Abstract
Adrenoleukodystrophy (ALD) is an X-linked disorder affecting primarily the white matter of the central nervous system occasionally accompanied by adrenal insufficiency. Despite the discovery of the causative gene, ABCD1, no clear genotype–phenotype correlations have been established. Association studies based on single nucleotide polymorphisms (SNPs) identified by comprehensive resequencing of genes related to ABCD1 may reveal genes modifying ALD phenotypes. We analyzed 40 Japanese patients with ALD. ABCD1 and ABCD2 were analyzed using a newly developed microarray-based resequencing system. ABCD3 and ABCD4 were analyzed by direct nucleotide sequence analysis. Replication studies were conducted on an independent French ALD cohort with extreme phenotypes. All the mutations of ABCD1 were identified, and there was no correlation between the genotypes and phenotypes of ALD. SNPs identified by the comprehensive resequencing of ABCD2, ABCD3, and ABCD4 were used for association studies. There were no significant associations between these SNPs and ALD phenotypes, except for the five SNPs of ABCD4, which are in complete disequilibrium in the Japanese population. These five SNPs were significantly less frequently represented in patients with adrenomyeloneuropathy (AMN) than in controls in the Japanese population (p = 0.0468), whereas there were no significant differences in patients with childhood cerebral ALD (CCALD). The replication study employing these five SNPs on an independent French ALD cohort, however, showed no significant associations with CCALD or pure AMN. This study showed that ABCD2, ABCD3, and ABCD4 are less likely the disease-modifying genes, necessitating further studies to identify genes modifying ALD phenotypes.
Electronic supplementary material
The online version of this article (doi:10.1007/s10048-010-0253-6) contains supplementary material, which is available to authorized users.
Keywords: Adrenoleukodystrophy, ABCD1, ABCD2, ABCD3, ABCD4, DNA microarray
Introduction
Adrenoleukodystrophy (ALD) is a demyelinating disease caused by mutations of ABCD1 [1]. This disease affects primarily the white matter of the central nervous system occasionally accompanied by adrenal insufficiency [2–4]. Diagnosis of ALD is usually made by the increased contents of very long chain saturated fatty acids (VLCFAs; >C22:0) in plasma as well as by mutational analysis of ABCD1 [5–7]. Since 15% of obligate female carriers have normal VLCFA levels [7], mutational analysis is essential for the diagnosis of the carriers. Since the first report of allogenic HSCT for childhood ALD, there has been an increasing number of reports showing efficacies of HSCT for the childhood cerebral form of ALD, if HSCT is performed at early stages of the disease [8–10]. Thus, availability of rapid molecular diagnosis for patients with ALD and carriers is mandatory in the clinical practice for ALD.
ALD is characterized by a broad spectrum of clinical presentations including childhood cerebral form, adrenomyeloneuropathy (AMN), AMN complicated by cerebral demyelination, adulthood cerebral form, and Addison disease. From clinical experience, patients with different clinical phenotypes can be observed even in a single pedigree. In support of this, no clear genotype–phenotype correlations have been observed [11–16], raising the possibility that other genetic or environmental factors are involved in the pleiomorphic clinical presentations of ALD.
ABCD1 gene encodes a half-ATP-binding cassette (ABC) transporter, adrenoleukodystrophy protein (ALDP), which is localized to the peroxisomal membrane. ABCD2, ABCD3, and ABCD4 genes are the closest homologues of the ABCD1 gene [17, 18]. It has been shown that the majority of mouse liver ALDP and the 70-kDa peroxisomal membrane protein (PMP70) that is encoded by ABCD3 are homomeric proteins [19]. Furthermore, it has been shown that ALDP can form homodimers or a heterodimer with the adrenoleukodystrophy-related protein (ALDR) that is encoded by ABCD2 or the PMP70 that is encoded by ABCD3 [16, 20–22], raising the possibility that these ABCD1-related genes function as disease-modifying genes for ALD.
To provide a rapid mutational analysis for ALD, we developed a microarray-based high-throughput resequencing system of ABCD1 (TKYPD01) [23]. Furthermore, to explore the possibility that these ABCD1-related genes function as disease-modifying genes, we established a comprehensive resequencing system for ABCD1-related genes, ABCD2, ABCD3, and ABCD4. On the basis of the comprehensive resequencing of ABCD1, ABCD2, ABCD3, and ABCD4 genes, we identified 11 novel single nucleotide polymorphism (SNPs). Using these novel SNPs as well as previously described SNPs of these genes, we conducted detailed association studies of these SNPs with the clinical phenotypes of ALD.
Materials and methods
Participants
Forty Japanese ALD patients, consisting of 14 patients with childhood cerebral ALD (CCALD), 8 patients with adulthood cerebral ALD (AdultCer), 2 patients with AMN with later development of cerebral ALD (AMN-Cer), 13 patients with AMN, 1 asymptomatic patient, and 2 patients with unknown form, were enrolled in this study. Among the patients, mutations were previously identified in 16 ALD patients by direct nucleotide sequence analysis, while no mutational analyses were conducted for 24 patients.
For replication studies of the results of association studies on Japanese ALD patients showing potential associations of SNPs in ABCD1-related genes with ALD phenotypes, an independent French ALD cohort with well-defined extreme phenotypes consisting of 118 patients with CCALD and 71 patients with pure AMN (AMN with age >45 years as well as with normal brain magnetic resonance imaging) was studied. In addition, 51 ALD patients with AMN-Cer were also analyzed in the French ALD cohort.
