by C. The cytokine was added for the first 3 d only and cells cultured for 32 d. Representative images are shown. All experiments were performed three times in triplicate using MDSCs from three donors in A, C, and D, and fracture supernatant from three donors in B. Values represent means from three experiments ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). One-way ANOVA with Bonferroni's multiple comparison test in A and B and Dunnett's multiple comparison test in C.
TNF-α and IL-6 Promote Migration of MDSCs.
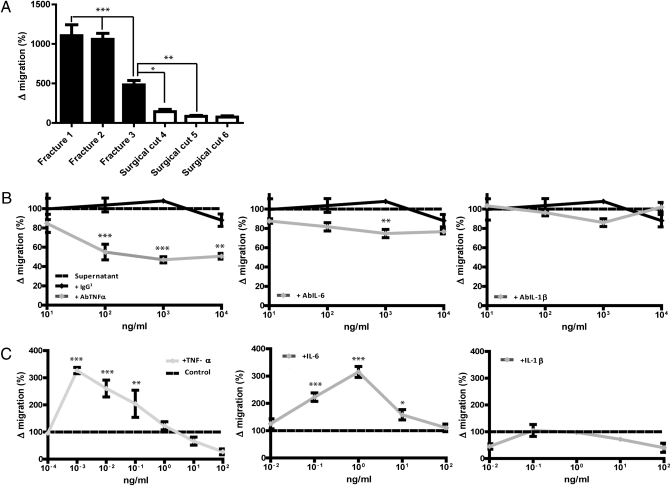
An in vitro model of cell migration in the fracture environment was developed. Human fracture supernatants promoted MDSC migration through an 8-μm pore transwell membrane significantly more than surgically cut bone supernatants (P < 0.001 for two of three fracture supernatants) (Fig. 4A). Neutralizing antibodies to TNF-α and IL-6 reduced fracture supernatant-induced cell migration in a dose-dependent manner. Neutralization of TNF-α resulted in the greatest inhibitory effect but neutralization of IL-1β had no effect (Fig. 4B). To confirm the chemoattractant effect of TNF-α and IL-6, the experiment was repeated using HSM with the addition of rhTNF-α, rhIL-6, and rhIL-1β. Both TNF-α and IL-6 induced cell migration (Fig. 4C). The optimal concentration of TNF-α for MDSC migration, at 1 pg/mL, was 1,000-fold less than the optimal concentration for osteogenic differentiation. The optimum osteogenic concentration of rhTNF-α of 1 ng/mL resulted in no net cell migration. At concentrations above this, there was less net cell migration. The optimal concentration for IL-6 at 1 ng/mL was also 1,000-fold less than the optimal osteogenic concentration (Fig. 3C). In contrast, IL-1β did not influence migration of MDSC within the dose range tested.
Fig. 4.
TNF-α and IL-6 promote supernatant-mediated cell migration and are chemoattractants for MDSCs. (A) MDSC migration in response to fracture and surgically cut supernatants, expressed relative to migration in HSM. (B) MDSC migration in response to fracture supernatant with the addition of AbTNF-α, AbIL-6, and AbIL-1β, expressed relative to migration in supernatant only (IgG1 as antibody control). (C) MDSC migration in response to rhTNF-α, rhIL-6, and rhIL-1β in HSM, expressed relative to migration in HSM. Each experiment was performed three times using three fracture supernatants (A and B) and MDSC from three donors (C). Results represent means of three experiments ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001). One-way ANOVA with Bonferroni's multiple comparison test in A and B and Dunnett's multiple comparison test in C.
PDGF is an established chemoattractant factor for cells of stromal origin (33). Moreover, chemokines including CCL-2 (MCP-1), CXCL-8 (IL-8), and CXCL-12 (SDF-1) have been implicated in stromal cell migration (34–36). The concentrations of chemokines in fracture and surgically cut supernatants were examined using the Luminex xMAP system. Only CXCL-12 was significantly elevated in fracture supernatants (Table S3). Naive MDSCs were highly responsive to migration in the presence of PDGF but not to any of the other chemokines tested (Figs. S3 and S4). The influence of TNF-α on chemokine-mediated MDSC migration was examined using MDSCs primed by culture for 72 h with rhTNF-α at a concentration of 1 pg/mL (the optimum concentration for cell migration shown in Fig. 4C). Prepriming with TNF-α resulted in migration in response to CCL-2 and CXCL-12 (Fig. S3A and S4). Migration was inhibited by AbPDGF, AbCCL-2, and AbCXCL-12 (Fig. S3B). Coneutralization of PDGF, CCL-2, or CXCL-12 with TNF-α inhibited cell migration in excess of the effect produced by neutralization of TNF-α alone (Fig. S3C). Proliferation of MDSCs did not vary according to culture in fracture or surgically cut supernatant and was not influenced by the presence of proinflammatory cytokines TNF-α, IL-1β, or IL-6 (Fig. S5).
