Abstract
Circadian (daily) rhythms are present in almost all plants and animals. In mammals, a brain clock located in the hypothalamic suprachiasmatic nucleus maintains synchrony between environmental light/dark cycles and physiology and behavior. Over the past 100 y, especially with the advent of electric lighting, modern society has resulted in a round-the-clock lifestyle, in which natural connections between rest/activity cycles and environmental light/dark cycles have been degraded or even broken. Instances in which rapid changes to sleep patterns are necessary, such as transmeridian air travel, demonstrate negative effects of acute circadian disruption on physiology and behavior. However, the ramifications of chronic disruption of the circadian clock for mental and physical health are not yet fully understood. By housing mice in 20-h light/dark cycles, incongruous with their endogenous ∼24-h circadian period, we were able to model the effects of chronic circadian disruption noninvasively. Housing in these conditions results in accelerated weight gain and obesity, as well as changes in metabolic hormones. In the brain, circadian-disrupted mice exhibit a loss of dendritic length and decreased complexity of neurons in the prelimbic prefrontal cortex, a brain region important in executive function and emotional control. Disrupted animals show decreases in cognitive flexibility and changes in emotionality consistent with the changes seen in neural architecture. How our findings translate to humans living and working in chronic circadian disruption is unknown, but we believe that this model can provide a foundation to understand how environmental disruption of circadian rhythms impacts the brain, behavior, and physiology.
Keywords: cognitive function, neuronal remodeling, structural plasticity, biological clock
Circadian (daily) rhythms in physiology and behavior are phylogenetically ancient and are present in almost all plants and animals (1). In mammals, these rhythms are generated by a master circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, which in turn synchronizes peripheral oscillators throughout the brain and body in almost all cell types and organ systems (2). Though circadian rhythms are phylogenetically ancient, modern industrialized society and the ubiquity of electric lighting has resulted in a fundamental alteration in the relationship between an individual's endogenous circadian rhythmicity and the external environment. The ramifications of this desynchronization for mental and physical health are not fully understood, although numerous lines of evidence are emerging that link defects in circadian timing with negative health outcomes (2, 3). Animal models have shown that chronic circadian disruption can alter mortality rates in tau mutant hamsters (4) and in aged mice (5). The current obesity epidemic in Western societies has also occurred hand-in-hand with a gradual decrease in sleep time and sleep quality (6), and although largely correlative, a potential causal link is plausible. Individuals reporting poor or disturbed sleep, including shift workers, show increased incidences of diabetes and risk factors for the development of cardiovascular disease (6, 7). It is also well documented that sleep deprivation has effects on cognitive function and emotionality (8, 9). However, the regulation of sleep is only one aspect of the multitude of circadian rhythms in the body, and thus investigations of how general circadian disruption can affect the brain and body are essential.
Rhythms generated by the SCN have wide-reaching effects throughout the rest of the brain and body. As such, the disruption of the circadian clock can have significant downstream effects in multiple cell types and multiple organ systems. In addition to the SCN, peripheral oscillators throughout the brain and body express circadian rhythms (2), and in many cases can persist for several cycles in vitro. However, most of these rhythms quickly dampen without input from the SCN master clock, or without an exogenous synchronizer, such as serum shock (10, 11). Tissues throughout the body show circadian rhythmicity, and are synchronized by the SCN in vivo or by other behaviors regulated by the brain clock, such as feeding. Conceptually, different tissues and organs are kept in synchrony so as to operate most efficiently with each other. When an organism undergoes a phase shift (i.e., experimental jet lag), a resynchronization of the circadian clock to a new phase is required, and a transient state of internal desynchronization between the SCN clock and peripheral oscillators occurs (10, 11). Eventually, a stable phase relationship between these oscillators and the SCN is reestablished after numerous cycles. However, the rate at which different oscillators reentrain following a phase shift varies (11), and because of this, continuous resynchronization could result in a chronic state of desynchronization.
The molecular basis of the circadian clockworks has been well studied, and rhythms in gene products throughout the body have been well characterized. Over the past decade, the mechanisms driving these diverse rhythms has become clearer, as it has been established that many of the circadian “clock genes” function as transcription factors that can further regulate hundreds of downstream elements (1). This presents an enlarged pool of factors within cells and tissues whose function could be compromised by circadian disruption. To add to this complexity, physiological rhythms, including endocrine function, are well documented (12, 13). It has been shown that some of these endocrine signals can feedback to modulate circadian behavior and the SCN (14), whereas others, such as the glucocorticoids, modulate peripheral oscillators and have little effect on the SCN (15). Thus, the ubiquity of clock genes, clock-controlled molecules, and circadian rhythms in physiological signals places the circadian clock at the center of a complex web of regulation that if perturbed could have effects in disparate brain and body systems.