Procedures
Primers specific for ABCD1, ABCD2, ABCD3, and ABCD4 were designed using BLAST search and Smith–Waterman method to avoid amplification of the related homologous genes (Fig. 1; ESM Tables 1, 2, 3, and 4). In particular, since there were many segments homologous to exons 8, 9, and 10 of ABCD1, a specific primer pair was designed (Fig. 1). Fifty nanograms of genomic DNA were subjected to polymerase chain reaction (PCR) amplification in a total volume of 50 μL. The PCR conditions were as follows: 94°C for 1 min, followed by five cycles consisting of 94°C for 30 s, 62°C for 30 s, and 68°C for 2 min; five cycles consisting of 94°C for 30 s, 60°C for 30 s, and 68°C for 2 min; and 25 cycles consisting of 94°C for 30 s, 58°C for 30 s, and 68°C for 2 min, followed by a final extension at 68°C for 7 min, using the LA Taq with GC Buffer PCR system (Takara Bio, Otsu, Shiga, Japan).
Fig. 1.
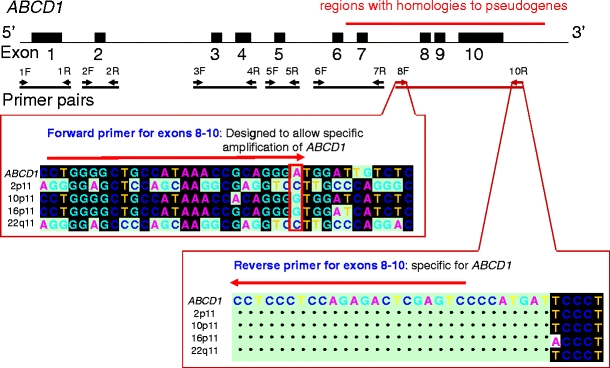
Primer design for ABCD1. All the exons of ABCD1 were amplified using six primer pairs. There were pseudogenes at 2p11, 10p11, 16p11, and 22q11, which were similar in sequence to exons 7–10 of ABCD1 gene (92–96%). The forward primer for exons 8–10 was designed to avoid amplification of the related homologous genes. We could design a specific reverse primer for exons 8–10
Resequencing DNA microarrays were used in the analyses of the sequences of ABCD1 (TKYPD01) and ABCD2 (TKYAD01). TKYPD01 and TKYAD01 were designed using the platform of GeneChip CustomSeqTM Resequencing Microarray (Affymetrix, Santa Clara, CA, USA). Since there are substantial homologies between ABCD1 and ABCD2, these genes were placed in independent microarrays (TKYPD01 and TKYAD01). Each PCR product of ABCD1 and ABCD2 was quantified using PicoGreen (Molecular Probes, Eugene, OR, USA) and equimolarly pooled. Pooled PCR products of ABCD1 and ABCD2 were fragmented using DNAse I, labeled with biotin, hybridized to DNA microarrays, and subjected to scan and analyses of nucleotide sequences of ABCD1 (TKYPD01) and ABCD2 (TKYAD01) according to the manufacturer's instructions (Affymetrix, Santa Clara, CA, USA). The base calls that were undetermined using the GDAS analysis software (Affymetrix, Santa Clara, CA, USA) were further analyzed by manual inspection. Identified mutations and SNPs were confirmed by the direct nucleotide sequence analysis of the PCR products. All the PCR products of ABCD3 and ABCD4 were analyzed by the direct nucleotide sequence analysis. Identified SNPs of ABCD2, ABCD3, and ABCD4 were examined as to whether they were novel SNPs or known SNPs using the J SNP (http://snp.ims.u-tokyo.ac.jp/index_ja.html) and DB SNP (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Snp&cmd=Limits).
Statistical analyses
We compared the allele frequencies of detected SNPs between the subgroups of ALD or between the individual subgroup and the controls by Fisher's exact test using the JMP 7 software (SAS Institute, Cary, NC, USA). Deviation of the SNP genotypes from the Hardy–Weinberg equilibrium was evaluated using the PEDSTATS program [24]. Linkage disequilibria among the neighboring SNPs were evaluated using Haploview version 4.1 [25].
Results
Resequencing DNA microarray-based mutational analysis of ABCD1 gene in Japanese ALD patients
All the mutations of ABCD1 were clearly identified using the resequencing DNA microarray system including 26 missense, 2 nonsense, and 12 insertion/deletion mutations of ABCD1 (Figs. 2 and 3; Tables 1 and 2). Mutations of ABCD1 gene were widely scattered in the entire region of ABCD1 gene (Fig. 3; Tables 1 and 2). All types of ABCD1 mutation were distributed among all the phenotypes of ALD (Fig. 3; Tables 1 and 2). Among the 40 mutations, 11 mutations were novel (Tables 1 and 2). Among the deletion/frameshift mutations that are expected to result in complete loss of ALDP functions, the mutations were distributed among all the phenotypes of ALD (Tables 1 and 2), supporting the previous observations of no genotype–phenotype correlations.
Fig. 2.
