Abstract
Copper (Cu) is essential for many biological processes, but is toxic when present in excessive amounts. In this study, we provide evidence that Cu plays a crucial role in controlling tuberculosis. A Mycobacterium tuberculosis (Mtb) mutant lacking the outer membrane channel protein Rv1698 accumulated 100-fold more Cu and was more susceptible to Cu toxicity than WT Mtb. Similar phenotypes were observed for a M. smegmatis mutant lacking the homolog Ms3747, demonstrating that these mycobacterial copper transport proteins B (MctB) are essential for Cu resistance and maintenance of low intracellular Cu levels. Guinea pigs responded to infection with Mtb by increasing the Cu concentration in lung lesions. Loss of MctB resulted in a 1,000- and 100-fold reduced bacterial burden in lungs and lymph nodes, respectively, in guinea pigs infected with Mtb. In mice, the persistence defect of the Mtb mctB mutant was exacerbated by the addition of Cu to the diet. These experiments provide evidence that Cu is used by the mammalian host to control Mtb infection and that Cu resistance mechanisms are crucial for Mtb virulence. Importantly, Mtb is much more susceptible to Cu than other bacteria and is killed in vitro by Cu concentrations lower than those found in phagosomes of macrophages. Hence, this study reveals an Achilles heel of Mtb that might be a promising target for tuberculosis chemotherapy.
Keywords: immune system, metal, accumulation
One of the most dangerous pathogens to mankind is Mycobacterium tuberculosis (Mtb). A key component of the immune response to Mtb is the IFN-γ–mediated activation of macrophages, resulting in efficient maturation of phagosomes with enhanced capacity to kill intracellular pathogens by using a range of hydrolytic enzymes, bactericidal peptides, and reactive oxygen and nitrogen intermediates (1). Copper (Cu) proteins are widely used for electron transfer reactions in the presence of oxygen because of the high redox potential of Cu(II)/Cu(I) (2). Hence, Cu is an essential nutrient for many bacteria, but it is also toxic because of the Cu(I)-catalyzed formation of hydroxyl radicals from hydrogen peroxide or other mechanisms (3). To avoid any free Cu ions cells use Cu-specific chaperones, storage proteins, and efflux systems (4). Early observations indicated that the toxicity of free Cu(I) in the presence of hydrogen peroxide may be used by the human immune system to fight bacterial pathogens (5, 6). Recent in vitro experiments with macrophages showed that IFN-γ–stimulated trafficking of the Cu transporter ATP7A to vesicles that fuse with phagosomes increasing their Cu content and their bactericidal activity against Escherichia coli (7). The first indication that the immune system might use Cu also to control growth of mycobacteria was provided by the finding that Cu concentrations are markedly increased within the phagosomal compartment of macrophages infected with M. avium (8).
Transcriptome analysis identified 30 Cu-responsive genes in Mtb (9), suggesting that Mtb faces critical concentrations of Cu during its life cycle. The ctpV gene (rv0969) is part of a Cu-induced operon controlled by the transcriptional regulator CsoR (10) and likely encodes a Cu-specific inner membrane efflux pump (9, 10). However, the ctpV mutant did not show a clear virulence defect in mice and guinea pigs (11). Further, Mtb produces the metallothionine MymT, a small protein that binds up to six Cu(I) ions and partially protects Mtb from Cu toxicity (12). The lack of MymT also did not reduce the virulence of Mtb in mice (12). These studies show that Cu resistance mechanisms exist in Mtb. However, it is unknown whether phagosomal Cu concentrations are sufficient to kill Mtb in vivo and how important Cu defense mechanisms are for virulence of Mtb.
Here, we present evidence that the outer membrane channel protein Rv1698 (13) is part of a Cu resistance mechanism that ensures low intracellular Cu levels in Mtb and protects the bacterium from the toxic effects of excess Cu. Importantly, we show that Rv1698 is required for full virulence of Mtb in guinea pigs and that guinea pigs respond to infections with Mtb by increasing Cu concentrations in lung lesions. This study provides experimental evidence that Cu resistance is crucial for survival of Mtb in animal hosts, establishes Cu as an antimycobacterial tool used by the immune system, and identifies a resistance mechanism by which excess Cu ions are limited within the bacterium.
Manuscript Text
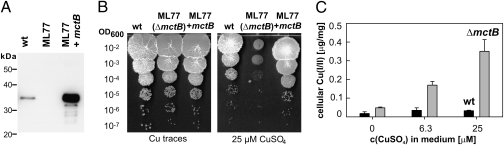
To examine the physiological functions of the outer membrane channel protein Rv1698 of Mtb and its homolog in M. smegmatis, Ms3747, we constructed the M. smegmatis mutant ML77 lacking expression of the ms3747 gene (SI Appendix, Fig. S1 A and B). Western blot experiments demonstrated the absence of the Ms3747 protein in detergent extracts of ML77 (Fig. 1A). ML77 exhibited a severe growth defect on Middlebrook 7H10 agar plates. This phenotype was restored by expression of ms3747 or rv1698 (SI Appendix, Fig. S2). Surprisingly, ML77 grew similarly to WT M. smegmatis SMR5 in Luria-Bertani (LB) medium, indicating that ML77 has no general growth defect, but rather might be more susceptible to a toxic compound present in Middlebrook 7H10 agar. Indeed, ML77 grew as well as WT M. smegmatis on plates made of 7H10 agar without added copper (Fig. 1B), but was impaired in the presence of 25 μM CuSO4 (Fig. 1B), demonstrating that the lack of Ms3747 made M. smegmatis susceptible to copper. This phenotype was abolished by expression of either ms3747 or rv1698 (SI Appendix, Fig. S2), indicating that the susceptibility of ML77 to Cu was solely due to the lack of Ms3747 and that the Mtb homolog Rv1698 has a similar function.
