Abstract
The Japanese beetle (JB), Popillia japonica, exhibits rapid paralysis after consuming flower petals of zonal geranium, Pelargonium x hortorum. Activity-guided fractionations were conducted with polar flower petal extracts from P. x hortorum cv. Nittany Lion Red, which led to the isolation of a paralysis-inducing compound. High-resolution–MS and NMR (1H, 13C, COSY, heteronuclear sequential quantum correlation, heteronuclear multiple bond correlation) analysis identified the paralytic compound as quisqualic acid (C5H7N3O5), a known but rare agonist of excitatory amino acid receptors. Optical rotation measurements and chiral HPLC analysis determined an l-configuration. Geranium-derived and synthetic l-quisqualic acid demonstrated the same positive paralytic dose–response. Isolation of a neurotoxic, excitatory amino acid from zonal geranium establishes the phytochemical basis for induced paralysis of the JB, which had remained uncharacterized since the phenomenon was first described in 1920.
The genus Pelargonium (Geraniaceae) consists of approximately 280 species of perennial small shrubs, most of which originate from South Africa, but also Australia, New Zealand, and the Far East (1). Extracts from Pelargonium species are used as a traditional medicine for treating dysentery, fever, respiratory infections, liver ailments, and wounds (2). Because of their colorful flowers, Pelargonium accessions are popular ornamental plants in North America and Europe (Fig. 1). Pelargonium zonale hybrids, Pelargonium x hortorum L. H. Bailey, include some of the most horticulturally desirable cultivars, and are commonly referred to as zonal geraniums.
Fig. 1.
Cluster of flower petals from P. x hortorum cv. Nittany Lion Red.
Pelargonium species possess chemically based defenses effective against insects (3) and pathogens (4). An intriguing example of a geranium phytochemical defense involves paralysis of the Japanese beetle (JB), Popillia japonica Newman (Scarabaeidae: Rutelinae), after consumption of one to two flower petals from zonal geranium (5–8). The phenomenon was first described in 1920 (5) and subsequently confirmed (6–8). Symptoms of paralysis first appear in the hind legs and progress anteriorly until the specimen eventually lies on its side or back with the legs extended rigidly outward (Fig. 2). Paralyzed beetles held under laboratory conditions typically recover within 24 h, but symptomatic beetles exposed to field conditions usually succumb to predation or desiccation (6–8).
Fig. 2.
Paralyzed JB after consumption of an agar plug infused with l-quisqualic acid from flower petals of zonal geranium. Arrow indicates portion of agar consumed.
Flower petals are more effective at inducing paralysis than the foliage (6, 8), which appears to support the optimal defense theory in that flowers are more valuable because of their role in sexual selection and therefore better defended than the foliage (9). Flowers of various colors are equally active (8) and their pigment chemistry has been well characterized (10). Previous attempts to elucidate the phytochemical basis for paralysis have not been reported since the phenomenon was first described in 1920 (5). Here we report on the isolation of a rare excitatory amino acid from flowers of zonal geranium with paralytic effects on the JB.
Results
Initial Activity-Guided Fractionations.
Experiments aimed at isolating a paralysis-inducing compound from flowers of zonal geranium initially focused on identifying a solvent with suitable extraction capabilities. Activity bioassays of crude extracts prepared by sequentially extracting flower petals with solvents of increasing polarity documented 44% paralysis of the JB 3 h after ingesting an agar plug infused with methanol extracts. No paralysis was associated with the chloroform, methylene chloride, or acetone extracts, or the solvent control (Fisher’s exact test; P < 0.0001).
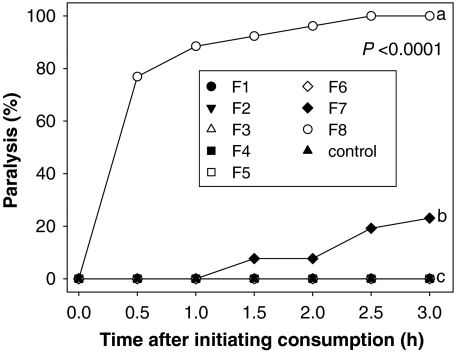
Because only a polar solvent was associated with activity, crude methanol∶water (3∶1) extracts were separated into eight fractions (F1–F8) using silica gel flash chromatography. Fractions F7 and F8 were associated with 24% and 100% paralysis, respectively, 3 h after the JB initiated consumption (Fig. 3).
Fig. 3.