In the present study, we asked if normal physiological and behavioral function would be compromised in mice exposed to environmental circadian disruption. Disruption was induced by housing male mice in 20-h light/dark (LD) cycles, whereas controls were maintained in normal 24-h LD cycles. We found that circadian disruption (CD) results in altered body temperature rhythms, increased weight gain, and elevated levels of plasma insulin and leptin. These physiological changes are accompanied by remodeling of neocortical neuronal structure, supporting the hypothesis that CD can affect brain morphology. The neural changes were associated with changes in cognitive function and emotionality, suggesting neurobehavioral ramifications of chronic CD.
Results
Altered Light/Dark Cycles Disrupt Circadian Rhythms in Body Temperature.
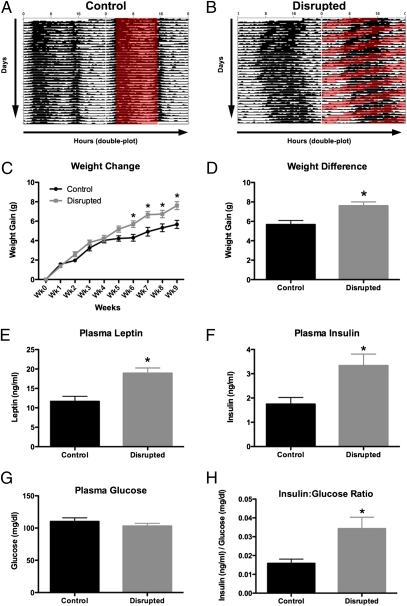
Body temperature rhythms are one of the myriad rhythms in the body regulated by the circadian clock (16). Housing in a 20-h LD cycle clearly disrupts body temperature rhythms (Fig. 1 A and B), with CD mice showing an inability to synchronize to the LD cycle, and repeated attempts to synchronize being unsuccessful. Examination of the body temperature actograms showed both a 20-h and a 24-h component to the activity rhythm.
Fig. 1.
Circadian disruption results in altered body temperature rhythms, increased weight gain, and altered metabolic hormone levels. Graphical depiction of daily changes in core body temperature in mice housed in congruent (A) 12:12 LD cycles or incongruous (B) 10:10 LD, with hours shown along the horizontal and subsequent days displayed vertically. Each day is plotted twice for ease of visualization. Whereas body temperature is synchronized in CON mice, CD mice showed a pattern in which attempts to synchronize to the shortened cycle failing repeatedly. CD mice show statistically significant weight gain starting at 6 wk (two-way RM ANOVA, interaction F9,180 = 3.05, P = 0.002; C). By the 10th week, CD mice weigh significantly more than CON mice (t test, P = 0.0037; D), and at the end of the experiment, show significantly higher plasma leptin (t test, P = 0.0009; E) and plasma insulin (t test, P = 0.01; F). CD animals do not have higher plasma glucose levels than controls (t test, P = 0.32; G), which results in a higher insulin:glucose ratio in CD animals (t test, P = 0.0124; H).
Circadian Disruption Results in Weight Gain and Increased Leptin and Insulin Levels.
CD mice show a gradual increase in body weight that becomes apparent after only 4–5 wk in the altered LD cycle. The increase becomes statistically significant by 6 wk, and accelerates through the 10th week (Fig. 1C). By the end of the experiment, CD mice have gained significantly more weight than controls (Fig. 1D). Interestingly, these effects occur even though no differences in overall food consumption were detected between groups over a 7-d period from the seventh to eighth week (Fig. S1). In addition to changes in body weight, we detected changes in the levels of the metabolic hormones insulin and leptin. Specifically, after chronic CD, mice show increased plasma leptin (Fig. 1E) and insulin levels (Fig. 1F). Because elevated insulin levels could be physiological in the face of elevated blood glucose, we assayed plasma glucose in the same samples. We found that CD and control animals had the identical levels of plasma glucose (Fig. 1G), resulting in a higher insulin:glucose ratio in CD mice (Fig. 1H).
