Abstract
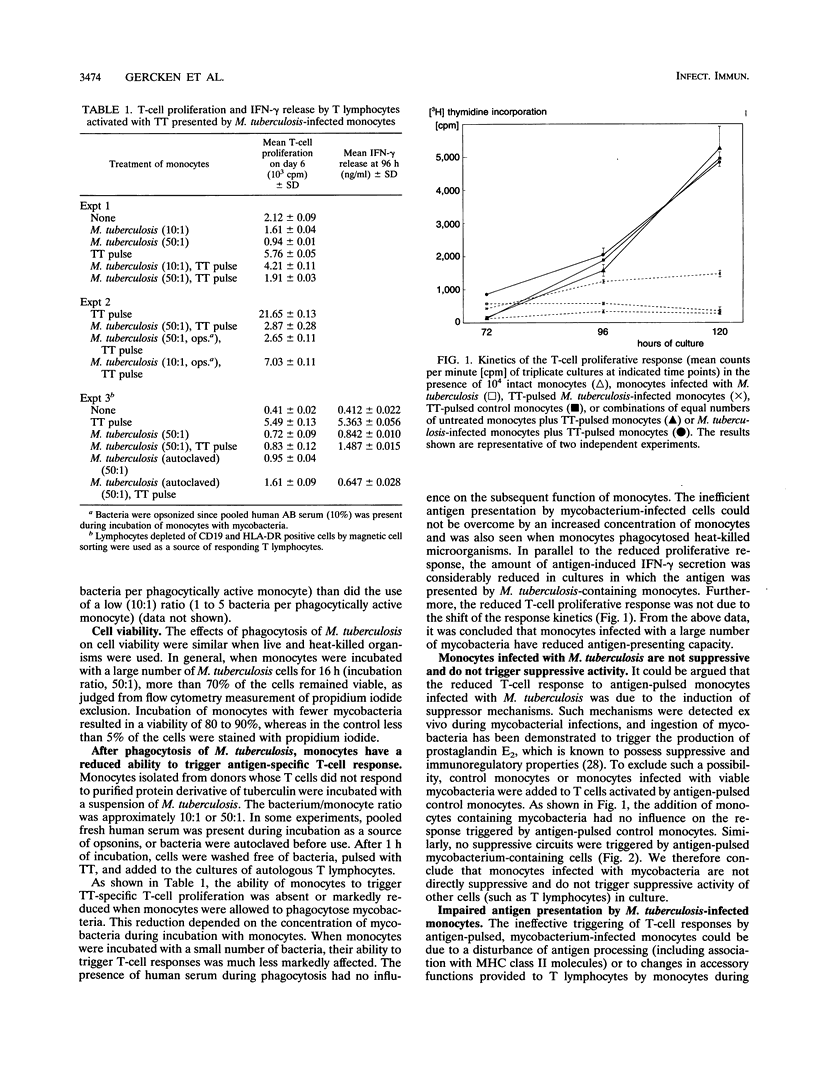
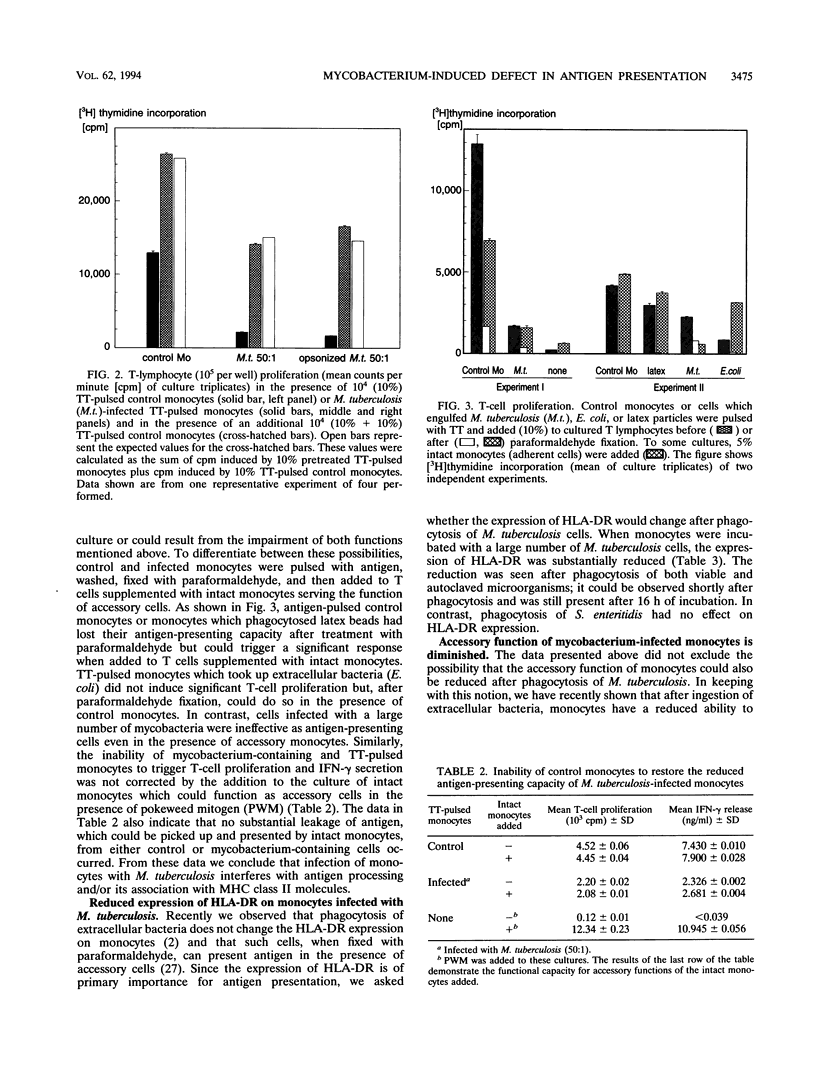
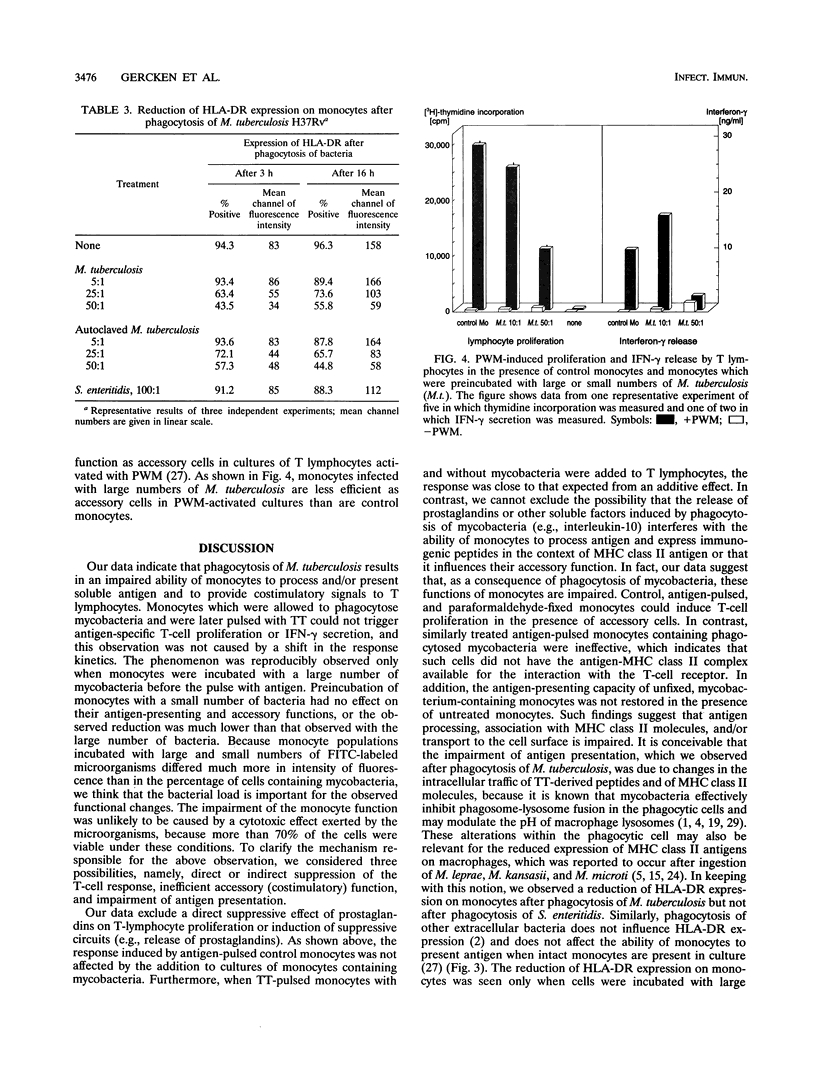
In this study we investigated the effect of an in vitro infection with Mycobacterium tuberculosis on the ability of human monocytes to present the soluble antigen tetanus toxoid to T cells. We observed that tetanus toxoid-specific T-cell proliferation was markedly reduced when monocytes were infected with large numbers (bacterium-to-monocyte ratio, 50:1) of both viable and heat-killed mycobacteria. The level of antigen-induced gamma interferon release also was decreased when M. tuberculosis-containing monocytes were used as antigen-presenting cells. However, mycobacterium-infected monocytes did not show or trigger suppressive activity, because the presence of mycobacterium-infected monocytes did not affect the T-cell response induced by tetanus toxoid-pulsed control monocytes. When M. tuberculosis-infected monocytes were fixed with paraformaldehyde, they were not able to serve as antigen-presenting cells even in the presence of untreated accessory monocytes. Moreover, the uptake of both viable and heat-killed M. tuberculosis cells reduced the expression of human leukocyte antigen DR on monocytes. With regard to accessory function, monocytes infected with large numbers of mycobacteria were less efficient as accessory cells than were control monocytes in cultures of T cells activated with pokeweed mitogen. These results indicate that infection with large numbers of M. tuberculosis cells impairs the ability of monocytes to process and/or present soluble antigen and to serve as accessory cells in T-cell activation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Malaviya A. N., Narayanan S., Rajgopalan P., Kumar R., Bharadwaj O. P. