Abstract
The majority of B lymphocytes in the adult mouse are generated in the bone marrow from hematopoietic stem cells (HSCs) that first appear in the aorta-gonado-mesonephros region of the fetus on embryonic day (E) 10.5–11. Comparatively less is known about B-cell development during embryogenesis. For example, which specific embryonic tissues participate in B lymphopoiesis and whether hematopoietic differentiation is skewed toward specific B-cell subsets in the embryo are unanswered questions, because the systemic circulation is initiated early during embryogenesis, resulting in an admixture of cells potentially originating from multiple sites. We demonstrate, using Ncx1−/− mice that lack systemic blood circulation, that the E9 yolk sac (YS) and the intra-embryonic para-aortic splanchnopleura (P-Sp) tissues independently give rise to AA4.1+CD19+B220lo-neg B progenitor cells that preferentially differentiate into innate type B-1 and marginal zone (MZ) B cells but not into B-2 cells upon transplantation. We have further demonstrated that these B-1 progenitor cells arise directly from YS and P-Sp hemogenic endothelium. These results document the initial wave of innate B lymphopoietic progenitor cells available for seeding the fetal liver at E11. The results of these studies expand our knowledge of hemogenic endothelial sites specifying distinct B-1 and MZ cell fates apart from B-2 cells and independent of an HSC origin during development.
Keywords: B-1 cell, marginal zone B cell, OP9 stromal cells, hematopoiesis
Hematopoiesis in the mouse embryo begins at embryonic day (E) 7.0 in the yolk sac (YS) (1–3). Both primitive and definitive erythro-myeloid progenitor cells are produced in this site by E10 (4). Hematopoietic stem cells (HSCs) that reconstitute all blood lineages in lethally conditioned adult mice first emerge at E10.5 in the aorta-gonado-mesonephros (AGM) region (5) with subsequent expansion in the fetal liver and other hematopoietic sites (6). Eventually, these HSCs migrate to the bone marrow (BM) where they maintain hematopoiesis, including production of B lymphocytes, throughout adult life. Although the role of HSCs in B-cell development in postnatal BM is well defined, comparatively less is known about the tissues involved in this process in the embryo and whether development is skewed to the production of specific B-cell subsets.
In this regard, murine B lymphocytes can be segregated into two main populations referred to as B-1 and B-2 cells. B-2 cells develop in postnatal BM from definitive HSCs and mature through transitional stages in the spleen before generating follicular (FO) B cells. FO cells are the predominant B-cell population in the adult that, in response to T-cell help, become activated, undergo class switching, and secrete Ig of multiple isotypes (7). B-1 cells, which are subdivided into CD5+ B-1a and CD5– B-1b subsets, are part of the innate immune system and preferentially localize in the pleural and peritoneal cavities to spontaneously secrete antibodies of limited specificity in a T cell-independent manner. B-1 cells were long hypothesized to be of fetal origin, and whether B-1 and B-2 cells have a distinct progenitor or not has been debated over decades (8). The recent description of a lin−AA4.1+CD19+B220lo-neg fetal liver B-1–specific progenitor cell has provided supportive evidence (9) for these hypotheses. Marginal zone (MZ) B cells, so named because of their localization in the splenic follicle, are functionally similar to B-1 cells in that they secrete IgM antibodies in response to blood-borne pathogens in a T cell-independent manner. Whereas MZ cells are thought to be HSC-derived B-2 cells generated in parallel with FO cells, it has recently been suggested that some are fetal derived (10–12), raising a question of the temporal and spatial origin of this B-cell subset.
The near simultaneous appearance of lin−AA4.1+CD19+B220lo-neg B-1 progenitor cells in E11 fetal liver (9, 13) with the known paucity of E11 HSCs in the whole conceptus [one to three HSCs among all hematopoietic sites at E11 (14, 15)] has raised the possibility that these cells are generated in a pre-HSC wave of hematopoiesis. However, little is known about when these progenitors first emerge and the tissue(s) in which this occurs. Evidence to support B-1 cell emergence from para-aortic splanchnopleura (P-Sp) tissue but not the YS has been previously reported (16). However, B lymphoid potential in the YS and P-Sp has long been controversial mainly because of the cell admixture that arises following onset of the systemic circulation (E8.25) in the embryos (17–21). To address this issue, we examined hematopoietic development in Ncx1−/− embryos (22) that fail to initiate a heartbeat and die at E11, providing a circulation-free environment for a short period of development. Using this approach, we demonstrate that the YS, along with the P-Sp, are autonomous sites of B-cell production. Our data further demonstrate that B lymphopoiesis in these tissues initiates from hemogenic endothelium and that the initial wave of development is biased toward the production of progenitor cells that reconstitute cells with B-1 and MZ characteristics, not B-2 cells, upon transplantation.
Results
YS and P-Sp Contain B-1 and MZ B-Cell Progenitors.