Mice Undergoing Circadian Disruption Show Reduction in Complexity of Neurons in the Medial Prefrontal Cortex.
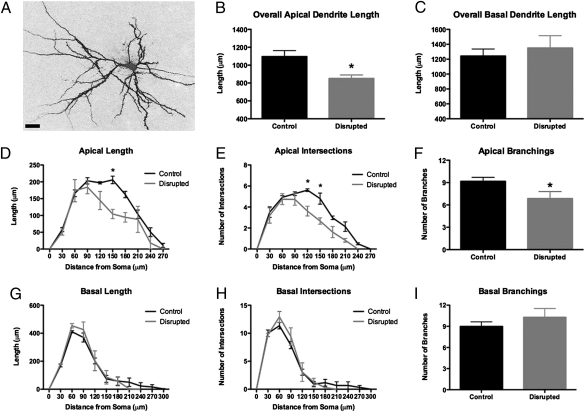
The prefrontal cortex is important for the regulation of higher-order executive function in rodents and humans (17–19). We investigated changes in the morphology of layer III prelimbic (PL) neurons of the medial prefrontal cortex (mPFC) by using dye-filling techniques and morphological reconstruction (Fig. 2A). Random cells within the PL of CD and control mice were filled with Lucifer yellow and then digitally reconstructed. There was no difference in the depth from the pial surface between control and CD mice (184.8 ± 29.76 μm and 180.5 ± 25.11 μm, respectively; two-tailed t test, P = 0.31). We determined that CD mice demonstrate a shortening of apical dendrites of PL neurons (Fig. 2B), whereas basal dendrites were largely unaffected (Fig. 2C). Specifically, in Scholl analysis, the amount of dendritic arborization between 90 and 210 microns from the soma was reduced (Fig. 2D). We also observed decreased dendritic complexity, as measured by fewer intersections (Fig. 2E) and fewer branch points (Fig. 2F), suggesting a loss of apical dendrite branches. The remodeling of the apical tree was selective in that basal dendrites were unaffected (Fig. 2 G–I). We extended our analysis to dendritic spines on the same Lucifer yellow-loaded neurons using confocal laser scanning microscopy and a custom-made software package (NeuronStudio) for spine analysis (20, 21). In contrast to the reorganization of dendritic morphology, systematic spine analysis of the apical and basal trees revealed that CD had no effects on dendritic spine density or dendritic spine morphology. However, within the context of the shorter apical dendrite lengths, we found an overall loss of apical, but not basal, dendritic spines per neuron following CD (Fig. S2).
Fig. 2.
Circadian disruption results in changes to the morphology of medial prefrontal neurons. Cells of layer III of prelimbic medial prefrontal cortex (PL) were labeled with Lucifer yellow (A). CD mice showed shrunken apical dendrites in the PL (t test; P = 0.0332; B) but with no effect on basal dendrites (t test, P = 0.5975; C). Sholl analysis revealed decreased complexity of the apical dendrites of PL neurons in CD mice (two-way RM ANOVA; interaction F9,36 = 2.191, P = 0.0462), with decreased length, particularly 150 μm from the soma (Bonferroni posttest, P < 0.001; D) and fewer intersections (two-way RM ANOVA; interaction F9,20 = 2.57, P = 0.0378), particularly at 120 and 150 μm from the soma (Bonferroni posttest, P < 0.01; E) as well as fewer overall branchings (t test, P = 0.048; F). There were no statistically significant differences in the basal dendrite in any of these measures (G–I). (Scale bar: 50 μm.)
Circadian Disrupted Animals Show Reduced Ability to Shift Learned Behaviors.