Percentage of JB specimens exhibiting paralysis after consuming an agar plug infused with fractions F1–F8. Active fractions F7 and F8 were tested at 2.0 and 0.68 mg per agar plug, respectively. Different letters indicate significant differences 3 h after initiating consumption (Fisher’s exact test, α = 0.05; n = 26 per treatment).
Isolation and Structural Determination.
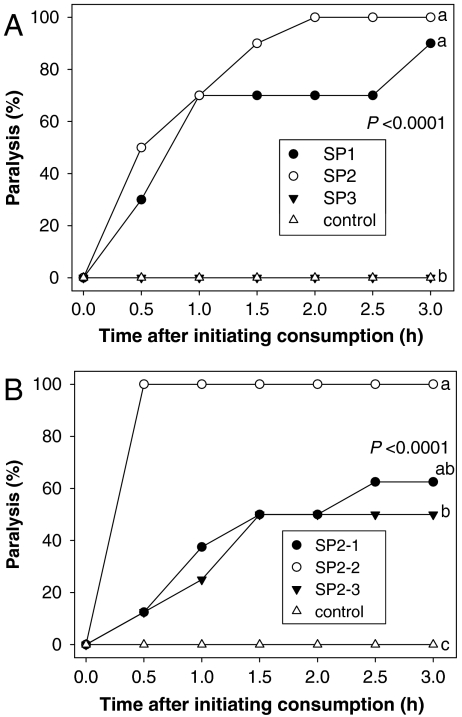
Based on results from the activity-guided fractionations, HPLC was used to isolate more effectively the paralytic compound. Semipreparative (SP) C18 HPLC was used to separate crude petal extracts into three fractions (SP1, SP2, and SP3). Bioassays demonstrated 90% and 100% paralysis after consumption of SP1 and SP2, respectively (Fig. 4A). Typical anthocyanidins and flavonols were present in the inactive fraction SP3.
Fig. 4.
Percentage of JB specimens exhibiting paralysis after consuming an agar plug infused with fractions SP1–SP3 (A) and SP2-1–SP2-3 (B). Active fractions SP1 and SP2 were tested at 3.0 and 2.3 mg per agar plug, respectively. Active fractions SP2-1, SP2-2, and SP2-3 were tested at 0.05, 0.4, and 0.03 mg per agar plug, respectively. Different letters indicate significant differences 3 h after initiating consumption (Fisher’s exact test, α = 0.05; n = 10, and n = 8 per treatment for A and B, respectively.)
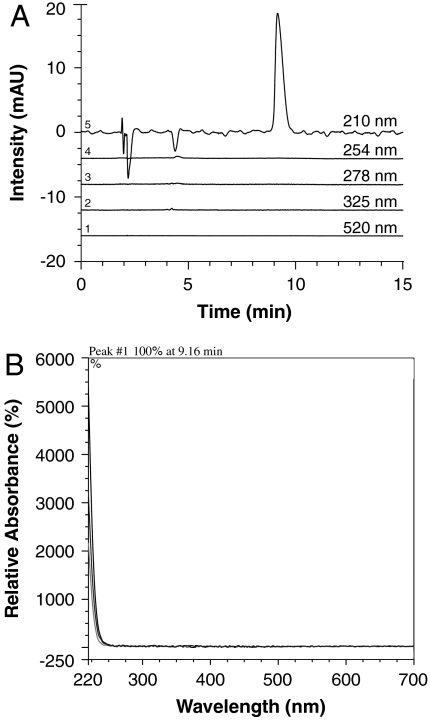
Highly polar analytes in SP2 were separated into three additional fractions (SP2-1–SP2-3) by hydrophilic interaction liquid chromatography (HILIC)–HPLC. Bioassays demonstrated the JB exhibited 100% paralysis 3 h after initiating consumption of SP2-2, whereas 62.5% and 50% paralysis were associated with SP2-1 and SP2-3, respectively (Fig. 4B). Analysis of SP2-2 by HILIC–HPLC revealed a single peak when monitored at 210 nm; no absorbance occurred at 254, 278, 325, or 520 nm (Fig. 5 A and B). The lack of absorbance at wavelengths other than 210 nm also excluded typical flavonoids as the source of activity. Lower levels of the 210 nm peak were detected in active fractions SP1, SP2-1, and SP2-3, but HILIC–HPLC analysis revealed the presence of impurities. Thus, only purified fraction SP2-2 was used for high-resolution (HR)–MS and NMR analysis.
Fig. 5.
UV-visible spectrum of purified fraction SP2-2 demonstrating an individual peak detected at 210 nm. Chromatogram was obtained by HILIC–HPLC.