Fig. 1.
MctB is required for copper resistance of and maintaining a low intracellular copper concentration in M. smegmatis. (A) Expression of ms3747 in M. smegmatis. Proteins were extracted with 2% SDS from WT M. smegmatis, the ms3747 mutant ML77, and ML77 complemented with the ms3747 expression vector pML451. Proteins were detected in a Western blot by using the monoclonal antibody 5D1.23. (B) Serial dilutions of cultures of M. smegmatis SMR5 (WT), ML77 (ΔmctB), and ML77 complemented with mctB were spotted on 7H10 agar plates without or with CuSO4 at a concentration of 25 μM. (C) M. smegmatis SMR5 (black bars) and the Δms3747 mutant ML77 (gray bars) were grown in self-made Middlebrook 7H9 medium with 0, 6.3, or 25 μM CuSO4. Samples were taken after growth for 36 h. Copper was determined by measuring the absorption of the Cu(II)–dithizone complex at 553 nm.
Minimal inhibitory concentrations of 100 and 250 μg/mL CuSO4 on 7H10 agar plates were determined for ML77 and WT M. smegmatis, respectively (SI Appendix, Fig. S3). Concentrations of 200 μM AgNO3 also impaired the growth of ML77 compared with WT M. smegmatis, whereas HgCl2 and CoCl2 had no effect (SI Appendix, Fig. S4). These results showed that Ms3747 is required for normal growth of M. smegmatis in the presence of elevated concentrations of Cu(II) and Ag(I) ions.
ML77 in Middlebrook 7H9 liquid cultures formed large clumps, in contrast to the parent WT strain (SI Appendix, Fig. S5). A similar phenotype is often caused by increased cell surface hydrophobicity. However, a complete lipid analysis did not reveal any differences between WT M. smegmatis and ML77. In addition, surface hydrophobicity was not changed as determined by Congo Red adsorption. These results suggest a specific defect of ML77 grown in liquid 7H9 medium as opposed to a general defect in membrane architecture or lipid properties.
To examine how Ms3747 contributes to Cu resistance of M. smegmatis, WT M. smegmatis, and the ms3747 mutant ML77 were grown in the absence or presence of 6.3 or 25 μM Cu in self-made 7H9 medium, and the Cu content of cell lysates was determined by measuring Cu(II)-specific absorption changes of dithizone (14). The Cu content of WT M. smegmatis did not change regardless of the external Cu(II) concentration. By contrast, the Cu content of ML77 increased by 11-fold at 25 μM external Cu(II) (Fig. 1C). These results show that Ms3747 is involved in maintaining low, homeostatic levels of intracellular copper.
Complementation of the Cu sensitivity of the ms3747 mutant by rv1698 and ms3747 (SI Appendix, Fig. S2), the channel activity of Rv1698 in vitro (13), and the fact that outer membrane channel proteins are involved in transport processes indicate that these proteins are involved in transport of copper. Efflux is a known Cu resistance mechanism in other bacteria (15). Hence, we propose to name these proteins MctB (mycobacterial Cu transport protein B).
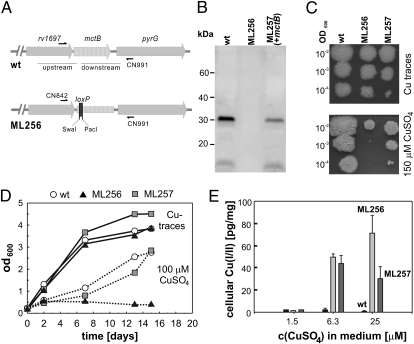
To examine the function of mctB in Mtb, we constructed a mutant in a similar manner as done for M. smegmatis (Fig. 2A). The unmarked mctB mutant Mtb ML256 was confirmed by specific restriction digests (SI Appendix, Fig. S6) and sequencing of a PCR fragment amplified from chromosomal DNA. To obtain an MctB-specific antibody, hybridomas were generated from B cells harvested from mice immunized with recombinant Mtb MctB protein purified from E. coli. The monoclonal antibody 5D1.23 was the only antibody that recognized the MctB proteins from both Mtb and M. smegmatis. A Western blot using the 5D1.23 antibody showed that mctB is expressed in Mtb H37Rv grown in Middlebrook 7H9 medium, but absent in ML256 (Fig. 2B). Expression of mctBMtb was restored by integration of the plasmid pML955 (SI Appendix, Table S2) at the attB site of mycobacteriophage L5 (Fig. 2B). No growth difference was observed for WT Mtb, ML256 (ΔmctB), and ML257 (ΔmctB, attBL5::mctBMtb) on 7H11 agar plates with Cu traces (Fig. 2C), demonstrating that the lack of mctB does not cause a general growth defect in Mtb. However, in the presence of 150 μM CuSO4, the mctB mutant ML256 showed a severe growth defect in contrast to WT Mtb (Fig. 2C). Growth of ML256 in the presence of CuSO4 was partially restored by expression of mctBMtb (Fig. 2C). This finding is consistent with the sixfold lower MctBMtb protein level compared with WT Mtb as determined by quantification of a Western blot of protein extracts (Fig. 2B). Growth inhibition of the mctB mutant ML256 by Cu was even more pronounced in liquid medium. Without Cu, all strains grew at a similar rate, whereas the mctB mutant did not grow in the presence of 100 μM CuSO4 in contrast to WT Mtb and the complemented mutant (Fig. 2D).