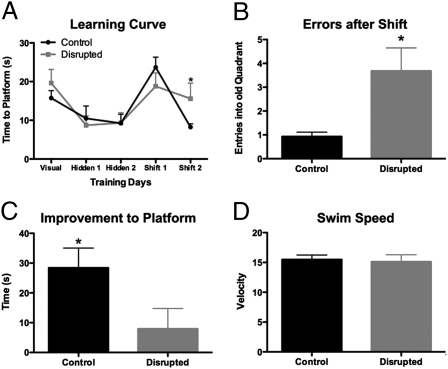
The neuroanatomical findings led to the hypothesis that prefrontal function would be impaired in CD mice. To test this hypothesis, we assessed cognitive flexibility by using a modified water maze task, a procedure that has been shown to be dependent on the mPFC (22, 23). CD mice showed normal acquisition of the task, as evidenced by their improvement from day 1 to day 2 of training, much as control mice, suggesting normal hippocampal function at this level of analysis (Fig. 3A). Both groups of mice also performed equally poorly on the first day the platform was shifted to the opposite quadrant (Fig. 3A). However, in the second shifted day, CD mice made more errors by returning to the former location of the platform than controls (Fig. 3B). In addition, CD mice show little improvement between the first and second days of the shift in platform location, whereas control mice show significant improvement (Fig. 3C). There was no difference in swim speed between groups on the day of the platform change (Fig. 3D). This is indicative of a change in the animal's ability to shift what it has learned, and thus a reduction in cognitive flexibility.
Fig. 3.
Disrupted circadian rhythms are associated with changes in cognition. CD mice showed normal acquisition of a modified Morris watermaze task, but were impaired when made to shift their learning to a new quadrant, with control animals performing better on the second reversal than CD mice (two-way RM ANOVA, F1,18 = 8.97, P = 0.0078; Bonferroni posttest P = 0.02; A). On the test day, CD mice made numerous errors by returning to the old platform location (t test with Welch's correction for unequal variances, P = 0.0212; B), and showed very little improvement in their ability to find the new platform location from the previous shift day (t test, P = 0.0237; C). There were no differences in swim speed on test day (t test, P = 0.7918; D).
Disrupted Animals Have Decreased Latency to Enter a Novel Environment.
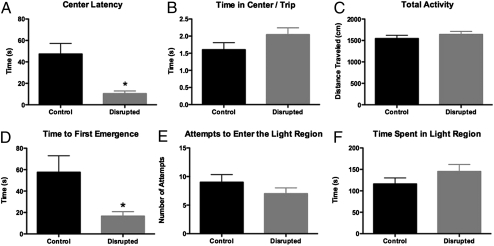
A separate group of mice were tested in the open field test (OFT) or the light/dark emergence task. These tasks evaluate an animal's behavior within a novel environment. CD mice entered the center of the OFT more rapidly than controls (Fig. 4A), but did not spend significantly more time in the center per entry (Fig. 4B), nor did they display more overall activity in the OFT (Fig. 4C). These data are similar to behaviors observed in animals with mPFC lesions (23). In the light/dark emergence task, CD mice had shorter emergence times than controls (Fig. 4D), but no difference in the number of emergences (Fig. 4E) or time spent in the lit region (Fig. 4F). This pattern of results is in agreement with the OFT data, and shows that CD alters the way mice respond within novel environments.
Fig. 4.
Circadian disruption results in changes in emotionality. Emotionality was assessed by OFT and LD box (LDB) task. CD mice entered the center of the OFT faster than controls (t test, P = 0.0006; A), while not spending any additional time in the center per entry (t test, P = 0.1331; B) or demonstrating any more locomotor activity (t test, P = 0.3735; C). In the LBD, CD mice emerged from the “safe” dark compartment into the bright compartment much faster than controls (unpaired t test with Welch's correction for unequal variance, P = 0.0277; D), and without making any more attempts to enter the region (t test, P = 0.2608; E) or spending any more time in the lit region (t test, P = 0.1905; F).
Discussion
In the present work we disrupted circadian rhythms by altering the LD cycle of normal C57 mice, such that the controls were exposed to a 24-h day (12 h light, 12 h dark), and the CD group was exposed to a significantly shortened 20-h day (10 h light, 10 h dark). It is important to note that the method of disruption used in this work has been applied successfully in mice to investigate the effects of circadian disruption on recovery in an induced pressure overload cardiac hypertrophy model, with results suggesting that, at least in an injury model, circadian disruption can hamper the body's restorative capacity (24). Here, we investigated effects of circadian disruption on metabolism, brain, and behavior in otherwise normal mice and found that this environmental manipulation leads to changes in metabolism, brain, and behavior. Together, these findings suggest that disruption of circadian rhythms through alterations of the environmental LD cycle can impact metabolic function and result in neural and behavioral changes, demonstrating the central role circadian rhythms play in both mental and physical health.