Lyophilized fraction SP2-2 was reconstituted in deuterated methanol (CD3OD). An off-white powder was recovered and redissolved in deuterated water (D2O) with ammonium formate. Analysis by HR–MS revealed quasimolecular ion peaks at m/z 190.0459 [M + H]+ and m/z 188.0317 [M - H]-, respectively. Analysis by HR–MS also showed m/z 212 [M + Na]+ and m/z 234 [M - H + 2Na]+ ions, along with m/z 379 [2M + H]+, m/z 401 [2M + Na]+, and m/z 423 [2M - H + 2Na]+ ions. Interpretation of the HR–MS data established C5H7N3O5 as the molecular formula ([M + H]+ calculated as 190.0466; [M - H]- calculated as 188.0307).
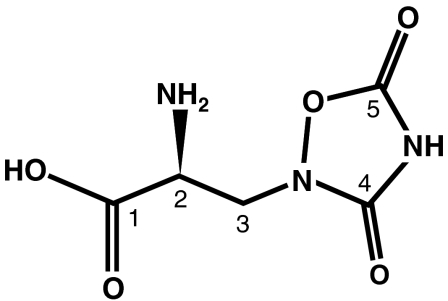
NMR analysis identified the paralysis-inducing compound in purified SP2-2 as quisqualic acid (Fig. 6), a known but rare excitatory amino acid (–12). NMR spectra (1H and 13C) of geranium-derived quisqualic acid matched the data for synthetic l-quisqualic acid (Table 1). NMR 13C analysis of quisqualic acid in D2O (spiked with CH3OD, δC 49.0) showed five carbon signals that corresponded to three carbonyl (δC1 172.50, δC4 174.61, and δC5 168.70), one methylene (δC3 51.13), and one methine (δC2 54.34) groups (Table 1). NMR 1H analysis showed a coupled three spin system in the region of 3.90–4.04 ppm, including one (δH 4.00, 1H, m) and two (δH 3.98, 2H, m) proton signals (Table 1). Cross peak correlations associated with COSY revealed the -CH2-CH unit, and heteronuclear sequential quantum correlation (HSQC) analysis revealed H2 and H3 were connected to C2 and C3, respectively. Heteronuclear multiple bond correlation (HMBC) analysis also demonstrated H3 was correlated with the carboxylic acid moiety at C1 and the urea moiety at C4. The carbamate moiety at C5 was established by considering the 13C NMR shift, the molecular formula (C5H7N3O5), and the degree of unsaturation.
Fig. 6.
Structure of l-quisqualic acid.
Table 1.
NMR spectroscopic data for natural, geranium-derived, and synthetic l-quisqualic acid (Fig. 6) (δ ppm)
| Natural |
Synthetic |
|||
| Position | δ 1H | δ 13C | δ 1H | δ 13C |
| 1 | 172.5 | 172.54 | ||
| 2 | 4.00 | 54.34 | 4.00 | 54.30 |
| 3 | 3.98 | 51.13 | 3.98 | 51.10 |
| 4 | 174.61 | 174.55 | ||
| 5 | 168.70 | 168.65 | ||
Comparisons of specific rotation for geranium-derived quisqualic acid 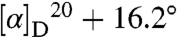 (c 0.6, 6M HCl) and synthetic l-quisqualic acid
(c 0.6, 6M HCl) and synthetic l-quisqualic acid 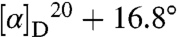 (c 0.6, 6M HCl) demonstrated the l-isomer was associated with zonal geranium. Comparison of retention times from chiral Crownpak Cr(+) HPLC analysis of geranium-derived (8.95 min), synthetic l-quisqualic acid (8.99 min), and synthetic d-quisqualic acid (6.90 min) also supported an l configuration.
(c 0.6, 6M HCl) demonstrated the l-isomer was associated with zonal geranium. Comparison of retention times from chiral Crownpak Cr(+) HPLC analysis of geranium-derived (8.95 min), synthetic l-quisqualic acid (8.99 min), and synthetic d-quisqualic acid (6.90 min) also supported an l configuration.
Dose–Response of Natural and Synthetic l-Quisqualic Acid.