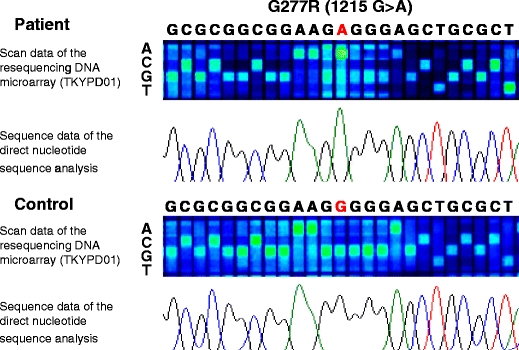
Scan data of the resequencing DNA microarray and sequence data of the direct nucleotide sequence analysis (upper panel: patient, lower panel: control). Each column shows a base position, and each row shows a base call DNA in the scan data of the resequencing DNA microarray. Here, a mutation (G277R) was detected, and the signal intensities around the mutation were reduced because of the mismatch of the mutation site. The sequence data of the direct nucleotide sequence analysis confirmed the scan data of the resequencing DNA microarray
Fig. 3.
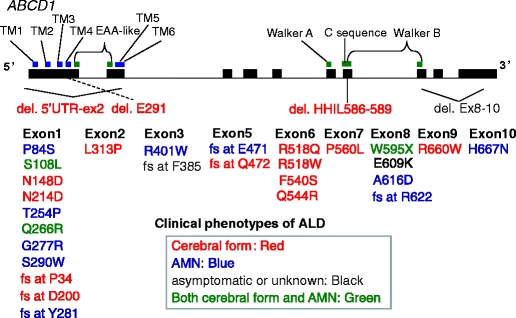
Identified mutations of ABCD1. Mutations of ABCD1 gene were widely scattered in the entire region of ABCD1 gene. All types of ABCD1 mutations were distributed among all the phenotypes of adrenoleukodystrophy. TM transmembrane domain, EAA-like EAA-like protein motif, Walker A Walker A motif, C sequence nucleotide binding fold conserved sequence, Walker B Walker B motif, fs frameshift
Table 1.
Identified ABCD1 mutations: mutations of ABCD1 that result in devastating effects (frame shifts or nonsense mutations) on adrenoleukodystrophy protein (ALDP)
| Patient number | Phenotype | Mutation of ABCD1 | Effect of mutation of ABCD1 |
|---|---|---|---|
| 1 | CCALD | 488C>AT | Frameshift at P34 |
| 2 | CCALD | 2171G>A | W595X |
| 3 | CCALD | 5′UTR-Ex2 1.4-kb deletiona | Disruption of gene structure |
| 4 | AdultCer | Del. 986Ca | Frameshift at D200 |
| 5 | AdultCer | Del. 1801–1802AGa | Frameshift at Q472 |
| 6 | AMN-Cer | Del. 2251 GGTG ins. TGTTCTa | Frameshift at R622 |
| 7 | AMN | Ins. 1237Ta | Frameshift at Y281 |
| 8 | AMN | Del. 1801–1802AGa | Frameshift at Q472 |
| 9 | AMN | 2171G>A | W595X |
| 10 | AMN | Del. 2251 GGTG ins. TGTTCTa | Frameshift at R622 |
| 11 | Unknown | Del. 1541Ca | Frameshift at F385 |
| 12 | Unknown | Ex8-10 0.3-kb deletiona | Disruption of gene structure |
Amino acid residue numbers in ALDP are based on Mosser et al. [1]. The domains and motifs in the ALDP are based on Mosser et al. [1]
CCALD childhood cerebral ALD, AdultCer adult with cerebral ALD, AMN-Cer AMN with cerebral ALD, AMN adrenomyeloneuropathy, TM transmembrane domain, Loop 1 loop 1 motif, EAA-like EAA-like protein motif, Walker A Walker A motif, Cons nucleotide binding fold conserved sequence, Walker B Walker B motif
aNovel mutation
Table 2.
Identified ABCD1 mutations: mutations of ABCD1 that result in amino acid substitutions or in-frame deletions
| Patient number | Phenotype | Mutation of ABCD1 | Effect of mutation of ABCD1 | Position of mutation |
|---|---|---|---|---|
| 13 | CCALD | 709C>T | S108L | Loop1 |
| 14 | CCALD | 709C>T | S108L | Loop1 |
| 15 | CCALD | 829A>G | N148S | TM2 |
| 16 | CCALD | 1026A>G | N214D | TM3 |
| 17 | CCALD | 1182G>A | G266R | Between TM4 and EAA-like |
| 18 | CCALD | 1324T>Ca | L313P | Between EAA-like and TM5 |
| 19 | CCALD | 1938C>T | R518W | Walker A |
| 20 | CCALD | 1939G>A | R518Q | Walker A |
| 21 | CCALD | 2017A>G | Q544R | Between Walker A and Cons |
| 22 | CCALD | 2017A>G | Q544R | Between Walker A and Cons |
| 23 | CCALD | 2065C>T | P560L | Between Walker A and Cons |
| 24 | CCALD | 2065C>T | P560L | Between Walker A and Cons |
| 25 | CCALD | Del. 2145–2156 | Del. HILQ587-590 | Between Walker A and Cons |
| 26 | AdultCer | Del. 1257–1259 | Del.E291 | EAA-like |
| 27 | AdultCer | 2005T>C | F540S | Between Walker A and Cons |
| 28 | AdultCer | 2358C>T | R660W | C-terminal to Walker B |
| 29 | AdultCer | 2385C>A | H667N | C-terminal to Walker B |
| 30 | AMN-Cer | 1146A>C | T254P | TM4 |
| 31 | AMN | 636C>T | P84S | TM1 |
| 32 | AMN | 709C>T | S108L | Loop1 |
| 33 | AMN | 1182G>A | G266R | Between TM4 and EAA-like |
| 34 | AMN | 1197G>A | E271K | Between TM4 and EAA-like |
| 35 | AMN | 1215G>Aa | G277R | Between TM4 and EAA-like |
| 36 | AMN | 1255C>G | S290W | EAA-like |
| 37 | AMN | 1581C>T | R401W | Between TM6 and Walker A |
| 38 | AMN | 2233C>A | A616D | Cons |
| 39 | AMN | 2385C>A | H667N | C-terminal to Walker B |