Fig. 2.
MctB is required for copper resistance and maintenance of a low intracellular copper concentration in M. tuberculosis. (A) Schematic representation of the chromosomal rv1698 region of M. tuberculosis H37Rv (WT). A fragment of 39 bp was replaced by the 45-bp loxP site in the 5′ part of the gene introducing stop codons in all three reading frames to construct the rv1698 deletion mutant ML256 by homologous recombination. The genes are drawn to scale. (B) Expression of mctB in Mtb. Proteins were extracted with 2% SDS from WT Mtb and the mctB mutant ML256. ML257 is an ML256 derivative carrying the integrative mctB expression vector pML955. Proteins were detected in a Western blot by using the MctB-specific monoclonal antibody 5D1.23. (C) Copper susceptibility. Serial dilutions of log-phase cultures of Mtb H37Rv (WT), ML256 (ΔmctB), and ML257 (+mctB) were spotted on 7H11/OADC agar without or with CuSO4 at a concentration of 150 μM. (D) Growth defect of Mtb ΔmctB mutant. Mtb WT, the ΔmctB mutant ML256, and the complemented mutant ML257 were grown in 7H10 medium supplemented with peptone (1 g/L) with or without 100 μM CuSO4. The optical density at 600 nm was measured at the indicated time points. (E) Mtb (black bars), the ΔmctB mutant ML256 (light gray bars), and the complemented mutant ML257 (dark gray bars) were grown in minimal medium with 0.8, 6.3, or 25 μM CuSO4. Copper was determined by measuring the absorption of the Cu(II)-dithizone complex at 553 nm.
To accurately assess the susceptibility of Mtb to copper, it was necessary to avoid albumin in the medium, which is known to sequester Cu ions in large quantities (3). Indeed, the high Cu susceptibility of the M. smegmatis mctB mutant ML77 was completely overcome by addition of either albumin or OADC, an albumin-containing supplement in media for Mtb. Therefore, Mtb was grown in Hartmans-de Bont minimal medium (HdB) and plated on HdB agar plates containing different CuSO4 concentrations (SI Appendix, Fig. S7). All Mtb strains grew normally at 1.5 μM CuSO4. No growth was observed ≥24 μM CuSO4, indicating that Mtb is more susceptible to Cu than M. smegmatis, which tolerated up to 100 μM Cu (SI Appendix, Fig. S3). Serial dilutions of cultures of Mtb H37Rv and of ML256 (ΔmctB) on 7H11/OADC plates showed that the addition of Cu(I)-chelating bathocuproine disulfonate (BCS) restored growth of WT Mtb and of the mctB mutant ML256 in the presence of 150 μM CuSO4 (SI Appendix, Fig. S8), indicating that Cu(I) is the Cu-species toxic for Mtb, which is consistent with previous findings (12).
The amount of Cu accumulated by WT Mtb was consistently low and independent of external CuSO4 concentrations as determined by the dithizone assay (Fig. 2E). By contrast, the Cu content of ML256 increased drastically by 100-fold with increasing CuSO4 concentrations in the medium (Fig. 2E), demonstrating the essential role of MctB for maintaining a low Cu concentration in Mtb. This effect was complemented in ML257 by expression of mctB, indicating that no other mutation caused the Cu sensitivity of the mctB mutant. However, this complementation was only partial, probably due to the lower expression of the integrated mctB gene in ML257 in contrast to the full complementation when mctB was expressed on a higher level in the M. smegmatis mutant ML77 (Fig. 1B).
It should be noted that the intracellular Cu concentration in Mtb was >10,000-fold lower than that measured in M. smegmatis. The decreased level of copper in Mtb might be due to its slower growth, which probably requires much less copper, compared to M. smegmatis. Taken together, these results demonstrate that MctB is required by Mtb and M. smegmatis for Cu resistance and for maintaining low intracellular Cu levels.