As a first approach to determining whether YS and P-Sp can generate B cells, we injected cells derived from both tissues of E9.5 [20–24 somite pairs (sp)] Ncx1−/− and wild-type (WT) embryos, with WT E15.5 fetal liver cells as a control, directly into the peritoneal cavity of sublethally irradiated CD45.1+NOD/SCID/IL2γcnull (NOG) neonates. As expected, E15.5 fetal liver cells exhibited multilineage reconstitution of B, T, and myeloid lineages (Fig. S1), indicating the integrity of our reconstitution protocol. In contrast, E9.5 YS or P-Sp preferentially generated CD45.2+IgMhighCD11b+CD5+ B-1a and CD45.2+IgMhighCD11b+CD5− B-1b cells, and a scant number of B-2 cells, in the peritoneal cavity of recipient mice but no other lineages of hematopoietic cells (Figs. 1A and 2 A and B and Table S1). Although few if any FO cells were derived from transplanted YS and P-Sp cells, a significant number of donor-derived cells with an IgMhighAA4.1−CD23−CD21high MZ cell phenotype were observed in the spleen (Figs. 1B and 2C). Thus, both E9.5 WT YS and P-Sp possess B-1 and MZ cell progenitor cell activity that can reconstitute these lymphocyte subsets in neonatal NOG recipients. In contrast to WT embryonic tissues, freshly isolated Ncx1−/− YS and P-Sp cells failed to reconstitute B-1 or MZ cells in the recipient mice.
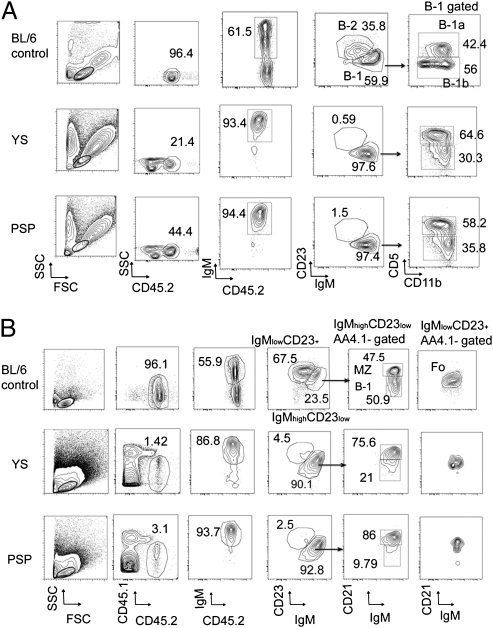
Fig. 1.
Representative phenotype of peritoneal and spleen cells in mice reconstituted with WT E9.5 YS and P-Sp cells. (A) Phenotypic analysis of B-1 and B-2 cells in the peritoneal cavity of nontransplanted control C57BL/6 mice (Top) and recipients of YS (Middle)- and P-Sp (Bottom)–derived cells. (B) Phenotypic analysis of B-1, FO B-2, and MZ cells in the spleen of nontransplanted control C57BL/6 mice (Top) and recipients of YS (Middle) and P-Sp (Bottom) cells. Lymphoid gate was determined from the forward scatter (FSC)/ side scatter (SSC) panel of WT BL/6 control peritoneal cells. The E9.5 WT YS and P-Sp cells were injected into 150 cGy-irradiated NOG neonates immediately after isolation. The number of animals examined in each group is listed in Table S1.
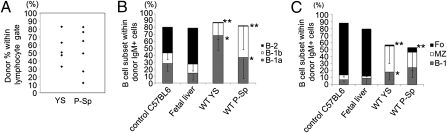
Fig. 2.
Progenitors that reconstitute B-1 and MZ cells are present in YS and P-Sp. The E9.5 WT YS and P-Sp cells were injected into 150 cGy-irradiated NOG neonates immediately after isolation. (A) The level of donor chimerism in the peritoneal cavity of recipient mice (CD45.1+) that received YS- and P-Sp–derived cells (CD45.2+) is depicted. (B) B-cell subsets within donor IgM+ cells in the peritoneal cavity of recipient mice. The percentage of B-1 cells in the peritoneal cavity of YS or P-Sp reconstituted mice was significantly higher than that observed in the peritoneum of nontransplanted adult C57BL/6 mice or E15.5 fetal liver reconstituted mice (P < 0.05). The percentage of B-2 cells in the peritoneal cavity of YS and P-Sp reconstituted animals was significantly less than that observed in the peritoneum of nontransplanted adult control mice or the E15.5 fetal liver reconstituted mice (P < 0.01). The IgMlowIgDhigh or IgMlowCD23high cells were defined as B-2 cells. (C) B-cell subsets within donor IgM+ cells in the spleen. In addition to B-1 cells, the spleen of mice reconstituted with YS and P-Sp cells also contained donor-derived MZ cells. The percentage of FO cells in the spleen of YS and P-Sp transplanted animals was significantly less than nontransplanted control C57BL/6 mice or fetal liver reconstituted mice (P < 0.01). The percentage of MZ cells in the spleen of YS and P-Sp transplanted mice was significantly higher than nontransplanted control mice or E15.5 fetal liver reconstituted mice (P < 0.05). The number of animals examined in each group is listed in Table S1.
YS and P-Sp Cells Can Independently Generate B-1 Progenitor Cells.