| 40 | Asymptomatic | 2211G>A | E609K | Cons |
Amino acid residue numbers in ALDP are based on Mosser et al. [1]. The domains and motifs in the ALDP are based on Mosser et al. [1]
CCALD childhood cerebral ALD, AdultCer adult with cerebral ALD, AMN-Cer AMN with cerebral ALD, AMN adrenomyeloneuropathy, TM transmembrane domain, Loop 1 loop 1 motif, EAA-like EAA-like protein motif, Walker A Walker A motif, Cons nucleotide binding fold conserved sequence, Walker B Walker B motif
aNovel mutation
Identification of SNPs of ABCD2, ABCD3, and ABCD4 genes by comprehensive resequencing
Comprehensive resequencing of ABCD2, ABCD3, and ABCD4 genes of the 40 Japanese patients with ALD revealed two novel SNPs, nine SNPs (six known and three novel SNPs), and 13 SNPs (seven known and six novel SNPs), respectively (Fig. 4; Tables 3, 4, 5, and 6; ESM Table 5). Hardy–Weinberg equilibrium was fulfilled for each SNP. The five known SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) were in complete linkage disequilibrium in the Japanese patients with ALD as well as in the controls, as determined using Haploview version 4.1 (Fig. 4).
Fig. 4.
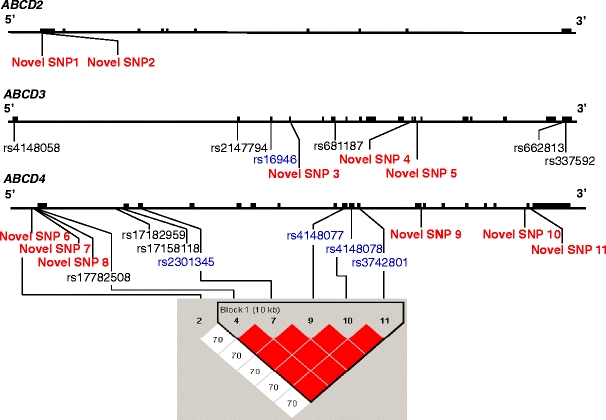
Identified single nucleotide polymorphisms (SNPs) of ABCD2, ABCD3, and ABCD4 (upper panel). Comprehensive resequencing of ABCD2, ABCD3, and ABCD4 genes of the 40 patients with adrenoleukodystrophy (ALD) revealed two novel SNPs, nine SNPs (six known and three novel SNPs), and 13 SNPs (seven known and six novel SNPs), respectively. Red characters indicate the novel SNPs, blue characters indicate the SNPs identified in the coding region, and black characters indicate the SNPs identified in the noncoding region. Linkage disequilibrium (LD) map of SNPs of ABCD4 in Japanese patients with ALD and the controls using the Haploview version 4.1 (lower panel). The five known SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) were in complete disequilibrium in Japanese patients with ALD and the controls (LOD = 43.97, r 2 = 1.0, D′ = 1.0). Novel SNP7 and the five known SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) were not in strong disequilibrium in Japanese patients with ALD and the controls (LOD = 1.15, r 2 = 0.037, D′ = 0.706), although novel SNP7 and the five known SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) were strong disequilibrium only in Japanese patients with ALD (LOD = 2.02, r 2 = 0.221, D′ = 1.0). The number in the box indicates the data of D′. The color of the box is determined from the LOD score and D′. The block was determined using a confidence interval algorithm [33]
Table 3.
Summary of identified single nucleotide polymorphism (SNPs) of ABCD2, ABCD3, and ABCD4 in 40 adrenoleukodystrophy patients: novel SNPs
| Gene | Name | Fragment | Position (UCSC hg18) | Base call | Category | Amino acid change |
|---|---|---|---|---|---|---|
| ABCD2 | Novel SNP1 | Exon1 | 38299954 | A/T | 5′ untranslated region | |
| Novel SNP2 | Exon1 | 38299659 | G/C | Coding nonsynonymous | A9G | |
| ABCD3 | Novel SNP3 | Exon4 | 94706096 | A/G | Coding nonsynonymous | M94V |
| Novel SNP4 | Exon14 | 94727816 | T/G | Intron | ||
| Novel SNP5a | Exon15 | 94728352 | G/C | Intron | ||
| ABCD4 | Novel SNP6 | 5′UTR | 73840784 | T/C | Upstream at the transcription start site | |
| Novel SNP7 | 5′UTR | 73839945 | T/C | Upstream at the transcription start site | ||
| Novel SNP8 | 5′UTR | 73839604 | G/A | Upstream at the transcription start site | ||
| Novel SNP9b | Exon12 | 73826720 | A/G | Intron | ||
| Novel SNP10 | Exon18 | 73823320 | A/G | Intron | ||
| Novel SNP11a | Exon18 | 73823116 | T/C | Intron |
A total of 24 SNPs of ABCD2, ABCD3, and ABCD4 were identified in 40 ALD patients. Among them, 11 SNPs (45.8%) were novel SNPs. The positions of these novel SNPs were based on the UCSC genome browser hg18
aThese SNPs were identified only in the cerebral form (childhood cerebral ALD and adult with cerebral ALD)
bThese SNPs were identified only in the AMN form
Table 4.