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis. 1977 Feb;115(2):207–212. doi: 10.1164/arrd.1977.115.2.207. [DOI] [PubMed] [Google Scholar]
- Chicurel M., García E., Goodsaid F. Modulation of macrophage lysosomal pH by Mycobacterium tuberculosis-derived proteins. Infect Immun. 1988 Feb;56(2):479–483. doi: 10.1128/iai.56.2.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings L. A., Tidman N., Poulter L. W. Quantitation of HLA-DR expression by cells involved in the skin lesions of tuberculoid and lepromatous leprosy. Clin Exp Immunol. 1985 Jul;61(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Drevets D. A., Campbell P. A. Roles of complement and complement receptor type 3 in phagocytosis of Listeria monocytogenes by inflammatory mouse peritoneal macrophages. Infect Immun. 1991 Aug;59(8):2645–2652. doi: 10.1128/iai.59.8.2645-2652.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruth U., Solioz N., Louis J. A. Leishmania major interferes with antigen presentation by infected macrophages. J Immunol. 1993 Mar 1;150(5):1857–1864. [PubMed] [Google Scholar]
- Geppert T. D., Davis L. S., Gur H., Wacholtz M. C., Lipsky P. E. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990 Oct;117:5–66. doi: 10.1111/j.1600-065x.1990.tb00566.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Harada Y., Harada S., Kajiki A., Kitahara Y., Takamoto M., Ishibashi T., Shinoda A. [Clinico-immunological study of the patients infected with Mycobacterium avium complex (MAC)]. Kekkaku. 1992 Jan;67(1):71–84. [PubMed] [Google Scholar]