B-1 developmental potential in fetal liver and BM has been previously associated with a lin−AA4.1+CD19+B220lo-neg progenitor. To determine if these progenitors were present in YS and P-Sp, we tested E9.0–9.5 (14–24 sp) WT YS and P-Sp cell suspensions for evidence of cells expressing the CD19 B cell marker but found very few “events” even from a total of 40 embryos (Fig. S2). Thus, at this gestational age, B lineage progenitors that may be present are below the limits of detection by flow cytometry.
We subsequently used an in vitro system to investigate the potential of E9.0–9.5 YS and P-Sp to generate lin−AA4.1+CD19+B220lo-neg cells. In these experiments, cultures were initiated with YS and P-Sp cells from WT and Ncx1−/− littermates. Because the systemic circulation is normally established in WT embryos by day 8.25, the use of Ncx1−/− embryos lacking circulating blood allowed us to determine the cell-autonomous potential of each tissue to generate B cells. YS and P-Sp cells were plated separately (1/2 embryo equivalent per well) on a confluent layer of OP9 stromal cells established in wells of six-well plates in medium supplemented with IL-7 and Flt-3 ligand. Eight to 10 d after culture initiation, one to two large cobblestone areas per each embryo equivalent of YS and P-Sp were observed (Fig. 3C). Nonadherent cells were generated from these foci, and they exhibited an AA4.1+CD19+B220lo-neg phenotype (Fig. 3A). Few if any cells with an AA4.1+CD19−B220+ B-2 progenitor phenotype were observed (Fig. 3A, Left). Genotyping of cells from the Ncx1−/− cultures confirmed that the CD19+B220low cells were Ncx1−/− YS/P-Sp derived and not the product of maternal blood cell contamination (Fig. 3B). Some AA4.1− cells were observed in the cultures, and these were primarily Mac1+ myeloid cells (Fig. S3A). AA4.1+CD19+B220lo-neg cells were also successfully generated from four to six sp (precirculation stage) YS and P-Sp cells (Fig. 3D).
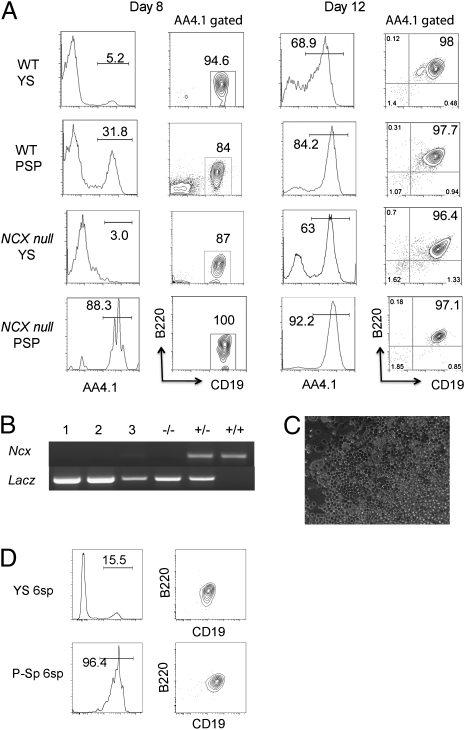
Fig. 3.
E9.0–9.5 Ncx1−/− YS and P-Sp can produce B-1 progenitor cells in vitro. (A) Cells from YS and P-Sp were isolated and placed in culture on OP9 stromal cells as described (19). At day 8, YS and P-Sp cells from WT and Ncx1−/− mice generated AA4.1+CD19+B220lo-neg B-1 progenitor cells. Cells with an AA4.1+CD19−B220+ B-2 progenitor phenotype were not detected. At day 12, the cultured cells became CD19+B220+. (B) Genotyping of cells from Ncx1−/− YS cocultures (lanes 1–3) and control for Ncx1−/−, Ncx1+/−, and WT embryos. All mutants express a Lac z transgene. (C) Cobblestone appearance of cells cultured for 12 d. An image of Ncx1−/− YS cell culture is depicted (200×). (D) E8.25 (four to six sp) YS and P-Sp cells can generate AA4.1+CD19+B220lo-neg cells in OP9 culture (representative of three independent experiments). Cell analysis was performed 8 d after culture initiation.
With increasing time in culture, the AA4.1+CD19+B220lo-neg cells acquired a AA4.1+CD19+B220+ phenotype (Fig. 3A, Right and Fig.S3B), suggesting a precursor–progeny relationship. In general, the frequency of AA4.1+CD19+B220+ cells produced in cultures from Ncx1−/− YS and P-Sp was lower compared with their WT counterparts (Table 1). Considering the fact that freshly isolated Ncx1−/− YS and P-SP failed to reconstitute B-1 or MZ B cells in the recipient mice, but that B progenitor potential was rescued in OP9 culture, these results are consistent with our prior report stating that the lack of blood flow in the Ncx1−/− embryonic tissues results in diminished production of blood cells overall, but does not prevent the emergence of hematopoietic potential (23).
Table 1.