To examine the specificity of MctB, we tested the susceptibility of the Mtb mctB mutant to other physiologically relevant transition metals such as Fe(III), Zn(II), Mn(II), and Ni(II) under similar conditions as for Cu(II). The chosen concentrations were at or above the concentrations previously determined in mycobacteria-containing phagosomes of infected macrophages (8). With the exception of copper, none of the tested metal ions impaired growth of the mctB mutant (SI Appendix, Fig. S9A). By contrast, growth of the mctB mutant was reduced by Cu(II) by two orders of magnitude, demonstrating that mctB is Cu-specific (SI Appendix, Fig. S9A).
Expression of the mymT gene encoding a copper-binding metallothionein of Mtb was induced up to 1,000-fold in response to copper and nitric oxide (12). Thus, an alternative explanation for the increase the Cu susceptibility of the mctB mutant ML256 could be that the lack of MctB might indirectly reduce mymT expression. However, a dot blot with a MymT-specific antibody revealed that mymT expression was strongly induced to similar levels in all three strains in the presence of 100 μM CuSO4 (SI Appendix, Fig. S10). In addition, agar dilution experiments also showed that the Mtb mctB mutant was not more susceptible to compounds that generate nitric oxide (SI Appendix, Fig. S9C), hydrogen peroxide (SI Appendix, Fig. S9D), or SDS (SI Appendix, Fig. S9B). Taken together, these experiments showed that mctB deletion does not cause a pleiotropic defect that would make Mtb more susceptible to reactive nitrogen and oxygen intermediates or cell-wall active compounds such as SDS, but rather it is the increased accumulation that makes the Mtb mctB mutant more susceptible to copper.
To assess the role of copper resistance in general and of MctB in the virulence of Mtb in particular, BALB/c mice were infected with aerosols containing Mtb H37Rv, the mctB mutant ML256, and the complemented mutant ML257. In the third week after infection, 10-fold fewer ML256 bacilli were detected compared with WT Mtb (SI Appendix, Fig. S11A). However, expression of mctB did not restore full virulence in the strain ML257, probably due to the sixfold lower MctB levels in ML257 compared with WT Mtb (Fig. 2B) and/or significantly lower initial inocula for the ML257-infected mice. Importantly, survival of the Mtb mctB mutant ML256 in the lungs of mice was severely compromised, resulting in a 100-fold decrease in bacterial burden compared with WT Mtb, in mice that were fed CuSO4 in their diet (SI Appendix, Fig. S11B). These observations indicate that the impaired survival of ML256 is a Cu-specific effect. Further support for the beneficial effect of Cu for mice infected with Mtb was provided by histopathology. Lung sections of mice infected with both WT Mtb and ML256 showed significantly fewer lymphocytic infiltrates and fewer overall lesions when the mice were fed additional Cu (SI Appendix, Fig. S12).
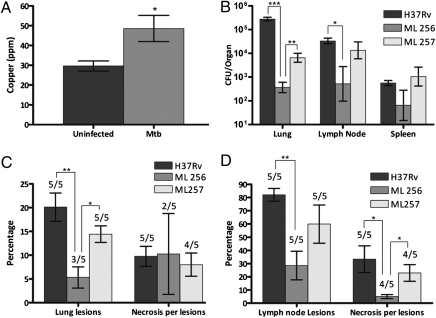
The finding that hypoxia induces Cu transport into macrophages and, thereby, increases their bactericidal potential (7, 16) and the fact that hypoxia does not exist in Mtb-infected mice (17) indicate that mice might not be a good animal model to examine the role of MctB in virulence of Mtb. By contrast, in guinea pigs, Mtb induces formation of discrete, granulomatous lesions with central necrosis and dystrophic mineralization similar to those of humans. We considered formation of these hypoxic granulomas as important for assessing the role of Cu in the mammalian immune system, because oxygen limitation was shown to stimulate Cu delivery to phagosomes containing bacteria (16). To examine the role of Cu in the response of guinea pigs to infection with Mtb, we isolated, microdissected and pooled granulomas from lungs of guinea pigs infected with WT Mtb H37Rv and analyzed trace minerals by flame atomic absorption spectrophotometry as described (18). The Cu concentration in isolated primary granulomas, where bacilli are concentrated, was significantly increased compared with unaffected lung parenchyma (Fig. 3A). These data show that Mtb is exposed to increased concentrations of Cu in vivo in lung lesions of guinea pigs and indicate that guinea pigs are a better animal model to evaluate the role of Cu resistance for virulence of Mtb. Therefore, guinea pigs were infected by low-dose aerosols with WT H37Rv, the isogenic ΔmctB mutant (ML256), or the complemented mutant ML257. Thirty days after infection, the bacterial burden of the ΔmctB mutant ML256 in the lungs of infected guinea pigs was reduced by >1,000-fold compared with WT Mtb. Low-level expression of mctB in ML257 partially complemented this virulence defect (Fig. 3B). The ΔmctB mutant was also significantly impaired in dissemination from the lung to the draining lymph nodes compared with the WT and complemented (ML257) strains, but was not affected in its ability to disseminate from the lung to the spleen (Fig. 3B). Despite its strong virulence defect, the ΔmctB mutant ML256 retained the ability to incite characteristic inflammatory lesions in the lung, lymph nodes and spleens of infected guinea pigs. Further, photomicrographs of representative lungs, lymph nodes, and spleens for ML256, ML257, and WT Mtb H37Rv show characteristic granulomatous inflammatory foci with central lesion necrosis (SI Appendix, Fig. S13, Fig. S14, and Fig. S15), indicating that the attenuation of the ΔmctB mutant did not significantly alter the host response compared with WT Mtb. Taken together these data show that the loss of MctB caused a drastic virulence defect of Mtb in guinea pigs, which is primarily due to a much lower bacterial burden in the lungs.