The relationship between circadian rhythms and metabolism is becoming an increasingly important area of research, and findings linking sleep loss and obesity are becoming increasingly numerous (6). Our findings demonstrate that chronic housing in a shortened LD cycle results in the alteration of body temperature rhythms, weight gain, and increases in both plasma leptin and insulin levels. The significance of these changes in metabolic factors is unclear, but both leptin and insulin are key hormones in the regulation of fat stores and glucose metabolism. Leptin is important in the regulation of food intake and is produced by adipocytes. Mice deficient in leptin or the leptin receptor become extremely obese (25). In animals undergoing 19 wk of diet-induced obesity, leptin levels are extremely high, and the sensitivity of the body to the leptin signal is reduced (26). Insulin is a crucial factor regulating glucose utilization and fat deposition. High levels of insulin, in the face of normal glucose load, denotes the potential for the development of type II diabetes, and dysregulation in insulin signaling contributes to fluctuations in plasma glucose and to obesity. Our findings suggest that even though plasma glucose levels are equivalent between CD and control animals, CD animals show higher levels of insulin. This could represent the development of insulin resistance. In humans, even acute misalignment of circadian and behavioral cycles can result in postprandial glucose responses that are within the prediabetic range (27). Whether the increased leptin and insulin levels observed in our model are a cause or an effect of the observed weight gain is unclear, although it should be evident that changes in these systems can go hand in hand, and could initiate a vicious cycle of metabolic dysregulation. Additionally, taken in the context of increased body weight, these effects are associated with the development of metabolic syndrome, a state that puts individuals at greater risk for cardiovascular disease.
Similarly convincing changes in weight and metabolic hormones have been observed in CLOCK mutant mice (28), suggesting that genetic disruption of the circadian clock can lead to changes in metabolism, a key finding that links the molecular clockwork to metabolic function. However, a caveat in using such genetic models is that the manipulation is both organism-wide and present from birth. These issues make it necessary to apply approaches such as those used in the present study to demonstrate how physiology and behavior are impacted by disruption of the normal day/night cycle. Indeed, the similarity in the effects of our environmentally driven circadian disruption model and the effects of a clock gene mutation model provides necessary, converging lines of evidence to aid in unraveling the interaction between genes and environment.
How these findings translate to humans is an important area of research, because such effects could put chronically disrupted individuals at risk for developing metabolic and cardiovascular problems. Long-term experiments in populations showing disrupted sleep/wake patterns are thus necessary to probe these findings further, but a longitudinal study in a nurses cohort of night-shift work has found that exposure to night work can lead to weight gain and obesity (29). More recently, it was found that alternating shift work is an independent risk factor for the development of obesity in a longitudinal (14-y) large cohort of Japanese male shift workers (30). Thus, the ability to model some of these effects in animals may provide methods to intervene and either reverse or protect an individual from the negative physiological ramifications of long-term shift work.
In addition to metabolic effects, we demonstrate neurobehavioral changes in CD animals. Specifically, we demonstrate changes in the complexity of neurons in the prelimbic region of the mPFC, and behaviors mediated by this brain area. Until the present study, nothing was known about how circadian disruption could affect the function and structure of the PFC, a brain region mediating higher-order executive functions in rodents and humans (17–19). This brain region is sensitive to stress and stress mediators, as stress-induced remodeling of neurons in the prelimbic mPFC of rats has been demonstrated (31–33), indicating that the PFC is sensitive to perturbations of normal homeostasis. Our behavioral findings indicate that though CD animals can learn a spatial memory task at the same rate as controls, their ability to change this learning is impacted (Fig. 3). A similar behavioral phenotype is observed in animals receiving lesions of the mPFC (22, 23), although the impact of a wholesale lesion on behavior is likely to be more severe than we observed in our animals. In terms of emotionality, CD animals demonstrate a lack of inhibition in mildly stressful behavioral tasks. In the OFT, CD animals more quickly enter the center of the arena, whereas not demonstrating increased overall locomotor activity, nor spending any more total time in the center overall, suggesting they overcome the initial neophobia or fear/anxiety responses when faced with the task (Fig. 4). Similarly, in the LD emergence task, CD mice emerge more quickly than their non-CD counterparts, while again not spending any additional time overall in the unprotected open area (Fig. 4). Though there are myriad explanations for such behaviors, most of which cannot be discerned in the present set of studies, the phenotype is consistent with that observed in animals that have received a PFC lesion (23, 34), suggesting that whatever the end interpretation of the emotional state of the animal, the phenotype is likely associated with a dysfunctional PFC. Though the effects we report on the PFC are unique, previous work in humans shows that short-recovery flight crews (those who are rapidly and repeatedly subjected to transmeridian air travel) have smaller medial temporal lobes and decreased performance on hippocampal memory and reaction-time tasks (35), though the study did not assess behaviors more directly related to PFC function.