Frequency of AA4.1+CD19+B220+ cell production by YS and P-Sp in vitro
| Frequency of AA4.1+CD19+B220+ cell production per tested embryos (%) |
||
| E9.0 (14–19 sp) | E9.5 (20–24 sp) | |
| WT YS | 4/7 (57.1) | 12/20 (60) |
| WT P-Sp | 3/6 (50) | 15/21 (71.4) |
| NCX1−/− YS | 3/23 (13) | 4/19 (21) |
| NCX1−/− P-Sp | 5/19 (26.3) | 8/20 (40) |
All of the cells were collected at day 12–14 of culture and analyzed by FACS. The number of embryos that produced AA4.1+CD19+B220− cells per total tested embryos is shown.
Lin−AA4.1+CD19+B220lo-neg Cells Differentiate into B-1 and MZ B Cells in Vivo.
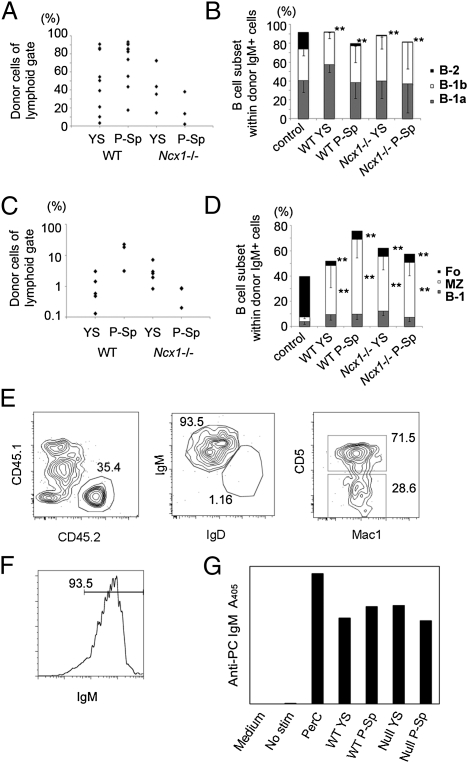
To confirm that the AA4.1+CD19+B220+ cells derived from YS and P-Sp were in fact B-1 progenitors, we injected them into neonatal NOG mice (Fig.S4A). Cells with the same phenotype from adult BM were transplanted in parallel (Fig.S4B). WT and Ncx1−/− YS- and P-Sp–derived CD45.2+donor cells engrafted in both the peritoneal cavity and spleen (Fig. 4 A and C and Table S2), but BM engraftment and the generation of T and myeloid cells was not observed (CD45.2+ cells in the BM were less than 0.01% for each lineage). Whereas adult BM-derived AA4.1+CD19+B220+ cells reconstituted mainly B-2 cells in the peritoneal cavity (Fig. S4B), YS- and P-Sp–derived cells showed scant B-2 or FO cell engraftment in these recipients (Fig. 4 B and D), and this was the case when recipients were examined at early (5 wk) and late (6 mo) time points post reconstitution. YS- and P-Sp–derived IgM+ B cells were present in the peritoneal cavity (Fig. S4C) and included IgMhighIgDlowMac1+CD5+ B-1a and IgMhighIgDlowMac1+CD5− B-1b cells (Fig. 4B). Interestingly, E9-9.5 YS- and P-Sp–derived AA4.1+CD19+B220+ cells generated AA4.1−IgMhighCD23−CD21high cells in the spleen, which is consistent with a MZ cell phenotype (Fig. 4D). The fact that these cells were generated from Ncx1−/− YS and P-Sp further demonstrates that intrinsic MZ cell developmental potential is associated with these tissues and that these data co-associate B1- and MZ-cell potential in the absence of B-2 potential to a defined phenotype of YS- and P-Sp–derived cells.
Fig. 4.
AA4.1+CD19+B220+ cells derived from Ncx1−/− YS and P-Sp are functional. E9-9.5 WT and Ncx1−/− YS or P-Sp were cultured in vitro, and 12 d later AA4.1+CD19+B220+ cells from the culture were injected into neonatal NOG mice. Recipients were examined 5 wk to 6 mo later (data presented are from 10 to 12 wk after injection). (A) The percentage of WT and Ncx1−/− donor YS- and P-Sp–derived cells in the recipient peritoneal cavity. (B) B-cell subsets within donor IgM+ cells in the peritoneal cavity. WT and Ncx1−/− YS- and P-Sp–derived cells reconstituted B-1 cells and a few B-2 cells (P < 0.01) in the peritoneal cavity. (C) The percentage of WT and Ncx1−/− donor YS- and P-Sp–derived cells in the recipient spleen. (D) B-cell subsets within donor IgM+ cells in the spleen. WT and Ncx1−/− YS- and P-Sp–derived cells primarily reconstituted MZ (P < 0.01) and B-1 cells, but not FO B-2 B cells (P < 0.01) in the spleen. Peritoneal (B) and spleen (D) cells from nontransplanted adult C57BL/6 mice are included as controls. (E) Peritoneal cells were harvested from the primary recipient NOG mice that had been reconstituted 4 mo previously with AA4.1+CD19+B220+ cells (CD45.2+) derived from E9.5 WT YS and P-Sp that emerged in vitro. Cells were then injected into the peritoneal cavity of 300 cGy-irradiated secondary adult NOG recipients (CD45.1+). Two to 4 mo after secondary transplantation, peritoneal cells were analyzed. Donor (CD45.2+ YS or P-Sp)-derived cells were detected as IgMhighIgDlowCD5+B-1a and IgMhighIgDlowCD5− B-1b cells in the secondary recipient animals (YS-derived cell transplanted: n = 3; P-Sp–derived cell transplanted: n = 4). Representative FACS plots are depicted. (F) Cells in the culture expressed surface IgM+ and were expanded upon antigen stimulation with PC to (G) secrete anti-PC–specific IgM antibodies in culture supernatants that were detected by ELISA (representative data from three experiments). PerC: peritoneal cells from C57BL/6 mouse as a positive control.