Fig. 3.
MctB-mediated copper resistance is required for virulence of M. tuberculosis in guinea pigs. (A) Cu concentrations in granulomatous lesions from guinea pigs infected with Mtb H37Rv. Tissue Cu was measured by atomic absorption spectroscopy in homogenates of lung parenchyma of five uninfected guinea pigs, and pooled primary granulomas were isolated by microdissection from lungs of five infected guinea pigs. *P ≤ 0.05. (B–D) Guinea pigs were infected with Mtb WT H37Rv (dark gray bars), the ΔmctB mutant ML256 (medium gray bars), and the complemented mutant ML257 (light gray bars). (B) The bacterial burden in lung, lymph node, and spleen was determined from tissue homogenates from guinea pigs 30 d after infection. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; number of infected animals per data point: n = 5. (C) Lung lesions were quantified from the area of total lung parenchyma affected by granulomatous inflammation. Data represents the mean percentage of affected lung for each treatment group or the mean percent area of lesions with necrosis. *P ≤ 0.05, **P ≤ 0.01; n = 5. (D) Lymph node lesions were quantified from the area of total lymph node parenchyma affected by granulomatous inflammation. Data represents the mean percentage of affected lymph node for each treatment group or the mean percent area of lesions with necrosis. *P ≤ 0.05, **P ≤ 0.01; n = 5.
Discussion
MctB (Rv1698, Ms3747) was discovered as an outer membrane channel protein with no homologs of known function (13). In this study, we show that MctB is required to maintain a nontoxic level of intracellular Cu in mycobacteria, implying that MctB is involved in Cu efflux. Support for this hypothesis is provided by the finding that mctB mutants are more susceptible to copper in contrast to porin mutants, which are typically more resistant to the transported solutes (19). Functionally similar proteins are known in Gram-negative bacteria. For example, the Cus efflux system is required for efficient Cu resistance by E. coli and consists of the inner membrane pump CusA, the outer membrane channel CusC, and the periplasmic membrane fusion protein CusB (20). Our finding that Ms3747 is required for resistance of M. smegmatis to Cu(I) and Ag(I) indicates that the main substrate of this efflux system is cytotoxic Cu(I) ions. Interaction of MctB with the putative Cu efflux pump CtpV (9) could provide the ion specificity and the energy for efflux against the concentration gradient and, hence, would explain the surprising specificity of the MctB pore for Cu(I) and Ag(I) despite its large single channel conductance of 4.3 nS (13). This principle is demonstrated by the tripartite metal and drug efflux systems in Gram-negative bacteria (21).
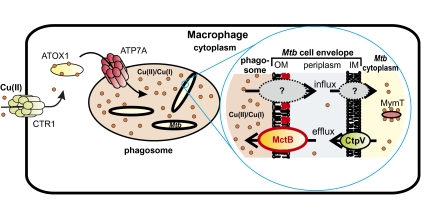
The minimal inhibitory concentration of Cu for Mtb is <24 μM (SI Appendix, Fig. S7), which is much lower than that of E. coli (≈3 mM) or of other bacteria (20). In addition, Cu appears to be bactericidal for Mtb (9) in contrast to M. smegmatis, whose growth is strongly delayed by high Cu concentrations (SI Appendix, Fig. S3), but eventually recovers to yield normal colony sizes. Importantly, Cu concentrations in the range of the minimal inhibitory concentration for Mtb have been determined in phagosomes of macrophages infected with M. avium and Mtb by microprobe X-ray fluorescence (8). Thus, Cu concentrations lethal to Mtb appear to exist in the phagosome of activated macrophages. These findings indicate that macrophages use Cu as a defensive weapon against Mtb and possibly other bacterial pathogens. Indeed, the Cu transporter ATP7A was required for full bactericidal activity of macrophages against E. coli (7). Interestingly, both IFN-γ and hypoxia stimulate trafficking of the Cu transporter ATP7A from the Golgi to phagosomes (7, 16). Hypoxia also increased expression of the Cu importer CTR1 (Fig. 4) and, hence, stimulated Cu uptake by macrophages resulting in increased Cu delivery to the ATP7A Cu transporter, in trafficking of ATP7A to cytoplasmic vesicles, and decreased expression of Cu-containing enzymes (16). Thus, upon stimulation with IFN-γ and under hypoxic conditions, both of which are crucial responses of the immune system to infection with Mtb (17, 22), macrophages increase Cu uptake and redirect the available Cu to ATP7A for delivery into phagosomes (Fig. 4).