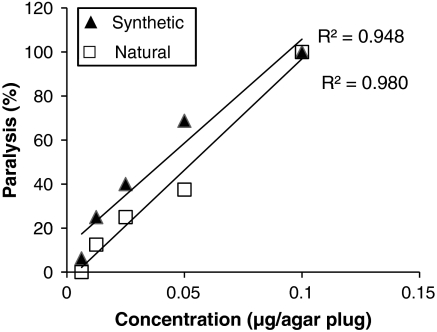
Geranium-derived and synthetic l-quisqualic acid exhibited the same (P = 0.213) positive paralytic dose–response (Fig. 7). All JB specimens exhibited paralysis after partially consuming agar plugs infused with 0.1 μg of natural and synthetic l-quisqualic acid (Fig. 7). At concentrations (microgram per plug) of 0.1, 0.05, 0.025, 0.0125, and 0.00625, quantification of the approximate amount of agar consumed after 3 h of exposure demonstrated a mean (± SE) amount (nanogram) of 4.63 ± 0.55, 2.48 ± 0.28, 1.95 ± 0.33, 1.62 ± 0.22, and 0.95 ± 0.10 of synthetic l-quisqualic acid were consumed, respectively. Similarly, at 0.1 - 0.00625 μg per plug, a mean (± SE) amount (nanogram) of 4.88 ± 1.15, 2.68 ± 0.25, 1.81 ± 0.26, 1.38 ± 0.11, and 0.87 ± 0.08 of natural l-quisqualic acid were consumed, respectively.
Fig. 7.
Dose–response of natural, geranium-derived, and synthetic l-quisqualic acid. Paralysis was assessed 3 h after the JB initiated consumption (n = 8 and 16 for natural and synthetic, respectively.)
Associating in Vivo Concentration of l-quisqualic Acid with Paralysis.
Subsequent experiments sought to determine if the concentration of l-quisqualic acid in zonal geranium flower petals was sufficient to induce the observed effect. Thus, a single lyophilized petal was first quantified to weigh a mean (± SE) of 3.78 ± 0.18 mg. Results from a petal feeding bioassay next determined a mean (± SE) of 5.55 ± 0.17 mg, or ∼11/2 petals, were ingested prior to 100% of JB specimens exhibiting paralysis. These results support earlier work (8) that ingestion of one to two petals is sufficient to induce extensive paralysis. HILIC–HPLC quantified the mean (± SE) concentration of l-quisqualic acid in the petals as 1.25 ± 0.02 mg/g of tissue, or 6.94 ± 0.11 μg in ∼11/2 petals. Because consumption of only 4.63 ± 0.55 ng of synthetic and 4.88 ± 1.15 ng of natural l-quisqualic acid was required to induce 100% paralysis (Fig. 7), the concentration of quisqualic acid in ∼11/2 petals is sufficient to induce the observed effect.
Discussion
These findings demonstrate zonal geranium is a previously unknown and unexpected source of l-quisqualic acid. This study also provides a unique look at the phytochemical basis for paralysis of the JB after consumption of zonal geranium flower petals. The basis for paralysis had remained unknown since the phenomenon was first described in 1920 (5). Paralysis of an insect induced by consumption of a plant-derived excitatory amino acid was also not previously demonstrated. By establishing zonal geranium as a natural source of l-quisqualic acid, it presents unique opportunities for the pursuit of botanically based formulations for insect pest management. Such information could assist with improving management strategies for the most destructive insect pest of ornamental and turf plants in the eastern United States (13).
l-quisqualic acid is known from Quisqualis indica Linnaeus (Combutaceae), the seeds of which are ground to form the traditional Chinese drug Shih-chun-tze for treating ascarisis (–12). Through spectral analysis, synthesis, and X-ray crystallography, the structure was elucidated as l-β-(3,5-dioxo-1,2,4-oxadiazolidin-2-yl)-alanine (–12, 14, 15). Quisqualic acid belongs to a distinct group of nonprotein amino acids, namely, the heterocyclic β-substituted alanine derivatives (16). Prior to our study, quisqualic acid had not been known from any genera other than Quisqualis.
l-quisqualic acid is thought to mimic l-glutamic acid, an amino acid that functions as a neurotransmitter in the insect neuromuscular junction and mammalian central nervous system (17, 18). l-quisqualic acid has been demonstrated to be an exceptionally potent agonist of excitatory amino acid receptors (19). The depolarizing action of l-quisqualic acid on the neuromuscular junction of crayfish was estimated to be 2–3 orders of magnitude more powerful than l-glutamic acid (19). Similarly, out of 120 analogues, l-quisqualic acid was the most potent agonist for excitatory amino acid receptors in the neuromuscular junction of the mealworm, Tenebrio molitor Linnaeus (20). It also acted as an agonist in the motoneuron of the American cockroach, Periplaneta americana Linnaeus (21), and the desert locust, Schistocerca gregaria (Forskal) (22).