Progeny of lin−AA4.1+CD19+B220lo-neg Progenitors Are Functional.
Next, we interrogated the functional potential of the B-1 cells that were produced in two ways. First, we tested the potential of these cells to self-renew, a distinguishing property of B-1 cells. Peritoneal cells were transplanted from primary into secondary adult recipient NOG mice. Analysis of these mice 4 mo later revealed the presence of a significant number of primary donor-type B-1 cells (Fig. 4E). Thus, the B-1 progenitor cells derived from E9.0–9.5 (14–24 sp) YS and P-Sp tissue can give rise to B-1 cells that reside long term in primary and secondary recipient animals. Finally, we examined T cell-independent antibody production from the in vitro-generated B cells. Phosphorylcholine (PC) is a common antigen recognized by B-1 cells, and anti-PC IgM is one of the natural antibodies secreted by B-1 cells. We sorted CD19+B220+ cells from the above cultures and stimulated them with PC for 7 d. During this time, the cultured cells matured to surface IgM expression (Fig. 4F), secreted anti-PC–specific IgM (Fig. 4G). Thus, the AA4.1+CD19+B220+ cells derived from E9.5 YS and P-Sp exhibited characteristics consistent with B-1 cells.
B-1 Progenitors Can Be Generated from Hemogenic Endothelium.
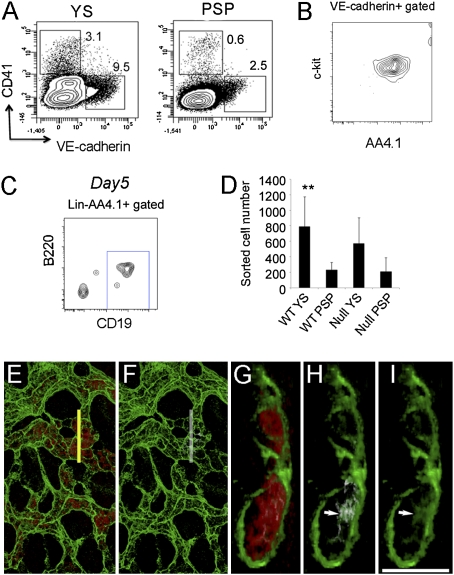
Lin−AA4.1+CD19+B220lo-neg B-1 progenitors have been identified in the E11.0 liver (9, 13), at which time only one to three HSCs are present in the entire conceptus (14, 15). This timing raises the possibility that the initial wave of B-1 progenitor production may not be HSC derived. Instead, we asked if B-1 progenitors could be generated from hemogenic endothelium in view of reports that endothelial cells expressing VE-cadherin or Tie-2+CD41− (24, 25) in E8.5–9.5 embryos have lymphoid potential (19, 21) and our results showing that AA4.1+CD19+B220lo-neg cells can be generated from E8.25 YS and P-Sp (Fig. 3D). We therefore initiated in vitro cultures with VE-cadherin+CD41− hemogenic endothelium derived from E9.0–9.5 (14–24 sp) WT and Ncx1−/− YS and P-Sp and determined whether B-1 progenitors could be produced (Fig. 5A). The VE-cadherin+CD41− cells plated coexpressed AA4.1 and c-kit (Fig. 5B), which were previously reported as markers for a lymphohematopoietic progenitor cell (26). VE-cad−CD41+ hematopoietic cells were tested in parallel. The VE-cad−CD41+ hematopoietic cells (Fig. 5A) failed to produce B cells and instead generated only erythroid cells and some myeloid cells (Fig. S5A). In striking contrast, AA4.1+CD19+B220lo-neg cells were generated from the VE-cad+CD41− hemogenic endothelium (Fig. 5C). At a later stage of development (>27 sp), YS- and P-Sp–derived VE-cad−CD41+ cells display B lymphoid potential (Fig. S5B), similar to findings previously reported (26). This observation may reflect the “endothelial-hematopoietic transition” that has been reported for emergence of HSCs at later stages (27).
Fig. 5.