Fig. 4.
Role of copper in bactericidal activity of macrophages and model of copper-resistance mechanisms of M. tuberculosis. Cu(II) is taken up across the plasma membrane of macrophages by the high-affinity Cu transporter Ctr1 (32). The Cu-chaperon Atox1 is a cytoplasmic Cu-binding and -transport protein that interacts with the Cu(II)-transporting ATPase Atp7A (33). In macrophages stimulated by IFN-γ or by hypoxia, cytoplasmic vesicles that contain Atp7A fuse with phagosomes (16). As a consequence, Cu is pumped into the phagosome, which contributes to its bactericidal activity (7). MctB is a pore-forming protein located in the outer membrane (OM) of Mtb (13) and prevents the accumulation of Cu within the mycobacterial cell probably by efflux of cuprous ions. Another important component of Cu homeostasis in Mtb is the cytoplasmic Cu-binding metallothionein MymT (12). The inner membrane (IM) transporter CtpV might be a Cu efflux protein (9, 11).
Considering the compelling in vitro data that Cu plays a key role in the bactericidal activity of macrophages, it is tempting to speculate that Cu resistance mechanisms of Mtb are important for bacterial virulence. However, previous studies showed only a modest increase in Cu susceptibility of Mtb in the absence of the copper-binding metallothionein MymT (12) and the putative inner membrane CtpV (11) and no or only a minor virulence defect in mice or guinea pigs. By contrast, the survival of the ΔmctB mutant ML256 in the lungs of guinea pigs was strongly impaired as compared with WT Mtb, resulting in a 1,000- and 100-fold reduced bacterial burden in lungs and lymph nodes (Fig. 3B). These results indicate that the role of MctB in Cu resistance of Mtb is less redundant than that of MymT or CtpV. Taken together, the increase of copper in the Mtb-containing granulomas of guinea pigs and the infection experiments in this study strongly indicate that Cu is used by mammalian immune systems to control growth of Mtb and demonstrate that, in turn, Cu resistance mechanisms are crucial for virulence of Mtb. The smaller virulence defect of the ΔmctB mutant in mice is likely due to the fact that mice do not form hypoxic granulomas (17). Because granuloma formation is a hallmark of the human immune response to infections with Mtb (22, 23), these findings also indicate that Cu might play a crucial role in humans infected with Mtb.
This study demonstrates that Cu resistance is crucial for survival of Mtb in the mammalian host. Based on the findings of this study, we propose the following model of how the mammalian immune system might control Mtb by using a Cu-dependent mechanism (Fig. 4). Macrophages generate an oxidative burst against intracellular pathogens that includes the release of hydrogen peroxide (24). Hydrogen peroxide, in contrast to other reactive oxygen species, can diffuse well through membranes (25). The immune system responds to infection with Mtb by secretion of IFN-γ by CD4+-T cells (26) that, in addition to other reactions, stimulates expression of the high-affinity copper importer CTR1 in macrophages (7). Hypoxic conditions, a hallmark of granulomas formed in humans after lung infection with Mtb, induce CTR1 expression and the chaperone ATOX1 and the copper transporter ATP7A (16) redirect Cu traffic to the Mtb-containing phagosome. Higher concentrations of Cu increases the formation of toxic hydroxyl radicals from hydrogen peroxide, a reaction that is catalyzed by Cu(I) (3). To protect itself from toxic effects of free Cu(I) Mtb utilizes at least two resistance mechanisms (Fig. 4), which might be partially redundant as observed for E. coli (15): sequestration of Cu by the metallothionein MymT (12) and probably efflux by the inner membrane transporter CtpV (11). The channel protein MctB might interact with CtpV to enable efflux of Cu across the outer membrane of Mtb. The observation that MctB is required for virulence of Mtb in guinea pigs suggests that the binding capacity of MymT might be eventually overwhelmed in the absence of MctB.
This study revealed an alternative target for tuberculosis chemotherapy, in which MctB might be inactivated by a drug and, thereby, makes Mtb more vulnerable to Cu ions used by macrophages to kill Mtb. Such an approach may lead to a new class of anti-infectives that could sensitize pathogens to host immune responses by inhibiting bacterial defense mechanisms as recently described by Carl Nathan and colleagues (27). Targeting MctB might be a promising strategy because the surface accessibility of MctB (28) may enable putative inhibitors to reach its target directly without the need to cross the notoriously impermeable outer membrane of Mtb, a major determinant of intrinsic drug resistance (29).
Materials and Methods
Bacterial Strains and Growth Conditions.
The strains used in this study are listed in SI Appendix, Table S3. Media and growth conditions are described in SI Appendix.
Plasmid Construction.
Construction of the plasmids is described in SI Appendix, Table S2.
Construction of the ΔmctB Mutants of M. smegmatis and Mtb.