To compare activity levels of quisqualic acid isomers, the actions of l-, d-, and dl-quisqualic acid at the glutamatergic nerve–muscle junction of S. gregaria were examined (22). The l-isomer exhibited the highest neuroexcitatory activity, and synthetic l-quisqualic acid exhibited the same level of activity as natural l-quisqualic acid isolated from Q. indica (22). The d- and dl-isomers were considerably less active, but they were more active than expected based on the known stereospecificity of the glutamatergic system for l- and d-glutamic acid. A possible explanation is that the affinities of l- and d-quisqualic acid for excitatory amino acid receptors are the same, but their efficacies are different (18). Results from our study also found synthetic and natural l-quisqualic acid exhibited the same levels of activity for paralyzing the JB.
Symptoms of paralysis exhibited by the JB resulting from the neurotoxic effects of l-quisqualic acid are similar to those produced by nicotine (23): sequential paralysis of the hind, middle, and front legs, followed by an inability to move steadily or maintain balance. An absence of food-aversion learning has also been demonstrated (7), such that the JB will choose geranium flowers over another suitable host (Tilia cordata Miller) and become repeatedly paralyzed. Although paralysis exhibited under laboratory conditions is usually reversible with most JB specimens recovering within 24 h, sustained overexcitation of receptors by excitatory amino acids can lead to neuronal degradation and eventual cell death (17).
In vivo concentrations of l-quisqualic acid in one to two flower petals of P. x hortorum cv. Nittany Lion Red were confirmed to be sufficient to induce the observed effect. Additional analyses are warranted to assess the localization of l-quisqualic acid in the seeds, foliage, and roots of zonal geranium and other Pelargonium species. The role of l-quisqualic acid in defending zonal geranium against other insect pests and pathogens also warrants further investigation.
Materials and Methods
Plant Material.
Clonal material of P. x hortorum cv. Nittany Lion Red were potted (Lightweight Mix 2; Conrad Fafard) and maintained under greenhouse conditions. Flower petals were hand collected and stored in polyacetate bags at -40 °C. Lyophilized petals were stored in the dark at 22–24 °C.
Insects.
Traps baited with phenethyl propionate∶eugenol∶geraniol (3∶7∶3) were used to collect adult JB in Wooster, Wayne County, OH. Adult females were maintained on insecticide-free soybean leaves at 22–24 °C and starved for 2 h prior to bioassays.
Initial Activity-Guided Fractionations.
To identify an appropriate solvent for extraction purposes, 25 g of lyophilized petals were sequentially extracted with chloroform, methylene chloride, acetone, and methanol. Petals were macerated in each solvent using a mortar and pestle and extracted for 1 h in the dark at 4 °C. Extracts were filtered through glass wool and the tissue was allowed to air dry before the next extraction. Crude extracts were concentrated under vacuum to a residue by rotary evaporation in a water bath held at 40 °C. All residues were reconstituted in the same volume of acetone∶methanol∶water (15∶15∶5) for bioassays (n = 16 per treatment).
Reconstituted extracts were bioassayed by their application to the surface of a solidified agar plug (10-mm diam, 3-mm height) (Sigma–Aldrich). Solvent control plugs were prepared accordingly. Extracts were allowed to infuse into the plugs under sterile fume hood conditions. After infusion, a single plug was placed in a Petri dish with an adult female JB. Beetles were briefly (∼1–2 min) held in place on the plugs and replaced within ∼2 min if feeding was not initiated. Petri dishes were kept on a laboratory bench at 22–24 °C during bioassays. Paralysis was recorded 3 h after consumption was initiated. Paralyzed beetles were characterized by an inability to right themselves after being inverted, along with the legs being extended and rigid rather than held closely to their bodies. Paralysis was also indicated by a lack of response after pinching the tarsi with fine forceps. Paralyzed beetles generally appeared deceased except for the occasional twitching of their tarsi and/or antennae.
After determining a polar solvent was most appropriate for extraction purposes, lyophilized petals (50 g) were combined with methanol∶water (3∶1) in a blender and ground for 30 s. The resulting slurry was extracted for 3 d in the dark at 4 °C. Filtered extracts were transferred by evaporation onto silica gel (18–32 μm, 60 Å, Sorbent Technologies) in a glass Pyrex® baking dish (22.8 × 33 cm). Extract-laden silica gel was loaded onto a silica gel-packed column and fractionated by flash chromatography using a gradient of 100% methylene chloride to 100% acetone to 100% methanol. Concentrated fractions (F1–F8) were reconstituted in acetone∶methanol∶water (15∶15∶5) for bioassays (n = 26 per treatment). Paralysis was assessed every 30 min for 3 h.