B-1 B progenitor cells are derived from VE-cadherin+CD41− hemogenic endothelial cells. (A) VE-cadherin+CD41− (endothelial) cells or VE-cadherin−CD41+ (hematopoietic) cells were sorted from E9.5 WT and Ncx1−/− YS and P-Sp cells and plated on OP9 with IL7 and flt-3 ligand. WT FACS dot plots are depicted. Ncx1−/− embryos displayed a similar phenotype. (B) All VE-cadherin+CD41− cells coexpressed AA4.1 and c-kit (n = 3). (C) After 5 d of culture, VE-cadherin+CD41− cells from Ncx1−/− YS produced AA4.1+CD19+B220dim cells in vitro (representative of four experiments). (D) The number of VE-cadherin+ cells obtained from one embryo equivalent (1 e.e.) is depicted. WT YS VE-cad+ cell number was significantly higher than WT P-Sp cell number (P < 0.01). (E–I) Emergence of CD19+ (white, white arrow) cells was detected as a small regionalized population of hematopoietic cells (red) within the E9.0 YS VE-cadherin+ vasculature (green). (E) Image of the E9.0 yolk sac showing hematopoietic cells (red) within the VE-cadherin+ (green) vasculature. (F) Same region as in E depicting small CD19dim (white) population. (G) Orthogonal α-projections (4× zoom) of the region indicated by the yellow line in E. (h and I) Arrows indicate rare CD19+ (white) VE-cadherin (green) double-positive cells. (I) VE-cad+ cells (green) are highlighted. (Scale bar: 100 μm in E and F and 25 μm in G–I.)
The numbers of sorted viable VE-cad+ cells recovered from each tissue (one embryo equivalent) were limiting (Fig. 5D), and at least 1,000 viable pooled VE-cad+CD41− cells were required for producing the B-1 B progenitors in the OP9 coculture. Thus, it appears that there are few B lineage cells in each tissue at the stages of development examined. To investigate this in more detail, we examined whole YS for the expression of CD19. Although we could not detect CD19+ cells in cell suspensions of E9.0 YS and P-Sp by flow cytometry, small clusters of CD19dim-expressing cells were detected in the VE-cad+ vascular network (Fig. 5 E–I). This observation, along with the data in Fig. 5C, suggests that B-cell progenitors emerge directly from hemogenic endothelium.
Discussion
HSCs, capable of stably repopulating all lymphoid and myeloid cells in irradiated recipients, appear at approximately day 10.5 of gestation in the AGM region (5, 6). However, blood cell production in various extra- and intra-embryonic tissues of the fetus initiates before this time (4, 28) and results in the generation of uni- and multipotential progenitors (1, 2, 6, 16–18, 29, 30). Numerous gaps in our understanding of B-cell development during this pre-HSC stage of fetal hematopoiesis remain. In particular, the identity of tissues with intrinsic B-cell developmental potential has been controversial, and little attention has been given to the types of B cell generated. In this study, we show that both YS and P-Sp have autonomous potential to generate B cells and that the first wave of fetal B-cell production is associated with the production of innate-type B cells without a B-2 cell phenotype at a pre-HSC stage.
Elucidating when and in which tissues fetal B lymphopoiesis initiates has been complicated by two significant technical obstacles. First, the early advent of the fetal circulation makes it difficult to distinguish tissues with intrinsic B-cell developmental potential from those that have been seeded by circulating progenitors. We addressed this issue by using Ncx1−/− mice in which circulation is obviated due to the lack of a heartbeat. Even with the use of the Ncx1−/− strain, a second technical challenge is that assay systems need to be sensitive enough to detect the B-cell potential of rare precursors. Thus, it was essential that in vitro and in vivo systems be optimized for doing so. In the former case, our in vitro cultures were performed on a supporting layer of OP9 stromal cells (31). We observed that these stromal cells were more sensitive than the widely used S17 line (32) in revealing the lymphoid development potential of rare YS- and P-Sp–derived progenitors. In addition, we tested the in vivo developmental potential of freshly isolated or cultured cells by injecting them into neonatal NOG mice in all experiments. The peritoneal injection of donor YS, P-Sp, and adult BM B progenitor cells into NOG neonates was apparently sufficient for B-1 and/or B-2 progenitors to engraft. In the present studies, the newborn peritoneal environment appeared to be more conducive for the growth, differentiation, and/or survival of the B-1 cells from the YS and P-Sp donors compared with an adult recipient. The peritoneal cavity of the NOG neonates also permitted engraftment of fetal liver HSCs that provided multilineage reconstitution. Under the conditions used in this study, none of the YS or P-Sp populations displayed HSC activity.
By combining the use of Ncx1−/− mice, the OP9 stromal cell culture system, and neonatal NOG recipients, we were able to consistently demonstrate the intrinsic tissue potential of YS and P-Sp to independently produce B lymphocytes. It has previously been reported that the B cells produced in E8.5–9.0 P-Sp are B-1 cells (16, 17), but these studies were conducted in embryos with an intact circulation. Our data confirm and extend this finding by demonstrating that the B-1 cell potential of that tissue, as well as the YS, is endowed with AA4.1+CD19+B220lo-neg B-1 progenitor and MZ cell potential. Importantly, in our study, there were few, if any, YS- or P-Sp–produced B-2 cells that engrafted in the recipient mice. Thus, the AA4.1+CD19+B220lo-neg cells derived from E9-9.5 YS and P-Sp are distinct progenitors from the B-2 lineage, which further supports the multilineage model of B-cell development that has been controversial (33).