Addition of rhTNF-α Locally Accelerates Fracture Healing in Vivo.
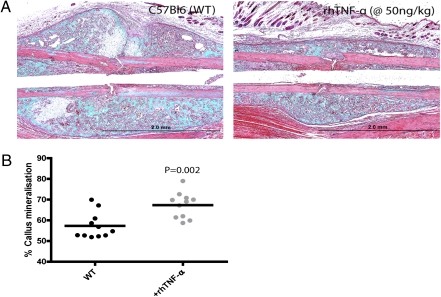
The influence of TNF-α on fracture healing in vivo was investigated using the murine model. Local injection of 20 μL of rhTNF-α at a concentration of 50 ng/mL in PBS on days 0 and 1 after surgery resulted in accelerated fracture healing at day 14 assessed histologically using Masson's Trichrome stain (Fig. 5A), as indicated by the resolution of the cartilaginous intermediary and remodeling of the mineralized callus. To confirm this observation quantitatively, limbs injected with rhTNF-α, or with PBS only, were harvested at day 28 and underwent microCT scanning, and percentage callus mineralization was determined. Fractured limbs with addition of local rhTNF-α exhibited significantly greater callus mineralization at day 28 compared with the PBS-only controls (Fig. 5B).
Fig. 5.
Addition of exogenous rhTNF-α locally accelerates fracture healing in vivo. Twenty microliters of rhTNF-α at 50 ng/mL was injected at the fracture site at days 0 and 1. (A) The mice were killed at day 14 and histological sections stained with Masson's Trichrome (n = 15). (B) At day 28, mice were harvested and limbs scanned by microCT in a blinded fashion. Percentage callus mineralization was determined from the fracture site to 1 mm proximally.
Discussion
Bone repair is needed not only for open fractures, but also for fragility (osteoporotic) fractures and orthopedic implants (e.g., joint replacement). Currently, the lifetime risk of fragility fracture is 40% (37) and by 2025 there will be ≈5 million hip fractures annually. These fractures are associated with a mortality rate exceeding 20% in the year following fracture. Thus, promoting bone formation is a major unmet need, for which there is currently no Food and Drug Administration-approved therapy.
A variety of current research approaches to augment fracture healing, such as the addition of BMPs, have been successful in the laboratory but disappointing in human trials. The ex vivo manipulation of autologous stem cells and their reimplantation is likely to be too expensive and impractical for primary fracture healing or implant fixation (9, 14). Hence, we sought to understand the molecular pathways of fracture repair to enable the development of simpler and more cost-effective approaches.
Analysis of tissue samples from our open-fracture model revealed a mobilizing stromal cell reservoir in adjacent skeletal muscle (Fig. 1). Consequently, molecular analyses began by investigating the bone-forming potential of supernatants from human fractured and surgically cut bone (Fig. 3A). This investigation revealed that only traumatically fractured bone fragments initiated osteogenesis in MDSCs. BMPs are known to be important in fracture healing (17–19) and were present in both the fracture and sliced bone fragments, with higher levels of BMP-2 and -4 in the former (Table S1). However, it was not BMPs or TGF-β, but rather proinflammatory cytokines TNF-α and IL-6 (present on account of trauma) that were responsible for the osteogenic effect of the supernatant (Fig. 3B and Fig. S2). Our data suggest that the proinflammatory cytokines are crucial in initiating osteogenesis of MDSCs. Proinflammatory cytokines have been shown to stimulate BMP synthesis (38–40) and osteogenic differentiation of human mesenchymal stromal cells (39).
There is published evidence that TNF-α is involved in fracture healing (19, 32). In TNF-α receptor knockouts (p55−/−, p75−/−) there was delayed bone repair, with impaired chrondrocyte differentiation, periosteal bridging, and callus resorption (27). However, the reported effects of TNF-α on marrow stromal cells are conflicting, being pro-osteogenic on human marrow stromal cells (39), but inhibitory on rat or mouse osteoblastic cells (41, 42). The difference may be species related, but is more likely TNF-α dose-dependent, as we showed a clear dose–response curve, with excess TNF-α reducing the migration and pro-osteogenic effects (Figs. 3 and 4). Furthermore, others have reported impaired fracture healing following high doses of TNF-α (43, 44), as well as up-regulated osteoclast numbers (45). Prior reports have not explored this TNF-α concentration effect. The evidence used for osteogenesis included the early phase marker ALP, as well as bone nodule formation (46). TNF-α, and to a lesser extent IL-6, stimulated both, but rhIL-1 only stimulated the initial phase but did not result in bone nodule formation by MDSCs (Fig. 3 C and D).