Isolation and Structural Determination.
Lyophilized petals (50 g) were ground in a blender with distilled deionized water and extracted in the dark for 1 h at 4 °C. Filtered extracts were lyophilized and the residue was reconstituted in 5% methanol. Extracts were filtered (Spin-X® Centrifuge Filters; 0.45 μm; Corning Inc.) before injection onto an HPLC system with a Dionex UltiMate 3000 binary pump (Dionex Corp.) fitted with a Phenomenex Gemini C18 column (250 × 10 mm, 5 μm, 110 Å) and a Dionex photodiode array detector set at 210, 254, 278, 308, and 520 nm. The following gradient at 2.0 mL/ min was used for solvent A (distilled deionized water) and solvent B (methanol): 0–10 min, 5% B; 10–15 min, 5–100% B; 15–30 min, 100% B. Fractions were manually collected according to: 0–6.5 min, SP1; 6.5–7.5 min, SP2; and 7.5–30 min, SP3. Data were processed using Chromeleon v6.8 Chromatography Management System. Concentrated fractions (SP1–SP3) were reconstituted in 10% methanol and bioassayed (n = 10 per treatment).
Fraction SP2 was concentrated, reconstituted in 50% acetonitrile (aq), and separated further using the aforementioned HPLC equipped with a Waters XBridge Amide HILIC column (150 × 4.6 mm, 3.5 μm) operated isocratically at 1 mL/ min with 80% B [A, 50% methanol (aq); B, 90% acetonitrile (aq) in 5 mM ammonium formate, pH 3.4]. Effluent was monitored at 210, 254, 278, 308, and 520 nm, but the peak of interest was only detected at 210 nm. Fractions were collected according to 0–8 min, SP2-1; 8–10 min, SP2-2; and 10–15 min, SP2-3. Concentrated fractions (SP2-1–SP2-3) were reconstituted in 10% methanol and bioassayed accordingly (n = 8 per treatment).
Lyophilized fraction SP2-2 was reconstituted in CD3OD, and an insoluble off-white powder (∼2 mg) was collected and redissolved in 300 μL of D2O with sodium formate (internal standard) (pH 4.0). HR–MS was performed using a Micro-TOF-Q instrument (Bruker Daltonics) equipped with an electrospray ionization source, operated in both positive and negative ion modes (scan range m/z 50–1,500; capillary voltage 4,500 V; collision cell 200 Vpp; drying gas N2 heated to 180 °C at 8 L/ min). Data were processed by Bruker Compass Data Analysis 4.0. NMR spectra were recorded at 298.5 K with a Bruker Avance DRX-500 NMR using a 5-mm broadband inverse probe (1H, COSY, multiplicity-edited HSQC, and HMBC spectra) and a broadband observe probe (13C spectra) equipped with z gradients. NMR (1H and 13C) analyses were performed at 500 MHz and 125 MHz, respectively. Spectra were calibrated to residual protonated solvent signals (CD2HOD 1H 3.30 and CD3OD δC 49.0).
Synthetic l-quisqualic acid was obtained commercially (> 99% enantiomeric purity; Sigma–Aldrich). To mimic experimental conditions, synthetic l-quisqualic acid was reconstituted in 80∶10∶10 acetonitrile∶methanol∶water in 5 mM ammonium formate (pH 3.4), lyophilized, and reconstituted in CD3OD. The CD3OD-insoluble powder was reconstituted in 300 μL of D2O with ammonium formate (internal standard) (pH 4.0). HR–MS of synthetic l-quisqualic acid was m/z 190.0468 [M + H]+.
NMR 13C shifts in the carbonyl regions of δ (150–180 ppm) for geranium-derived and synthetic l-quisqualic acid differed slightly compared to previous reports (11, 14). Assignment of proton chemical shifts appeared to be reversed from those previously reported (11, 14), but were in the same order as a more recent study (24). Variability in NMR solvent conditions and pH between our study and others (11, 14) are considered to be the basis for slight differences in chemical shifts.