Our findings show that disruption of circadian timing results in changes in body temperature, increased obesity, and changes in the metabolic hormones, leptin and insulin, which themselves have effects on cognition and mood (36–40). The metabolic effects are accompanied by changes in mPFC neuronal morphology, with layer III neurons showing a reduction in overall complexity. In addition to the neural changes, we observed concomitant changes in behaviors mediated by the mPFC, with a decrease in cognitive flexibility and an alteration in emotionality. Though the mechanisms mediating the circadian disruption induced changes in this metabolism-brain-behavior relationship are unknown, we hypothesize that the interactions are both interrelated and likely nonlinear. An important mediator of these effects could be changes in corticosterone (CORT), the primary corticosteroid in mice and an important synchronizer of peripheral oscillators throughout the brain and body (15). Chronic stress, or exposure to mediators of stress such as CORT, results in morphological changes in many neural structures, including the mPFC, similar to those described here (41). Dysregulation of the HPA axis could therefore be an important intermediary in the genesis of these effects, although this would require significant further exploration. Furthermore, in addition to changes in the PFC, it is likely that CD results in structural changes in other brain regions as well. It will be important to probe changes in areas regulating emotionality and cognition, such as the amygdala and hippocampus, as well as hypothalamic nuclei, which are important in metabolic regulation.
The ramifications of this work for individuals undergoing circadian disruption remain unclear. However, because many occupations with high cognitive demand also force individuals to operate in conditions where circadian disruption is present (e.g., airline pilots, medical practitioners, armed forces), determining the effects of disrupted clocks on the brain and body is imperative. As the intricacies of the molecular mechanisms of circadian timing and the wide range of tissues that express oscillators become clearer, we are also starting to understand the relationship between normal circadian clocks and health. As a corollary, disruption of circadian timing could lead to dysfunction of physiological and behavioral processes that contribute to disease. We believe that our model has the potential to contribute to our understanding by providing a noninvasive way to investigate the downstream ramifications of circadian disruption on metabolism, neuronal plasticity, and behavior in otherwise normal animals. Such models are necessary to provide a test bed to evaluate the efficacy of treatments (pharmacological or otherwise) aimed at reducing or rescuing the effects of chronic circadian disruption.
Materials and Methods
Animals and Housing.
Male C57BL/6 mice (Charles River, Inc.) were group housed (n = 5 per cage) and allowed 1 wk to acclimate to the facility. Food and water were available ad libitum for the duration of the experiment. Body temperature was continuously recorded in randomly selected mice (two per cage) using temperature data-loggers implanted into the peritoneal cavity (SubCue, Inc.), programmed to record body temperature every 30 min, with a resolution of 0.0625 °C. Following implant, animals were allowed 1 wk to recover. Body temperature was visualized using Clock Lab (Actimetrics) for MatLab (MathWorks, Inc.). For circadian manipulation, one group of animals was transferred to a 20-h LD cycle, with 10 h of light and 10 h of dark. The control group remained in a 12:12 LD cycle. Animals were weighed weekly during cage change and otherwise left undisturbed. All procedures with animals were undertaken with the approval of The Rockefeller University Institutional Animal Care and Use Committee.
Behavioral Analyses.
In all cases, the experimenters undertaking and analyzing the behavior were blind to the animals’ conditions until the code was broken at the end of the study. OFT (n = 20 per group) and LDB (n = 10 per group) experiments were undertaken 2–4 h before lights-on in both groups. Briefly, both OFT and LDB were measured using EthoVision software (Noldus, Inc.). For OFT, animals were placed into a Plexiglas arena (45 × 45 × 15 cm) under dim light. The animals’ behavior was recorded for 5 min and scored automatically. Similarly, for LDB, animals were placed into the closed and dark compartment of a Plexiglas arena (60 × 30 × 30 cm) with a small opening cut into the wall separating light and dark sides. The amount of time it took the animal to fully emerge into the light side and the time spent ambulating in the light side were recorded for 10 min and scored by two independent experimenters blind to the conditions.