- Hayward A. R., Read G. S., Cosyns M. Herpes simplex virus interferes with monocyte accessory cell function. J Immunol. 1993 Jan 1;150(1):190–196. [PubMed] [Google Scholar]
- Kaye P. M., Sims M., Feldmann M. Regulation of macrophage accessory cell activity by mycobacteria. II. In vitro inhibition of Ia expression by Mycobacterium microti. Clin Exp Immunol. 1986 Apr;64(1):28–34. [PMC free article] [PubMed] [Google Scholar]
- Leopardi R., Ilonen J., Mattila L., Salmi A. A. Effect of measles virus infection on MHC class II expression and antigen presentation in human monocytes. Cell Immunol. 1993 Apr 1;147(2):388–396. doi: 10.1006/cimm.1993.1078. [DOI] [PubMed] [Google Scholar]
- Madsen M., Johnsen H. E. A methodological study of E-rosette formation using AET-treated sheep red blood cells. J Immunol Methods. 1979 May 10;27(1):61–74. doi: 10.1016/0022-1759(79)90239-4. [DOI] [PubMed] [Google Scholar]
- Makonkawkeyoon S., Kasinrerk W. In vitro suppression of interleukin 2 production by Mycobacterium leprae antigen. Clin Exp Immunol. 1989 Jun;76(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- McDonough K. A., Kress Y., Bloom B. R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993 Jul;61(7):2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Molloy A., Gaudernack G., Levis W. R., Cohn Z. A., Kaplan G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Feb;87(3):973–977. doi: 10.1073/pnas.87.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Mshana R. N., Hastings R. C., Krahenbuhl J. L. Infection with live mycobacteria inhibits in vitro detection of Ia antigen on macrophages. Immunobiology. 1988 Apr;177(1):40–54. doi: 10.1016/S0171-2985(88)80090-1. [DOI] [PubMed] [Google Scholar]