The mctB mutants of M. smegmatis (ms3747) and Mtb (rv1698) were constructed by using a two-step selection strategy as described in SI Appendix.
Preparation and Analysis of Protein Extracts.
An overnight culture of M. smegmatis grown in self-made 7H9 (7H9sm) medium was used to inoculate 7H9sm medium with or without copper. Proteins were extracted from M. smegmatis by using 2% SDS in PBS at 40 °C as described (13). Proteins were analyzed in Western blots by using specific antibodies raised against MctB (5D1.23), MymT (kindly provided by Ben Gold), and horseradish peroxidase-coupled anti-mouse or anti-rabbit secondary antibodies as described (30). For further details, see SI Appendix.
Metal Susceptibility Assays.
To determine the susceptibility of WT M. smegmatis and ML77 to copper, self-made 7H10 (7H10sm) plates were prepared containing different concentrations of CuSO4. The strains were grown in 7H9sm medium without Cu overnight. The cultures were filtered through a 5-μm filter to obtain single-cell suspensions. Plates with appropriate dilutions were incubated at 37 °C. The susceptibility of M. smegmatis to silver, mercury, and cobalt was measured on Mueller-Hinton agar plates to prevent formation of water-insoluble salts. The concentration of AgNO3, HgCl, and CoCl2 in these plates was 1.6, 8, 40, and 200 μM. Copper sulfate was used as a control. Mtb was grown in 7H9/OADC medium for 7 d. Appropriate dilutions were dropped on 7H11 agar plates containing CuSO4, ammonium iron (III) citrate, MnCl2, ZnCl2, and NiCl2 at the indicated concentrations.
Copper Accumulation.
Precultures of M. smegmatis strains were grown in 7H9sm medium without copper. Then, three cultures of each M. smegmatis strain were inoculated in 7H9sm medium with 0, 6.3, or 25 μM CuSO4 and shaken at 37 °C. Samples were taken 36 h after inoculation. Precultures of each Mtb strain were grown in HdB medium (30 mL) with 0.8 μM CuSO4 and used to inoculate triplicate cultures for each Cu concentration by using the same medium (25 mL). All Mtb cultures were incubated for 8 d at 37 °C before the CuSO4 concentration was either kept at 0.8 μM or adjusted to 6.3 or 25 μM. Samples were taken after 10 d. Generally, samples were centrifuged at 3,250 × g and washed twice in the appropriate medium without added Cu. For Mtb, the washed pellets were resuspended in 0.5 mL of ultra-pure water and boiled for 1 h to inactivate Mtb. All samples were dried at 60 °C overnight under vacuum. Dried pellets were suspended in 600 μL of H2O and sonicated for 20 min in a sonicator bath. The Cu content of the cells was analyzed by using the photometric dithizone assay as published (14). For details, see SI Appendix.
Experimental Infections in Mice.
A preliminary Cu tolerance study was performed in which three groups of BALB/c mice (Charles River) were given water ad libitum containing 118 mg/L, 1180 mg/L, or 11.8 g/L Cu sulfate pentahydrate (Sigma). The mice were monitored for 2 wk. A concentration of 118 mg/L Cu sulfate pentahydrate did not cause any signs of distress in mice and was chosen for further experiments.
Midlog phase cultures of WT Mtb, the mctB mutant ML256, and the complemented mutant ML257 were diluted to OD600 ≈ 0.08 to implant 500–1,000 bacilli in the lungs of mice by using a Middlebrook inhalation exposure system (Glas-Col). Eighty 4- to 6-wk-old BALB/c mice were infected with WT Mtb H37Rv or ML256, and 40 mice were infected with ML257. One day after infection, half of the infected mice were separated and given water ad libitum containing 118 mg/L Cu sulfate pentahydrate. Four mice from each group were weighed and killed at days 1, 7, 14, 28, 84, and 112 after infection to determine the number of bacilli in the lung and spleen. Mouse organs were aseptically removed, homogenized, and serially diluted. Appropriate dilutions were plated onto Middlebrook 7H11 agar plates to determine the colony-forming units. For histological analysis, representative tissue samples from each group were fixed in 10% formaldehyde, embedded in parraffin, sectioned, and stained with hematoxylin and eosin by using standard procedures.
Experimental Infections in Guinea Pigs.
Guinea pigs were infected with M. tuberculosis strains H37Rv, ML 256, and ML257 by using an aerosol chamber, and determination of the bacterial load and histological analysis were done as described (31). Five animals were analyzed at each time point. For further details see SI Appendix. Grossly visible primary lung lesions for chemical analysis were dissected from paraformaldehyde fixed lungs of five guinea pigs infected with Mtb. Lungs were embedded in low melting point agar and sectioned into 3-mm slices. A subset of slices was selected by uniform random sampling. From those slices, individual primary lesions were dissected from lungs and pooled (>1 gram per animal). Equal quantities of samples collected in a similar fashion from paraformaldehyde-fixed lungs of five noninfected animals were used as negative controls. Pooled tissue samples from each animal were dried overnight in a drying oven at 85 °C, weighed to determine total dry weight per sample, and ashed overnight in a muffle furnace at 600 °C. The ashed samples were allowed to cool and then dissolved in nitric acid. The solutions were sonicated to complete dissolution. The resulting acid solution was diluted with deionized water for Cu analyses. Copper concentrations were determined via flame atomic absorption spectroscopy as described (19). The values for each of the five animals were averaged, and tissue concentrations were reported as parts per million of pooled samples on a dry weight basis.