MZ cells share overlapping functional properties with B-1 cells (34). Although this population has traditionally been thought to be the progeny of the same transitional B cells from which FO B cells arise (35), more recently the view that MZ cells may be developmentally heterogeneous has been advanced (11). Our data show an embryonic origin of at least some MZ cells and indicate that they are generated in the same initial wave of fetal B-cell development from which B-1 cells arise. The fact that the same AA4.1+CD19+B220lo-neg B-1 progenitor cells also produced MZ cells with few B-2 cells upon transplantation into NOG neonates suggests a common developmental pathway of these B-cell subsets during embryogenesis.
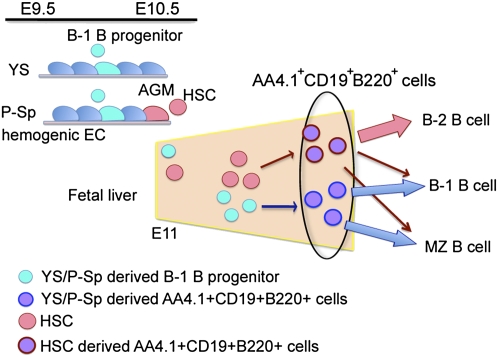
On the basis of our results, we suggest a model of fetal B-cell development in which the first B lineage cells generated during embryogenesis are endothelial-cell–derived B-1 progenitors (Fig. 6). These YS/P-Sp–derived cells then migrate to the fetal liver via the systemic circulation. It has been suggested that some AA4.1+CD19+B220 lo-neg cells in the adult are derived from an HSC-generated common lymphocyte progenitor cell (36). In this case, the AA4.1+CD19+B220lo-neg B-1 progenitors in fetal liver would be a mixture derived from at least two distinct sources: one HSC derived and one HSC independent (Fig. 6). Additional in vivo cell labeling and fate mapping approaches will be required to confirm the heterogeneous developmental origin of fetal liver B-1 progenitors. Our data provide a foundation on which such studies can be based.
Fig. 6.
Suggested origin of B-1 progenitor cells in the mouse embryo. B-1 progenitor cells emerge from YS and P-Sp hemogenic endothelium at E9–9.5. These cells then migrate into the fetal liver and mature into AA4.1+CD19+B220+ cells that can differentiate into B-1 and MZ cells. HSCs subsequently emerge at E10.5 in the AGM region and migrate into the fetal liver where they differentiate into AA4.1+CD19+B220+ cells that are primarily the precursors of B-2 cells. We thus propose that the AA4.1+CD19+B220+ population in the E11 fetal liver is developmentally heterogeneous. A proportion of these cells is independently derived from YS and P-Sp sites that generate B-1 and MZ B cells. Other B progenitor cells are primarily HSC-derived and will generate B-2 cells.
Materials and Methods
Mice.
Ncx1 heterozygous male mice (22) on a C57BL/6 background were mated with Ncx1 heterozygous females for timed pregnancy. The embryos were harvested and the somite number was counted. The anterior embryo was used for genotyping as described before (4).
Cell Suspension Preparation.
Staged 14–19 sp embryos were isolated at day E9.0 and 20–24 sp embryos at E9.5. The YS and P-Sp tissues were isolated and digested with 0.1% collagenase (Stemcell Technologies) for 10 min followed by incubation in cell dissociation buffer (Invitrogen) to generate a single-cell suspension. The cell suspension was used for transplantation or culture on OP9 stromal cells.
Transplantation.
Freshly isolated WT YS and P-Sp cells or cultured WT or Ncx1 null YS/P-Sp–derived AA4.1+CD19+B220+ cells were suspended in 25 μL of medium and injected into the peritoneal cavity of sublethally (150 cGy) irradiated NOG neonates (1–3 d old). For secondary transplantation, the peritoneal cells were collected from the primary recipient mice and injected into the peritoneal cavity of sublethally (350 cGy) irradiated adult NOG mice. Five to 16 wk after transplantation, the peritoneal cells, spleen, and BM were harvested and single-cell suspensions from each tissue were made. These cells were stained with various antibodies as described below to detect donor-derived B-cell lineages.
In Vitro Cultures.
WT or NCX1−/− YS and P-Sp cells were plated on confluent OP9 stromal cells (a gift of Toru Nakano, Osaka University, Osaka, Japan) in six-well plates in induction medium (α-MEM, 10% FBS, and 5 × 10−5 M 2-mercaptoethanol) supplemented with 50 units/mL IL-7 and 10 ng/mL flt-3 ligand. The supernatant were collected every other day, and the phenotype of the nonadherent cells were analyzed by flow cytometry.
Cell Surface Analysis and Sorting by Flow Cytometry.