Summary of identified single nucleotide polymorphism (SNPs) of ABCD2, ABCD3, and ABCD4 in 40 adrenoleukodystrophy patients: known SNPs
| Gene | Fragment | SNP ID | Category | Amino acid change |
|---|---|---|---|---|
| ABCD3 | Exon1 | rs4148058 | 5′ untranslated region | |
| Exon2 | rs2147794 | Intron | ||
| Exon3 | rs16946 | Coding synonymous | ||
| Exon7 | rs681187 | Intron | ||
| Exon23 | rs662813 | 3′ untranslated region | ||
| Exon23 | rs337592 | 3′ untranslated region | ||
| ABCD4 | 5′UTR | rs17782508a | Upstream at the transcription start site | |
| Intron1 | rs17182959 | Intron | ||
| Intron1 | rs17158118 | Intron | ||
| Exon3 | rs2301345a | Coding synonymous | L62L | |
| Exon9 | rs4148077a | Coding nonsynonymous | A304T | |
| Exon10 | rs4148078a | Coding synonymous | L320L | |
| Exon11 | rs3742801a | Coding nonsynonymous | E368K |
A total of 24 SNPs of ABCD2, ABCD3, and ABCD4 were identified in 40 ALD patients. Among them, 11 SNPs (45.8%) were novel SNPs. The positions of these novel SNPs were based on the UCSC genome browser hg18
aThese SNPs were in complete disequilibrium in the Japanese population
Table 5.
Association studies of detected single nucleotide polymorphism (SNPs) with the clinical phenotypes of Japanese adrenoleukodystrophy patients: novel SNPs
| Gene | SNP name | Allele frequency (number) | p valuea | ||||
|---|---|---|---|---|---|---|---|
| Cerebral form (a total of 44 chromosomes) | AMN (a total of 26 chromosomes) | Control (number of detected SNPs/total number) | Cerebral form vs AMN | Cerebral form vs control | AMN vs control | ||
| ABCD2 | Novel SNP1 | 3 | 0 | 5/164 | 0.2894 | 0.3700 | 1.0000 |
| Novel SNP2 | 1 | 0 | 3/164 | 1.0000 | 1.0000 | 1.0000 | |
| ABCD3 | Novel SNP3 | 1 | 0 | 0/164 | 1.0000 | 0.2115 | 1.0000 |
| Novel SNP4 | 0 | 0 | 3/134 | 1.0000 | 1.0000 | 1.0000 | |
| Novel SNP5 | 1 | 0 | 0/160 | 1.0000 | 0.2157 | 1.0000 | |
| ABCD4 | Novel SNP6 | 17 | 9 | 67/164 | 0.8019 | 0.8635 | 0.6680 |
| Novel SNP7 | 2 | 1 | 2/164 | 1.0000 | 0.1974 | 0.3585 | |
| Novel SNP8 | 1 | 0 | 5/164 | 1.0000 | 1.0000 | 1.0000 | |
| Novel SNP9 | 0 | 1 | 0/164 | 0.3714 | 1.0000 | 0.1368 | |
| Novel SNP10 | 4 | 5 | 14/160 | 0.2766 | 1.0000 | 1.0000 | |
| Novel SNP11 | 1 | 0 | 5/160 | 1.0000 | 1.0000 | 1.0000 | |
aResults of two-sided Fisher's exact test
Table 6.
Association studies of detected single nucleotide polymorphism (SNPs) with the clinical phenotypes of Japanese adrenoleukodystrophy patients: known SNPs
| Gene | SNP ID | Allele frequency (number) | p valueb | ||||
|---|---|---|---|---|---|---|---|
| Cerebral form (a total of 44 chromosomes) | AMN (a total of 26 chromosomes) | Control (number of detected SNPs/total number) | Cerebral form vs AMN | Cerebral form vs control | AMN vs control | ||
| ABCD3 | Rs4148058 | 11 | 5 | 22/164 | 0.7697 | 0.1010 | 0.3820 |
| Rs2147794 | 6 | 2 | 34/152 | 0.7009 | 0.2880 | 0.0740 | |
| Rs16946 | 6 | 3 | 35/164 | 1.0000 | 0.2933 | 0.3019 | |
| Rs681187 | 17 | 9 | 75/158 | 1.0000 | 0.3109 | 0.2890 | |
| Rs662813 | 18 | 10 | 42/152 | 1.0000 | 0.0984 | 0.1435 | |
| Rs337592 | 2 | 4 | 19/152 | 0.1855 | 0.1714 | 0.7512 | |
| ABCD4 | Rs17782508a | 9 | 2 | 42/164 | 0.1921 | 0.5575 | 0.0468 |
| Rs17182959 | 10 | 7 | 40/128 | 0.5599 | 0.3386 | 0.8163 | |
| Rs17158118 | 10 | 7 | 33/162 | 0.5599 | 0.8344 | 0.4454 | |
| Rs2301345a | 9 | 2 | 42/164 | 0.1921 | 0.5575 | 0.0468 | |
| Rs4148077a | 9 | 2 | 42/164 | 0.1921 | 0.5575 | 0.0468 | |
| Rs4148078a | 9 | 2 | 42/164 | 0.1921 | 0.5575 | 0.0468 | |
| Rs3742801a | 9 | 2 | 42/164 | 0.1921 | 0.5575 | 0.0468 | |
aFive SNPs (rs17782508, rs2301345, rs4148077, rs4848078, and rs3742801) were in complete disequilibrium in the Japanese population
bResults of two-sided Fisher's exact test
Association studies of SNPs of ABCD2, ABCD3, and ABCD4 with the clinical phenotypes of ALD
Using the 11 novel SNPs and 13 previously described SNPs in ABCD2, ABCD3, and ABCD4, we conducted association studies of these SNPs with the clinical phenotypes of ALD (Tables 5 and 6).