Optical rotation of synthetic l-quisqualic (> 99%; Sigma–Aldrich) and the natural analyte were measured using an Autopol IV polarimeter (Rudolph Research Analytical). Specific rotation values 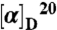 (c 2.0, 6M HCl) were calculated and are reported. Isomeric purity was also determined by HPLC using an Agilent 1200 binary pump with a variable wavelength detector set at 205 nm, equipped with a chiral Crownpak Cr(+) column (100 × 4.6 mm; 5-mm particle size; Daicel Chemical Industries, Ltd.), and operated isocratically at 0.2 mL/ min with 100% perchloric acid (pH 1.0). Synthetic l- and d-quisqualic acid (> 99% enantiomeric purity; Ascent Scientific Ltd.) were used for comparing retention times with geranium-derived quisqualic acid.
(c 2.0, 6M HCl) were calculated and are reported. Isomeric purity was also determined by HPLC using an Agilent 1200 binary pump with a variable wavelength detector set at 205 nm, equipped with a chiral Crownpak Cr(+) column (100 × 4.6 mm; 5-mm particle size; Daicel Chemical Industries, Ltd.), and operated isocratically at 0.2 mL/ min with 100% perchloric acid (pH 1.0). Synthetic l- and d-quisqualic acid (> 99% enantiomeric purity; Ascent Scientific Ltd.) were used for comparing retention times with geranium-derived quisqualic acid.
Dose–Response of Natural and Synthetic l-Quisqualic Acid.
Natural and synthetic l-quisqualic acid was obtained as previously noted and serial dilutions were prepared using distilled deionized water. Solutions were applied at 0.1, 0.05, 0.025, 0.0125, and 0.00625 μg per agar plug for bioassays (n = 8 and n = 16 for natural and synthetic, respectively). To quantify the amount of l-quisqualic acid consumed for each dose, agar plugs were weighed immediately before and after 3 h of exposure to the JB.
Associating in Vivo Concentration of l-Quisqualic Acid with Paralysis.
To determine if l-quisqualic acid is localized at a sufficient concentration to induce paralysis, the mean weight of an individual lyophilized flower petal (n = 10) was first measured. A feeding bioassay was then conducted to determine the amount of petal tissue that needed to be consumed to induce paralysis in 100% of JB specimens. Individual JB specimens were confined to Petri dish arenas (n = 10) with three lyophilized petals, which were weighed immediately before and after 3 h of exposure.
Concentration of l-quisqualic acid in petals was next quantified. Lyophilized petals (1 g) were ground in a blender with distilled deionized water and extracted (× 2) in the dark for 1 h at 4 °C. Six individual 1-g batches were prepared (n = 6). Crude extracts from both extractions were pooled, filtered, lyophilized, and reconstituted in 20 mL of water. Prior to analysis of the crude extracts, six serial dilutions of synthetic l-quisqualic acid (0.078–2.5 mg/mL) were prepared and 20-μL volumes were injected onto the HILIC–HPLC system. l-quisqualic acid was detected at 210 nm and a standard curve was prepared by plotting standard concentration against peak area. The resulting line equation was used to calculate concentrations of l-quisqualic acid in the petal extracts, which was then related to tissue weight. Standard and natural samples of l-quisqualic acid were injected in triplicate.
Statistical Analysis.
Contingency (R × C) tables and Fisher’s exact test (two-tail) were used to compare proportions of paralyzed beetles (α = 0.05) (25). Regression analysis was used to test the equality of slopes among dose–response values for natural and synthetic l-quisqualic acid (25).
ACKNOWLEDGMENTS.