Cognitive Flexibility Task.
A modified Morris watermaze (n = 10 per group) task was used to probe changes in cognitive flexibility in disrupted mice. Testing occurred in a plastic pool filled with water made opaque by the addition of white Tempra Paint (Dixon Co.), and maintained between runs at 23 ± 3 °C. EthoVision software was used for the acquisition and analysis of all behaviors. Training and testing occurred at the same relative time (1 h after lights off) in both groups. The same experimenter ran all groups of animals, in a random fashion, each day. Briefly, on day 1, animals received four 90-s (max) trials with a visible platform, released from one of four release points (north, south, east, west) in a randomized order each day. To avoid unintended cues, after placing the animal into the maze, the experimenter would stand in the same part of the room. This procedure was repeated on all training and testing days. On days 2 and 3, animals received four trials with a hidden platform (1-min intertrial interval). On day 4, the platform was moved to the diagonally opposite quadrant of the maze, and animals were given a further four trials. On day 5, the platform was kept in the new location. Latency to reach the platform each day, swim speed, and time spent in the old and new quadrants on probe day were analyzed.
Blood Analyses.
Leptin, insulin, and glucose were assayed from the same animal. Disrupted and control mice were rapidly decapitated after 16 wk of CD. Trunk blood was collected in EDTA-coated tubes and immediately placed on ice. Plasma was obtained via rapid centrifugation, aliquoted, and stored at −70 °C until used. Plasma leptin and insulin levels were assayed using ELISAs (Millipore, Inc.) following the manufacturer's instructions. Samples were run in duplicate, with lower levels of detectability of <2 ng and <1 ng/mL, for leptin and insulin, respectively. Plasma glucose levels were assayed using a colormetric glucose oxidation assay, using the manufacturer's instructions (Caymen Chemical).
Cell Filling and Morphological Analysis.
Methods used to assess neural morphology were similar to previously established protocols (32). After 10 wk of circadian disruption, animals were transcardially perfused with 1% paraformaldehyde (para), followed by 4% para. Brains were then removed and postfixed in 4% para overnight at 4 °C in coded vials to allow the remaining part of the study to be blind to experimental condition. Brains were carefully blocked to ensure sectioning was undertaken in the same plane between animals, and serial coronal PFC sections cut on a Vibratome (VT1000S; Leica Inc.) at 250 μm. Sections for intracellular dye-filling were stored in 0.1 M PBS (pH 7.4) at 4 °C until processing. For neuronal reconstructions, slices were labeled with DAPI in PBS for 5 min before visualize cortical layers under epifluorescence. Random cells in layer III of the prelimbic (PL) cortex were loaded with a glass micropipette containing 5% Lucifer yellow (Molecular Probes) at 1–6 nA for ∼5 min to completely fill all distal aspects of the dendritic tree (at least six neurons per animal). Morphological tracing of filled PL neurons was undertaken on a Nikon Eclipse microscope at 60× using the NeuroLucida software package (MicroBrightfield, Inc.). Following tracing, only neurons (i) within layer II/III and within the boundary of the PL, (ii) exhibiting complete filling of dendritic tree, as evidenced by well-defined endings, (iii) displaying intact primary and secondary branches, and (iv) having apical dendrites reaching the pial surface were used for analysis. Mean dendritic length and branching patterns were computed, and Sholl analysis was undertaken. The code was then broken to allow for statistical analysis.
Dendritic Spines.
Spines were analyzed using a Zeiss 510 confocal scanning laser microscope (Carl Zeiss MicroImaging, Inc.) with a Neo-PLAN 100× objective and a digital zoom of 3.6, such that the pixel dimensions in the XYZ were 0.05 × 0.05 × 0.1 μm. At 200 μm and 100 μm from the soma in the apical tree, and at 100 μm from the soma on the basal tree, confocal z-stacks were taken by exciting an Argon laser at 453 nm and capturing the emissions through a bandpass filter from 505 to 530 nm. Conditions such as laser power, pinhole size, and pixel dwell time were set initially then held constant throughout the study. Z-stacks were deconvolved using AutoDeblur, imported into NeuronStudio, and analyzed using a custom-made Rayburst algorithm for automated spine analysis of spine density, spine head diameter, spine surface area, and spinehead volume (20, 21). Data were then imported into Microsoft Excel and analyzed by an experimenter blind to the experimental groups.