For ABCD2, we analyzed two novel SNPs (novel SNP1 and novel SNP2). There were no significant differences in the allele frequencies between patients with cerebral form and those with AMN, or between the patients with individual phenotypes of ALD and the controls. For ABCD3, we analyzed three novel SNPs (novel SNP3, novel SNP4, and novel SNP5) and six previously described SNPs (rs4148058, rs2147794, rs16946, rs681187, rs662813, and rs337592). However, we did not also detect any significant associations.
For ABCD4, we analyzed six novel SNPs (novel SNP6, novel SNP7, novel SNP8, novel SNP9, novel SNP10, and novel SNP11) and seven previously described SNPs (rs17782508, rs17182959, rs17158118, rs2301345, rs4148077, rs4148078, and rs3742801). Interestingly, the five previously described SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) that are in complete linkage disequilibrium were significantly less frequently represented in the patients with Japanese AMN than in the controls in the Japanese population (p = 0.0468), whereas there were no significant differences in the Japanese patients with the cerebral form compared with the controls (Tables 5 and 6).
Given the significant association of the five SNPs (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) with the phenotypes of AMN, we then conducted a replication study on an independent French ALD cohort with extreme phenotypes (117 CCALD cases and 71 pure AMN cases). However, we did not find any significant association of these five SNPs with AMN or CCALD. Interestingly, the combination of two intronic SNPs (A (rs17182959) and G (rs7158118)) was significantly more frequently represented in the 51 patients with AMN-Cer than in those with CCALD in the French ALD cohort (p = 0.0049). The combination of these two intronic SNPs (A (rs17182959) and G (rs7158118)), however, was not present in any of the Japanese patients with ALD, although one combination of two intronic SNPs (A (rs17182959) and G (rs7158118)) was present in the Japanese controls (Tables 5 and 6 ; ESM Table 5).
Discussion
Our microarray-based high-throughput mutational analysis system was accurate to detect all the mutations, which were confirmed by direct nucleotide sequence analysis. This system should be highly useful for the mutational analysis of ABCD1 for the diagnosis of patients with ALD, and the diagnosis of the carriers with ALD.
Although reverse transcription (RT)-PCR has been preferentially used for the analysis of ABCD1 gene [11, 12, 26] to overcome the difficulty of specifically amplifying the ABCD1 gene owing to the existence of the related highly homologous genes [13, 27], primers allowing the specific amplification of ABCD1 enable the PCR analysis of genomic DNA, which is much easier than RT-PCR analysis. Similar approaches have also been used for SSCP-based [13, 28] and DNA-based diagnostic testing methods [29].
In the Japanese ALD patients, mutations of ABCD1 gene were widely scattered in the entire region of ABCD1 gene. All types of ABCD1 mutation were distributed among all the phenotypes of ALD, including childhood cerebral form, AMN, and adulthood cerebral form, suggesting that there is no association of a particular phenotype of ALD with individual mutations as previously observed in other ALD populations [1, 11–13, 15] (http://www.x-ald.nl/). Even among the frameshift mutations that are clearly expected to cause a complete loss of ALDP functions, such ABCD1 mutations were distributed among all the phenotypes of ALD.
On the basis of the comprehensive resequencing of ABCD2, ABCD3, and ABCD4 genes, we searched for SNPs of these related genes to explore the possibility of these genes as candidate disease-modifying genes for ALD, although it was shown that ALD phenotypes are independent of ABCD2 genotype in two independent association studies of ABCD2 polymorphisms and ALD phenotypes [30]. Although our study did not reveal SNPs significantly associated with the clinical phenotypes irrespective of the ethnic background, the SNPs in ABCD4 with suggestive association in the Japanese patients (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801) and French patients (rs17182959 and rs7158118) may still deserve further investigation including association studies on other independent cohorts and studies on the biological effects related to these SNPs. Among the SNPs with suggestive association in the Japanese patients (rs17782508, rs2301345, rs4148077, rs4148078, and rs3742801), rs4148077 (A304T) substitutes a hydrophilic amino acid for a hydrophobic amino acid, and rs3742801 (E368K) substitutes a basic amino acid for an acidic amino acid. It would be interesting to investigate if these amino acid substitutions may have relevance to the function of ABCD4.
In this study, we identified as many as 11 novel SNPs in ABCD2, ABCD3, and ABCD4 genes in addition to the 13 previously described SNPs. These findings indicate that there are still numerous novel SNPs with the number comparable to that of previously described SNPs; furthermore, this study places great emphasis on the role of comprehensive resequencing in the discovery of novel SNPs in relevant genes. The novel SNPs as well as previously described ones in ABCD2, ABCD3, and ABCD4 genes should be useful for further association studies on ALD and other peroxisome diseases and on the biological implications associated with these SNPs.