We thank Gerald Hammel, Jennifer Barnett, James Moyseenko, David Benninger, Alane Robinson, Betsy Anderson, and Abigail Hart for technical assistance with activity-guided fractionations and insect and plant maintenance. We thank Pablo Jourdan and Susan Stieve of the Ohio Plant Germplasm Center for providing plant material. This research was supported by base funds associated with US Department of Agriculture–Agricultural Research Service Research Project 3607-22000-010-00D and contributes to National Program 304-Crop Protection and Quarantine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Van der Walt JJA, Vorster PJ. Pelargoniums of Southern Africa. Vol. 3. Cape Town, South Africa: Natl Botanical Garden; 1988. p. 54. [Google Scholar]
- 2.Watt C, Breyer-Brandwijk MG. The Medicinal and Poisonous Plants of Southern and Eastern Africa. London: Livingstone; 1962. p. 1457. [Google Scholar]
- 3.Walters DS, Craig R, Mumma RO. Effects of mite resistance mechanisms of geraniums on mortality and behavior of foxglove aphid (Acyrthosiphon solani Kaltenbach) J Chem Ecol. 1990;16:877–886. doi: 10.1007/BF01016497. [DOI] [PubMed] [Google Scholar]
- 4.Kayser O, Kolodziej H. Antibacterial activity of extracts, constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997;63:508–510. doi: 10.1055/s-2006-957752. [DOI] [PubMed] [Google Scholar]
- 5.Davis JJ. The green Japanese beetle. New Jersey Department Agriculture Circular. 1920;30:33. [Google Scholar]
- 6.Ballou CH. Effects of geranium on the Japanese beetle. J Econ Entomol. 1929;22:289–293. [Google Scholar]
- 7.Potter DA, Held DW. Absence of food-aversion learning by a polyphagous scarab, Popillia japonica, following intoxication by geranium, Pelargonium x hortorum. Entomol Exp Appl. 1999;91:83–88. [Google Scholar]
- 8.Held DW, Potter DA. Characterizing toxicity of Pelargonium spp. and two other reputedly toxic plant species to Japanese beetles (Coleoptera: Scarabaeidae) Environ Entomol. 2003;32:873–880. [Google Scholar]
- 9.Strauss SY, Irwin RE, Lambrix V. Optimal defense theory and flower petal color predict variation in the secondary chemistry of wild radish. J Ecol. 2004;92:132–141. [Google Scholar]
- 10.Mitchell KA, Markham KR, Boase MR. Pigment chemistry and color of Pelargonium flowers. Phytochem. 1998;47:355–361. [Google Scholar]
- 11.Takemoto T, Nakajima T, Arihara S, Koike K. Studies on the constituents of Quisqualis Fructus. II. Structure of quisqualic acid. Yakugaku Zasshi. 1975;95:326–332. doi: 10.1248/yakushi1947.95.3_326. [DOI] [PubMed] [Google Scholar]
- 12.Pan PC, Fang SD, Tsai CC. The chemical constituents of shih-chun-tze, Quisqualis indica L. Sci Sin. 1976;19:691–701. [Google Scholar]
- 13.Potter DA, DW Held. Biology and management of the Japanese beetle. Annu Rev Entomol. 2002;47:175–205. doi: 10.1146/annurev.ento.47.091201.145153. [DOI] [PubMed] [Google Scholar]
- 14.Takemoto T, Koike K, Nakajima T, Arihara S. Studies on the constituents of Quisqualis Fructus. III. Synthesis of quisqualic acid and the related compounds. Yakugaku Zasshi. 1975;95:448–452. doi: 10.1248/yakushi1947.95.4_448. [DOI] [PubMed] [Google Scholar]
- 15.Flippen JL, Gilardi RD. Quisqualic acid. Acta Crystallogr B. 1976;32:951–953. [Google Scholar]
- 16.Spiteller P, Von Nussbaum F. In: β-Amino Acids in Natural Products. Enantioselective Synthesis of β-Amino Acids. Juaristi E, Soloshonok VA, editors. New York: Wiley; 2005. pp. 19–91. [Google Scholar]
- 17.Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- 18.Usherwood PNR. Insect glutamate receptors. Adv Insect Physiol. 1994;24:309–341. [Google Scholar]
- 19.Shinozaki H, Shibuya I. Effects of kainic acid analogues on crayfish opener muscle. Neuropharm. 1974;15:145–147. doi: 10.1016/0028-3908(76)90052-6. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto T, et al. Agonistic action of synthetic analogues of quisqualic acid at the insect neuromuscular junction. Arch Insect Biochem Physiol. 1985;2:65–73. [Google Scholar]
- 21.Wafford KA, Sattelle DB. Effects of amino acid neurotransmitter candidates on an identified insect motoneurone. Neurosci Lett. 1986;63:135–140. doi: 10.1016/0304-3940(86)90050-9. [DOI] [PubMed] [Google Scholar]
- 22.Boden P, et al. The action of natural and synthetic isomers of quisqualic acid at a well-defined glutamatergic synapse. Brain Res. 1986;385:205–211. doi: 10.1016/0006-8993(86)91065-6. [DOI] [PubMed] [Google Scholar]
- 23.Thurston R, Smith WT, Cooper BP. Alkaloid secretion by trichomes of Nicotiana species and resistance to aphids. Entomol Exp Appl. 1966;9:428–432. [Google Scholar]
- 24.Baldwin JE, Adlington RM, Birch DJ. Synthesis of l-quisqualic acid: A general method for enantio-efficient synthesis of β-aminoalanine derivatives. J Chem Soc Chem Comm. 1985;5:256–257. [Google Scholar]
- 25.SAS Institute. SAS Procedures Guide. Cary, NC: SAS Inst; 2001. p. 685. [Google Scholar]