Statistics.
All statistical analyses were accomplished using Prism 5 (GraphPad Software, Inc.). Two-tailed t tests, one-way or two-way repeated-measures (RM) ANOVAs were undertaken where appropriate, and Tukey or Bonferroni posttests, respectively, were used to probe interactions. If variances between groups were statistically different, t tests with Welch's correction were used. In all cases, results were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Russell Romeo, Dr. Matthew Hill, and Christopher Wilson for their helpful discussion on previous versions of this manuscript. This work was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (to I.N.K.), a National Research Service Award graduate fellowship from National Institute on Aging Grant F31 AG034794 (to E.B.B.), National Institute of Mental Health Grants 5RO1 MH41256 (to B.S.M.) and 5P5 MH58911 (to B.S.M. and J.H.M.), and an investigator-initiated grant from Sepracor, Inc. (to B.S.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018375108/-/DCSupplemental.
References
- 1.Roenneberg T, Merrow M. Circadian clocks—the fall and rise of physiology. Nat Rev Mol Cell Biol. 2005;6:965–971. doi: 10.1038/nrm1766. [DOI] [PubMed] [Google Scholar]
- 2.Hastings MH, Reddy AB, Maywood ES. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 3.Bechtold DA, Gibbs JE, Loudon AS. Circadian dysfunction in disease. Trends Pharmacol Sci. 2010;31:191–198. doi: 10.1016/j.tips.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- 5.Davidson AJ, et al. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol. 2007;22(Suppl 2):S1–S8. doi: 10.1097/01.yic.0000277956.93777.6a. [DOI] [PubMed] [Google Scholar]
- 9.Van Cauter E, Turek FW. Depression: A disorder of timekeeping? Perspect Biol Med. 1986;29:510–519. doi: 10.1353/pbm.1986.0033. [DOI] [PubMed] [Google Scholar]
- 10.Abe M, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 12.Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- 13.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 14.Karatsoreos IN, Silver R. Minireview: The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 16.Scheer FA, Pirovano C, Van Someren EJ, Buijs RM. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 19.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS ONE. 2008;3(4):e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc. 2006;1:2152–2161. doi: 10.1038/nprot.2006.313. [DOI] [PubMed] [Google Scholar]
- 22.de Bruin JP, Sànchez-Santed F, Heinsbroek RP, Donker A, Postmes P. A behavioural analysis of rats with damage to the medial prefrontal cortex using the Morris water maze: Evidence for behavioural flexibility, but not for impaired spatial navigation. Brain Res. 1994;652:323–333. doi: 10.1016/0006-8993(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix L, White I, Feldon J. Effect of excitotoxic lesions of rat medial prefrontal cortex on spatial memory. Behav Brain Res. 2002;133:69–81. doi: 10.1016/s0166-4328(01)00442-9. [DOI] [PubMed] [Google Scholar]
- 24.Martino TA, et al. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension. 2007;49:1104–1113. doi: 10.1161/HYPERTENSIONAHA.106.083568. [DOI] [PubMed] [Google Scholar]
- 25.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 26.Van Heek M, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–390. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niedhammer I, Lert F, Marne MJ. Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. Int J Obes Relat Metab Disord. 1996;20:625–633. [PubMed] [Google Scholar]
- 30.Suwazono Y, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- 31.Cerqueira JJ, et al. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lacroix L, Broersen LM, Weiner I, Feldon J. The effects of excitotoxic lesion of the medial prefrontal cortex on latent inhibition, prepulse inhibition, food hoarding, elevated plus maze, active avoidance and locomotor activity in the rat. Neuroscience. 1998;84:431–442. doi: 10.1016/s0306-4522(97)00521-6. [DOI] [PubMed] [Google Scholar]
- 35.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 36.Harvey J. Leptin regulation of neuronal excitability and cognitive function. Curr Opin Pharmacol. 2007;7:643–647. doi: 10.1016/j.coph.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moult PR, Harvey J. Hormonal regulation of hippocampal dendritic morphology and synaptic plasticity. Cell Adh Migr. 2008;2:269–275. doi: 10.4161/cam.2.4.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Malley D, et al. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Convit A. Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Starr VL, Convit A. Diabetes, sugar-coated but harmful to the brain. Curr Opin Pharmacol. 2007;7:638–642. doi: 10.1016/j.coph.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwen BS. Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.