The diverse phenotypic variations of ALD still remain enigmatic. Recent studies suggest the role of perosixomes of oligodendrocytes in axonal loss and neuroinflammation [31] and microglial apoptosis as an early pathogenic change in CCALD [32]. With the advancement in our understanding of the pathophysiology of ALD, we hope that we can further probe into the disease-modifying factors on the basis of the molecular pathogenesis of ALD. Genome-wide association studies may well serve as an alternative approach for the identification of disease-modifying genetic factors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Primers for amplification of ABCD1 (DOC 38 kb)
Primers for amplification of ABCD2 (DOC 46 kb)
Primers for amplification of ABCD3 (DOC 56 kb)
Primers for amplification of ABCD4 (DOC 63 kb)
Identified single nucleotide polymorphisms in each patient (XLS 34 kb)
Acknowledgements
This study was partially supported by KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas, Applied Genomics, the Twenty-first Century COE Program, Center for Integrated Brain Medical Science, and Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a Grant-in-Aid for “the Research Committee for Ataxic Diseases” of the Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare, Japan; and a grant from the Takeda Foundation. MA and PA were supported by grants from INSERM and European Leukodystrophy Foundation (2008-001C4A).
Disclosures
The experiments comply with the current laws of the country in which they were performed. The authors report no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser HW, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 2.Schaumburg HH, Powers JM, Raine CS, Suzuki K, Richardson EP., Jr Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975;32:577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- 3.Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997;120:1485–1508. doi: 10.1093/brain/120.8.1485. [DOI] [PubMed] [Google Scholar]
- 4.van Geel BM, Assies J, Wanders RJA, Barth PG. X-linked adrenokeukodystrophy: clinical presentation, diagnosis, and therapy. J Neurol Neurosurg Psychiatry. 1997;63:4–14. doi: 10.1136/jnnp.63.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igarashi M, Schaumburg HH, Powers J, Kishimoto Y, Kolodny EH, Suzuki K. Fatty acid abnormality in adrenoleukodystrophy. J Neurochem. 1976;26:851–860. doi: 10.1111/j.1471-4159.1976.tb04462.x. [DOI] [PubMed] [Google Scholar]
- 6.Moser HW, Moser AB, Kawamura N, Murphy J, Suzuki K, Schaumburg H, Kishimoto Y. Adrenoleukodystrophy: elevated C26 fatty acid in cultured skin fibroblasts. Ann Neurol. 1980;7:542–549. doi: 10.1002/ana.410070607. [DOI] [PubMed] [Google Scholar]
- 7.Moser AB, Kreiter N, Bezman L, Lu S, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3, 000 peroxisome disease patients and 29, 000 controls. Ann Neurol. 1999;45:100–110. doi: 10.1002/1531-8249(199901)45:1<100::AID-ART16>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 8.Aubourg P, Blanche S, Jambaqué I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, Rolland MO, Debre M, Chaussain JL, Griscelli C, Fischer A, Bougnères PF. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990;322:1860–1866. doi: 10.1056/NEJM199006283222607. [DOI] [PubMed] [Google Scholar]
- 9.Malm G, Ringden O, Anvret M, von Dobeln U, Hagenfeldt L, Isberg B, Knuutila S, Nennesmo I, Winiarski J, Marcus C. Treatment of adrenoleukodystrophy with bone marrow transplantation. Acta Paediatr. 1997;86:484–492. doi: 10.1111/j.1651-2227.1997.tb08918.x. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro E, Krivit W, Lockman L, Jambaque I, Peters C, Cowan M, Harris R, Blanche S, Bordigoni P, Loes D, Ziegler R, Crittenden M, Ris D, Berg B, Cox C, Moser H, Fischer A, Aubourg P. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356:713–718. doi: 10.1016/S0140-6736(00)02629-5. [DOI] [PubMed] [Google Scholar]
- 11.Krasemann EW, Meier V, Korenke GC, Hunneman DH, Hanefeld F. Identification of mutations in the ALD-gene of 20 families with adrenoleukodystrophy/adrenomyeloneuropathy. Hum Genet. 1996;97:194–197. doi: 10.1007/BF02265264. [DOI] [PubMed] [Google Scholar]
- 12.Takano H, Koike R, Onodera O, Sasaki R, Tsuji S. Mutational analysis and genotype-phenotype correlation of 29 unrelated Japanese patients with X-linked adrenoleukodystrophy. Arch Neurol. 1999;56:295–300. doi: 10.1001/archneur.56.3.295. [DOI] [PubMed] [Google Scholar]
- 13.Kok F, Neumann S, Sarde CO, Zheng S, Wu KH, Wei HM, Bergin J, Watkins PA, Gould S, Sack G, Moser HW, Mandel J, Smith KD. Mutational analysis of patients with X-linked adrenoleukodystrophy. Hum Mutat. 1995;6:104–115. doi: 10.1002/humu.1380060203. [DOI] [PubMed] [Google Scholar]
- 14.Ligtenberg MJ, Kemp S, Sarde CO, van Geel BM, Kleijer WJ, Barth PG, Mandel JL, van Oost BA, Bolhuis PA. Spectrum of mutations in the gene encoding the adrenoleukodystrophy protein. Am J Hum Genet. 1995;56:44–50. [PMC free article] [PubMed] [Google Scholar]