TNF-α has previously been shown to enhance the effect of chemokines on stromal cells (35) and we showed this effect for CCL-2 and CXCL-12. Thus, TNF-α was both directly and indirectly responsible for MDSC recruitment. This finding explains the fact that supernatants were more potent than recombinant TNF-α and only partly blocked by anti–TNF-α. IL-6 appears to have a more modest effect than TNF-α on promoting MDSC recruitment and bone formation. In contrast, IL-1β appeared to have almost no influence on MDSC migration or osteogenic differentiation. The overlapping effects of TNF-α and IL-6 may arise from IL-6 being downstream of TNF-α in cell recruitment and differentiaton (47, 48). These results suggest that addition of TNF-α locally to achieve levels in the order of 1 ng/mL at the fracture site would augment fracture repair. This theory was tested in our murine open-fracture model, injecting TNF-α at the fracture site at day 0 and 24 h later. Local administration of 50 ng/kg in 20 μL (equivalent to around 1 ng locally) resulted in significantly accelerated fracture healing and remodeling.
Our data suggest that resident stromal cells present in muscle are an important source of osteoprogenitor cells. Uniquely, we have demonstrated that proinflammatory cytokines, in particular TNF-α, are crucial in fracture healing. Moreover, this occurs in a concentration-dependent manner. In vivo there is likely to be a concentration gradient of TNF-α, with progressive reduction radially from the fracture site. Our data would suggest that the lower concentrations in the muscle distal to the fracture may promote cell migration both directly and by potentiating the effects of other chemokines. Following cell migration to the fracture site, TNF-α, encountered at a higher concentration, may then inhibit further cell migration and promote local osteogenesis. These results suggest that promoting inflammation, and specifically TNF-α, at the site of fracture or implant would promote bone formation and encourage a favorable clinical outcome. The challenge is to apply this to humans, where different types of fractures may result in varying levels of endogenous TNF-α production. The greatest clinical need is in osteoporotic fractures and the models described here are being modified to investigate whether repair of osteoporotic fractures might also be enhanced by up-regulating TNF-α to augment mesenchymal stromal cell recruitment and differentiation.
In summary, this article describes a previously unexplored approach to accelerating bone-fracture repair, a major medical need for which there is little current research. Molecular analysis of human fracture samples revealed that TNF-α, produced at the fracture site, can (at the appropriate concentration) recruit stem cells and promote repair in mouse models. These results provide a basis for human clinical trials.
Materials and Methods
Detailed methods for obtaining cell populations, staining for alkaline phosphatase and bone nodules, ALP quantification, flow cytometry, supernatant production, migration assay, histology, and microCT analyses are available in SI Materials and Methods. All work was undertaken with ethical approval and patient consent. Our murine model has been published previously (20).
Supplementary Material
Acknowledgments
We are grateful for support from the Kennedy Institute of Rheumatology Trustees and the National Institute for Health Research Biomedical Research Centre funding scheme.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018501108/-/DCSupplemental.