Supplementary Material
Acknowledgments
We thank Mamadou Daffe for lipid analysis, Adrie Steyn for providing the plasmids pCreSacB1 and pBS346; Ben Gold for providing MymT peptide and MymT-specific antibodies; and Jennifer Rowland, Jason Huff, and Ursula Niederweis for editing the manuscript. The Tuberculosis Animal Research and Gene Evaluation Taskforce program was supported by the National Institutes of Health Contract N01-AI30036. This work was supported by National Science Foundation Grant “Experimental Program to Stimulated Competitive Research” 4166 (to S.H.B.) and National Institutes of Health Grants AI083856 (to R.J.B.), AI083632 (to M.N.), and AI063432 (to M.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009261108/-/DCSupplemental.
References
- 1.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crichton RR, Pierre JL. Old iron, young copper: From Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 5.Prohaska JR, Lukasewycz OA. Copper deficiency suppresses the immune response of mice. Science. 1981;213:559–561. doi: 10.1126/science.7244654. [DOI] [PubMed] [Google Scholar]
- 6.Newberne PM, Hunt CE, Young VR. The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br J Exp Pathol. 1968;49:448–457. [PMC free article] [PubMed] [Google Scholar]
- 7.White C, Lee J, Kambe T, Fritsche K, Petris MJ. A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J Biol Chem. 2009;284:33949–33956. doi: 10.1074/jbc.M109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner D, et al. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell's endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 9.Ward SK, Hoye EA, Talaat AM. The global responses of Mycobacterium tuberculosis to physiological levels of copper. J Bacteriol. 2008;190:2939–2946. doi: 10.1128/JB.01847-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, et al. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007;3:60–68. doi: 10.1038/nchembio844. [DOI] [PubMed] [Google Scholar]
- 11.Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: A putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol Microbiol. 2010;77:1096–1110. doi: 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold B, et al. Identification of a copper-binding metallothionein in pathogenic mycobacteria. Nat Chem Biol. 2008;4:609–616. doi: 10.1038/nchembio.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siroy A, et al. Rv1698 of Mycobacterium tuberculosis represents a new class of channel-forming outer membrane proteins. J Biol Chem. 2008;283:17827–17837. doi: 10.1074/jbc.M800866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar B, Singh HB, Katyal M, Sharma RL. Spectrophotometric and derivative spectrophotometric determination of copper (II) with dithizone in aqueous phase. Microchim Acta. 1991;105:79–87. [Google Scholar]
- 15.Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev. 2003;27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 16.White C, et al. Copper transport into the secretory pathway is regulated by oxygen in macrophages. J Cell Sci. 2009;122:1315–1321. doi: 10.1242/jcs.043216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Via LE, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basaraba RJ, et al. Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis (Edinb) 2008;88:69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke S, Grass G, Rensing C, Nies DH. Molecular analysis of the copper-transporting efflux system CusCFBA of Escherichia coli. J Bacteriol. 2003;185:3804–3812. doi: 10.1128/JB.185.13.3804-3812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 22.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 23.Ulrichs T, Kaufmann SH. New insights into the function of granulomas in human tuberculosis. J Pathol. 2006;208:261–269. doi: 10.1002/path.1906. [DOI] [PubMed] [Google Scholar]
- 24.Miller RA, Britigan BE. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004;84:93–101. doi: 10.1016/j.tube.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C, et al. A philosophy of anti-infectives as a guide in the search for new drugs for tuberculosis. Tuberculosis (Edinb) 2008;88:S25–S33. doi: 10.1016/S1472-9792(08)70034-9. [DOI] [PubMed] [Google Scholar]
- 28.Song H, Sandie R, Wang Y, Andrade-Navarro MA, Niederweis M. Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2008;88:526–544. doi: 10.1016/j.tube.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederweis M, Danilchanka O, Huff J, Hoffmann C, Engelhardt H. Mycobacterial outer membranes: In search of proteins. Trends Microbiol. 2010;18:109–116. doi: 10.1016/j.tim.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephan J, et al. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol Microbiol. 2005;58:714–730. doi: 10.1111/j.1365-2958.2005.04878.x. [DOI] [PubMed] [Google Scholar]
- 31.Ordway DJ, et al. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob Agents Chemother. 2010;54:1820–1833. doi: 10.1128/AAC.01521-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134:1003–1006. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- 33.Hamza I, Prohaska J, Gitlin JD. Essential role for Atox1 in the copper-mediated intracellular trafficking of the Menkes ATPase. Proc Natl Acad Sci USA. 2003;100:1215–1220. doi: 10.1073/pnas.0336230100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.