The following commercial antibodies were used: anti-mouse Ter119 (TER-119), CD11b (M1/70), CD41 (MWreg30), VE-cadherin (BV14), CD19 (1D3), B220 (RA3-6B2), AA4.1 (AA4.1), CD5 (53-7.3), CD45.1 (A20), CD45.2 (104), IgM (121-15F9), IgD (11–26), CD23 (B3B4), and CD21(8D9). These antibodies were conjugated with FITC, phycoerythrin (PE), PerCPCY5.5, PE-Cy7, allophycocyanine (APC), or APC-Cy7 in various combinations, and each was used at concentrations that were titrated before use. Antibodies were all purchased from eBioscience. Flow cytometric detection was performed on an LSR II (Becton Dickinson) instrument with analysis performed using Flow Jo software (Tree Star). For transplantation, AA4.1+CD19+B220+ cells were sorted by FACS Aria (Beckton Dickinson) from day 12 of YS or P-Sp coculture with OP9 cells.
Cell Stimulation in Vitro.
Cultured YS- or P-Sp–derived B cells at a density of 2 × 105 were distributed in triplicate in 96-well plates in RPMI 1640 medium supplemented with 10% FCS, 10 μM β-mercaptoethanol, 25 mM Hepes, 10 ng/mL IL5, 10 μg/mL lipopolysaccharide (Sigma), and 10 μg/mL PC (Biosearch Technologies). Cells were incubated for 7 d at 37 °C in 5% CO2. Then supernatants of each well were collected, pooled, and examined for the presence of the anti-PC–specific IgM by ELISA.
Whole-Embryo Staining and Imaging.
E9.0 whole embryos were harvested and fixed with −20 °C acetone for 10 min, washed extensively with PBS, and blocked for 4 h in 10% normal rat serum in PBS. Embryos were stained with donkey anti-mouse VE-cadherin antibody (R&D Systems product no. AF1002) conjugated to Alexafluor 647 (Invitrogen), per manufacturer's instructions, and with purified rat anti-mouse CD19 antibody (1D3, eBioscience). An anti-rat Alexa 546 antibody was used for secondary antibody. Intact embryos were gradually cleared to 80% glycerol and dissected, and the yolk sacs were flat mounted. Images were generated with an Olympus FV1000-MPE confocal/multiphoton microscope mounted on an Olympus IX81 inverted microscope stand with a UPLSAPO 40× (oil) 0.90 n. a. objective. Nonspecific autofluorescence of the hematopoietic cells was generated with the 488 laser. All three channels of the 102-slice stack with 0.62 μm xyz resolution was processed with the despeckle median filter of ImageJ ver. 1.44e. Three-dimensional images were produced by Voxx, a volume rendering program using the α-rendering algorithm.
Statistical Analysis.
All of the statistical analyses were performed using unpaired two-tailed Student's t test assuming experimental samples of equal variance.
Supplementary Material
Acknowledgments
Funding was provided in part from National Institutes of Health Grant AI080759 (to M.C.Y.) and Grant AI21256 (to K.D.) and from grants from the Riley Children's Foundation (to M.C.Y.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015841108/-/DCSupplemental.
References
- 1.Weissman IL, Baird S, Gardner RL, Papaioannou VE, Raschke W. Normal and neoplastic maturation of T-lineage lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41:9–21. doi: 10.1101/sqb.1977.041.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Weissman IL, Papaioannou V, Gardner R. Differentiation of Normal and Neoplastic Hematopoietic Cells. Vol. 5. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1978. Fetal hematopoietic origin of the adult hematolymphoid system; pp. 33–47. [Google Scholar]
- 3.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 4.Lux CT, et al. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 6.Dzierzak E, Speck NA. Of lineage and legacy: The development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 8.Herzenberg LA. B-1 cells: The lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 9.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Andrés B, et al. The first 3 days of B-cell development in the mouse embryo. Blood. 2002;100:4074–4081. doi: 10.1182/blood-2002-03-0809. [DOI] [PubMed] [Google Scholar]
- 14.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): Role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 15.Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 17.Godin I, Dieterlen-Lièvre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci USA. 1995;92:773–777. doi: 10.1073/pnas.92.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 19.Nishikawa SI, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama D, et al. B cell potential can be obtained from pre-circulatory yolk sac, but with low frequency. Dev Biol. 2007;301:53–61. doi: 10.1016/j.ydbio.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Yokota T, et al. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133:2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- 22.Koushik SV, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- 23.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferkowicz MJ, et al. CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development. 2003;130:4393–4403. doi: 10.1242/dev.00632. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Ferkowicz MJ, Johnson SA, Shelley WC, Yoder MC. Endothelial cells in the early murine yolk sac give rise to CD41-expressing hematopoietic cells. Stem Cells Dev. 2005;14:44–54. doi: 10.1089/scd.2005.14.44. [DOI] [PubMed] [Google Scholar]
- 26.Yamane T, Hosen N, Yamazaki H, Weissman IL. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci USA. 2009;106:8953–8958. doi: 10.1073/pnas.0904090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 29.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15:477–485. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 30.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 31.Kodama H, Nose M, Niida S, Nishikawa S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 32.Collins LS, Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987;138:1082–1087. [PubMed] [Google Scholar]
- 33.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Curr Opin Immunol. 2007;19:150–155. doi: 10.1016/j.coi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 35.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 36.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.