References
- 1.Bosse MJ, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347:1924–1931. doi: 10.1056/NEJMoa012604. [DOI] [PubMed] [Google Scholar]
- 2.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ, LEAP Study Group Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie EJ, et al. Early predictors of long-term work disability after major limb trauma. J Trauma. 2006;61:688–694. doi: 10.1097/01.ta.0000195985.56153.68. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy ML, et al. LEAP study group Psychological distress associated with severe lower-limb injury. J Bone Joint Surg Am. 2003;85-A:1689–1697. doi: 10.2106/00004623-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Friedlaender GE, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Pt 2, Suppl 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 6.Govender S, et al. BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Lane JM. BMPs: Why are they not in everyday use? J Bone Joint Surg Am. 2001;83-A(Pt 2, Suppl 1):S161–S163. [PubMed] [Google Scholar]
- 8.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg Am. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Corsi KA, Schwarz EM, Mooney DJ, Huard J. Regenerative medicine in orthopaedic surgery. J Orthop Res. 2007;25:1261–1268. doi: 10.1002/jor.20432. [DOI] [PubMed] [Google Scholar]
- 10.Dragoo JL, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 11.Baltzer AW, et al. Potential role of direct adenoviral gene transfer in enhancing fracture repair. Clin Orthop Relat Res. 2000;379(379, Suppl):S120–S125. doi: 10.1097/00003086-200010001-00016. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Yang Y. Innate immune recognition of viruses and viral vectors. Hum Gene Ther. 2009;20:293–301. doi: 10.1089/hum.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu-Petersen Z, et al. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuk PA, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 16.Toma JG, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 17.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 18.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60-B:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 19.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;355(355, Suppl):S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 20.Harry LE, et al. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 21.Harry LE, Sandison A, Pearse MF, Paleolog EM, Nanchahal J. Comparison of the vascularity of fasciocutaneous tissue and muscle for coverage of open tibial fractures. Plast Reconstr Surg. 2009;124:1211–1219. doi: 10.1097/PRS.0b013e3181b5a308. [DOI] [PubMed] [Google Scholar]
- 22.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 23.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 25.Kon T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann W, et al. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Gerstenfeld LC, et al. Impaired fracture healing in the absence of TNF-alpha signaling: The role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 28.Heymann D, Rousselle AV. gp130 Cytokine family and bone cells. Cytokine. 2000;12:1455–1468. doi: 10.1006/cyto.2000.0747. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–936. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yano S, et al. Functional expression of beta-chemokine receptors in osteoblasts: Role of regulated upon activation, normal T cell expressed and secreted (RANTES) in osteoblasts and regulation of its secretion by osteoblasts and osteoclasts. Endocrinology. 2005;146:2324–2335. doi: 10.1210/en.2005-0065. [DOI] [PubMed] [Google Scholar]
- 31.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 32.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 33.Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet-derived growth factor: Biology and clinical applications. J Bone Joint Surg Am. 2008;90(Suppl 1):48–54. doi: 10.2106/JBJS.G.01231. [DOI] [PubMed] [Google Scholar]
- 34.Blades MC, et al. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis Rheum. 2002;46:824–836. doi: 10.1002/art.10102. [DOI] [PubMed] [Google Scholar]
- 35.Ponte AL, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 36.Stich S, et al. Human periosteum-derived progenitor cells express distinct chemokine receptors and migrate upon stimulation with CCL2, CCL25, CXCL8, CXCL12, and CXCL13. Eur J Cell Biol. 2008;87:365–376. doi: 10.1016/j.ejcb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Harvey N, Dennison E, Cooper C. Osteoporosis: Impact on health and economics. Nat Rev Rheumatol. 2010;6:99–105. doi: 10.1038/nrrheum.2009.260. [DOI] [PubMed] [Google Scholar]
- 38.Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85A(Suppl 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 39.Rifas L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J Cell Biochem. 2006;98:706–714. doi: 10.1002/jcb.20933. [DOI] [PubMed] [Google Scholar]
- 40.Yeh LC, Zavala MC, Lee JC. Osteogenic protein-1 and interleukin-6 with its soluble receptor synergistically stimulate rat osteoblastic cell differentiation. J Cell Physiol. 2002;190:322–331. doi: 10.1002/jcp.10064. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert L, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 42.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005;288:E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto J, et al. Inhibitory effects of tumor necrosis factor alpha on fracture healing in rats. Bone. 1989;10:453–457. doi: 10.1016/8756-3282(89)90078-1. [DOI] [PubMed] [Google Scholar]
- 44.Yoshikawa H, Hashimoto J, Masuhara K, Takaoka K, Ono K. Inhibition by tumor necrosis factor of induction of ectopic bone formation by osteosarcoma-derived bone-inducing substance. Bone. 1988;9:391–396. doi: 10.1016/8756-3282(88)90121-4. [DOI] [PubMed] [Google Scholar]
- 45.Balga R, et al. Tumor necrosis factor-alpha: Alternative role as an inhibitor of osteoclast formation in vitro. Bone. 2006;39:325–335. doi: 10.1016/j.bone.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 46.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 47.Franchimont N, Wertz S, Malaise M. Interleukin-6: An osteotropic factor influencing bone formation? Bone. 2005;37:601–606. doi: 10.1016/j.bone.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Kozawa O, Suzuki A, Kaida T, Tokuda H, Uematsu T. Tumor necrosis factor-alpha autoregulates interleukin-6 synthesis via activation of protein kinase C. Function of sphingosine 1-phosphate and phosphatidylcholine-specific phospholipase C. J Biol Chem. 1997;272:25099–25104. doi: 10.1074/jbc.272.40.25099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.