- 15.Berger J, Molzer B, Fae I, Bernheimer H. X-linked adrenoleukodystrophy (ALD): a novel mutation of the ALD gene in 6 members of a family presenting with 5 different phenotypes. Biochem Biophys Res Commun. 1994;205:1638–1643. doi: 10.1006/bbrc.1994.2855. [DOI] [PubMed] [Google Scholar]
- 16.Smith KD, Kemp S, Braiterman LT, Lu JF, Wei HM, Geraghty M, Stetten G, Bergin JS, Pevsner J, Watkins PA. X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem Res. 1999;24:521–535. doi: 10.1023/A:1022535930009. [DOI] [PubMed] [Google Scholar]
- 17.Broccardo C, Troffer-Charlier N, Savary S, Mandel JL, Chimini G. Exon organisation of the mouse gene encoding the adrenoleukodystrophy related protein (ALDRP) Eur J Hum Genet. 1998;6:638–641. doi: 10.1038/sj.ejhg.5200233. [DOI] [PubMed] [Google Scholar]
- 18.Lombard-Platet G, Savary S, Sarde CO, Mandel JL, Chimini G. A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc Natl Acad Sci USA. 1996;93:1265–1269. doi: 10.1073/pnas.93.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimarães CP, Domingues P, Aubourg P, Fouquet F, Pujol A, Jimenez-Sanchez G, Sá-Miranda C, Azevedo JE. Mouse liver PMP70 and ALDP: homomeric interactions prevail in vivo. Biochim Biophys Acta. 2004;1689:235–243. doi: 10.1016/j.bbadis.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Hillebrand M, Verrier SE, Ohlenbusch A, Schäffer A, Söling HD, Wouters FS, Gärtner J. Live cell FRET microscopy: homo- and heterodimerization of two human peroxisomal ABC transporters, the adrenoleukodystrophy protein (ALDP, ABCD1) and PMP70 (ABCD3) J Biol Chem. 2007;282:26997–27005. doi: 10.1074/jbc.M702122200. [DOI] [PubMed] [Google Scholar]
- 21.Liu LX, Janvier K, Berteaux-Lecellier V, Cartier N, Benarous R, Aubourg P. Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J Biol Chem. 1999;274:32738–32743. doi: 10.1074/jbc.274.46.32738. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka AR, Tanabe K, Morita M, Durisu M, Kasiwayama Y, Matsuo M, Kioka N, Amachi T, Imanaka I, Ueda K. ATP binding/hydrolysis by and phosphorylation of peroxisomal ATP-binding cassette proteins PMP70 (ABCD3) and adrenoleukodystrophy protein (ABCD1) J Biol Chem. 2002;277:40142–40147. doi: 10.1074/jbc.M205079200. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y, Seki N, Ishiura H, Mitsui J, Matsukawa T, Kishino A, Onodera O, Aoki M, Shimozawa N, Murayama S, Itoyama Y, Suzuki Y, Sobue G, Nishizawa M, Goto J, Tsuji S. Development of high-throughput microarray-based resequencing system for neurological disorders and its application to molecular genetics of amyotrophic lateral sclerosis. Arch Neurol. 2008;65:1326–1332. doi: 10.1001/archneur.65.10.1326. [DOI] [PubMed] [Google Scholar]
- 24.Wigginton JE, Abecasis GR. PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data. Bioinformatics. 2005;21:3445–3447. doi: 10.1093/bioinformatics/bti529. [DOI] [PubMed] [Google Scholar]
- 25.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 26.Lachtermacher MB, Seuanez HN, Moser AB, Moser HW, Smith KD. Determination of 30 X-linked adrenoleukodystrophy mutations, including 15 not previously described. Hum Mutat. 2000;15:348–353. doi: 10.1002/(SICI)1098-1004(200004)15:4<348::AID-HUMU7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Sarde CO, Mosser J, Kioschis P, Kretz C, Vicaire S, Aubourg P, Poustka A, Mandel JL. Genomic organization of the adrenoleukodystrophy gene. Genomics. 1994;22:13–20. doi: 10.1006/geno.1994.1339. [DOI] [PubMed] [Google Scholar]
- 28.Feigenbaum V, Lombard-Platet G, Guidoux S, Sarde C, Mandel JL, Aubourg P. Mutational and protein analysis of patients and heterozygous women with X-linked adrenoleukodystrophy. Am J Hum Genet. 1996;58:1135–1144. [PMC free article] [PubMed] [Google Scholar]
- 29.Boehm CD, Cutting GR, Lachtermacher MB, Moser HW, Chong SS. Accurate DNA-based diagnostic and carrier testing for X-linked adrenoleukodystrophy. Mol Genet Metab. 1999;66:128–136. doi: 10.1006/mgme.1998.2779. [DOI] [PubMed] [Google Scholar]
- 30.Maier EM, Mayerhofer PU, Asheuer M, Köhler W, Rothe M, Muntau AC, Roscher AA, Holzinger A, Aubourg P, Berger J. X-linked adrenoleukodystrophy phenotype is independent of ABCD2 genotype. Biochem Biophys Res Commun. 2008;377:176–180. doi: 10.1016/j.bbrc.2008.09.092. [DOI] [PubMed] [Google Scholar]
- 31.Kassmann CM, Lappe-Siefke C, Baes M, Brügger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J, Nave KA. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- 32.Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Naqpal S, Moser AB, Frosch MP, Ransohoff RM. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol. 2008;63:729–742. doi: 10.1002/ana.21391. [DOI] [PubMed] [Google Scholar]
- 33.Grabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adenemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for amplification of ABCD1 (DOC 38 kb)
Primers for amplification of ABCD2 (DOC 46 kb)
Primers for amplification of ABCD3 (DOC 56 kb)
Primers for amplification of ABCD4 (DOC 63 kb)
Identified single nucleotide polymorphisms in each patient (XLS 34 kb)