- Nash D. R., Douglass J. E. Anergy in active pulmonary tuberculosis. A comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980 Jan;77(1):32–37. doi: 10.1378/chest.77.1.32. [DOI] [PubMed] [Google Scholar]
- Pancholi P., Mirza A., Bhardwaj N., Steinman R. M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science. 1993 May 14;260(5110):984–986. doi: 10.1126/science.8098550. [DOI] [PubMed] [Google Scholar]
- Pryjma J., Baran J., Ernst M., Woloszyn M., Flad H. D. Altered antigen-presenting capacity of human monocytes after phagocytosis of bacteria. Infect Immun. 1994 May;62(5):1961–1967. doi: 10.1128/iai.62.5.1961-1967.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Bachelet M., Carvalho de Sousa J. P. Intracellular growth of Mycobacterium avium in human macrophages is linked to the increased synthesis of prostaglandin E2 and inhibition of the phagosome-lysosome fusions. FEMS Microbiol Immunol. 1992 Jul;4(5):273–279. doi: 10.1111/j.1574-6968.1992.tb05006.x. [DOI] [PubMed] [Google Scholar]
- Sasaki D. T., Dumas S. E., Engleman E. G. Discrimination of viable and non-viable cells using propidium iodide in two color immunofluorescence. Cytometry. 1987 Jul;8(4):413–420. doi: 10.1002/cyto.990080411. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Kawasumi H., Takashima T., Tsuyuguchi T., Kishimoto S. Mycobacterium avium-Mycobacterium intracellular complex-induced suppression of T-cell proliferation in vitro by regulation of monocyte accessory cell activity. Infect Immun. 1990 May;58(5):1369–1378. doi: 10.1128/iai.58.5.1369-1378.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]



