Abstract
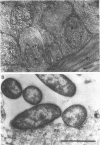
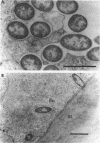
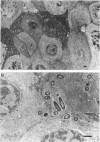
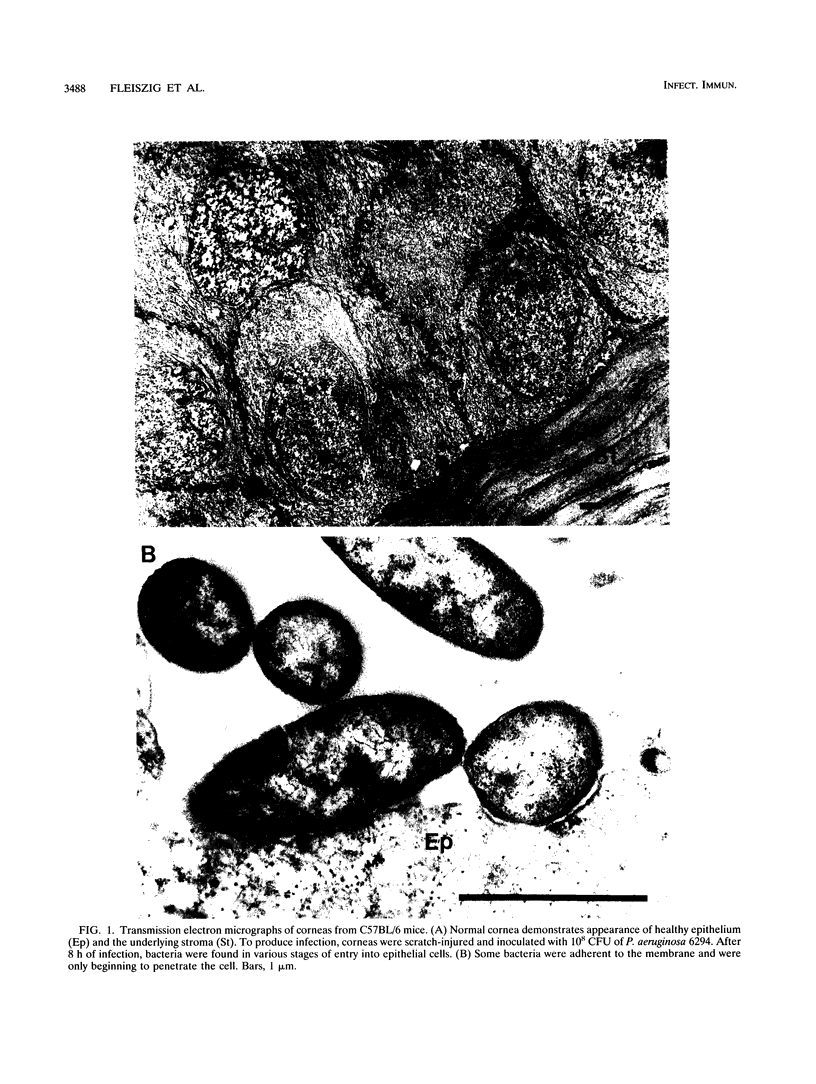
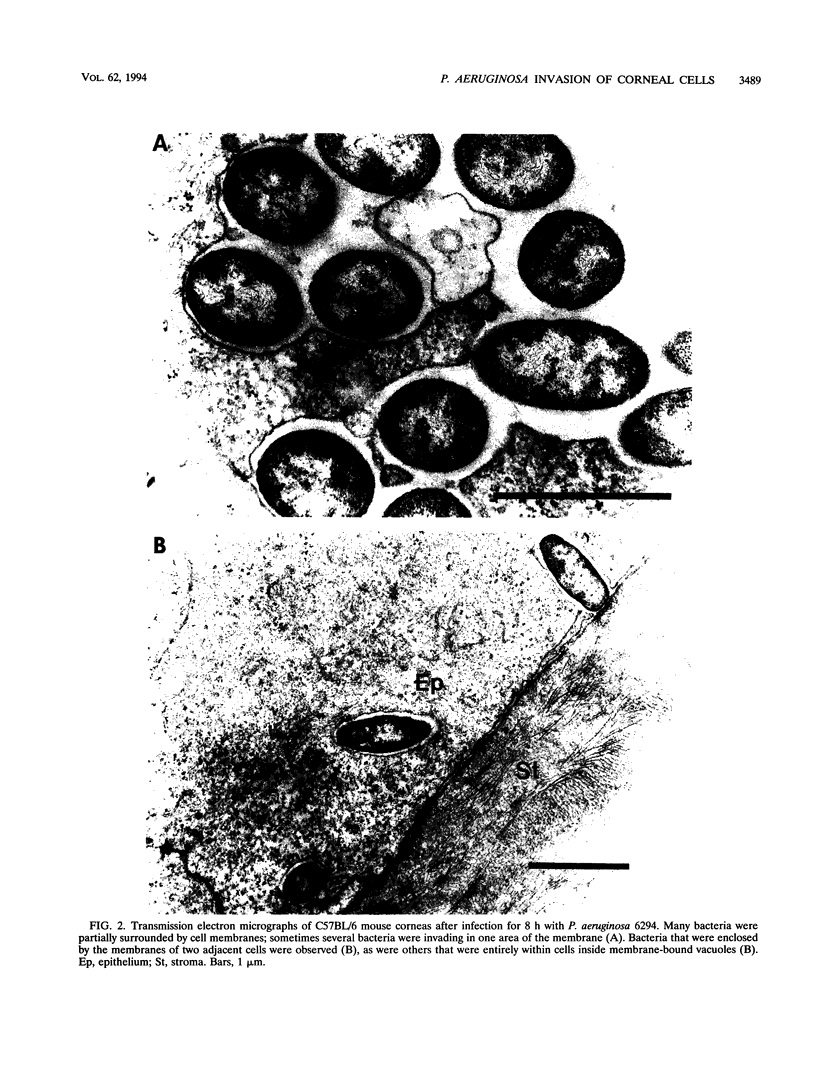
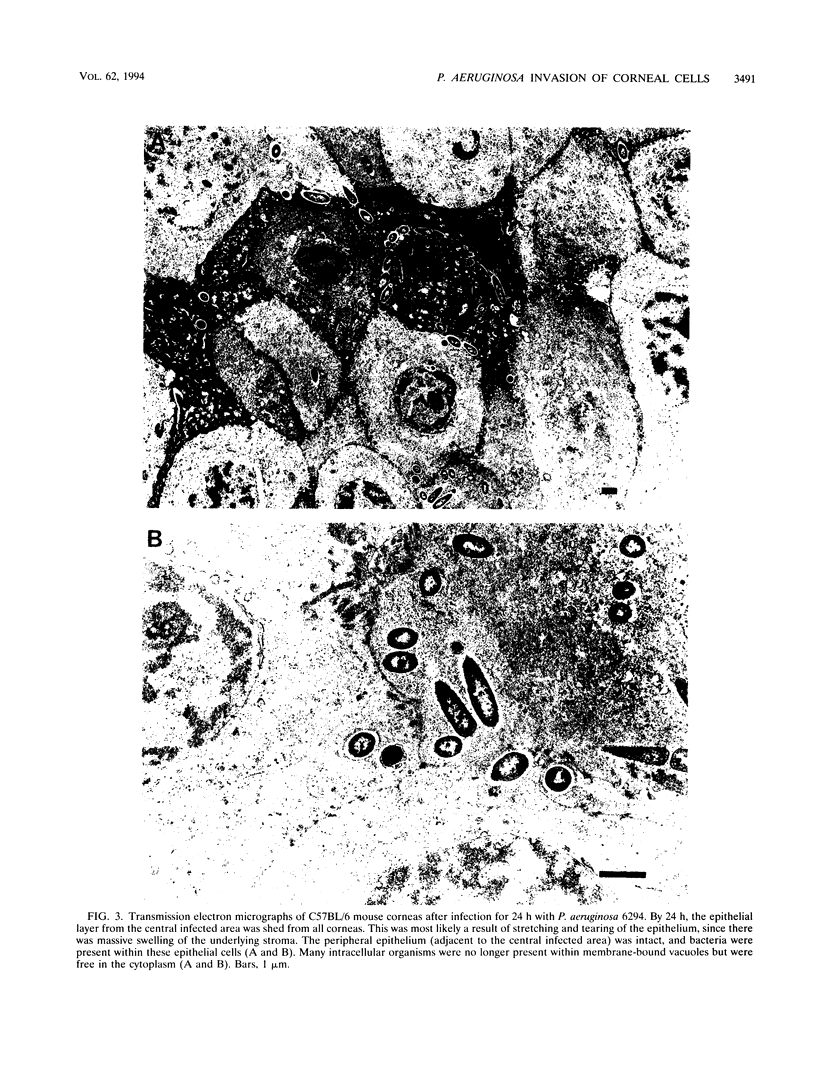
Pseudomonas aeruginosa is considered an extracellular pathogen. Using assays to determine intracellular survival in the presence of gentamicin, we have demonstrated that some strains of P. aeruginosa are able to invade corneal cells during experimental bacterial keratitis in mice. Although intracellular bacteria were detectable 15 min after inoculation, the number of intracellular bacteria increased in a time-dependent manner over a 24-h period. Levels of invasion were similar when bacteria were grown as a biofilm on solid medium and when they were grown in suspension. Intracellular bacteria survived in vitro for at least 24 h, although only minimal bacterial multiplication within cells was observed. P. aeruginosa PAK and Escherichia coli HB101 did not cause disease in this model and were not isolated from corneas after 24 h even when an inoculum of 10(8) CFU was applied. Transmission electron microscopy of corneal epithelium from eyes infected for 8 h revealed that intracellular bacteria were present within membrane-bound vacuoles, which suggests that bacterial entry was an endocytic process. At 24 h, the observation of many bacteria free in the cytoplasm indicated that P. aeruginosa was able to escape the endocytic vacuole. The ability of some P. aeruginosa strains to invade corneal epithelial cells may contribute to the pathogenesis or to the progression of disease, since intracellular bacteria can evade host immune effectors and antibiotics commonly used to treat infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfonso E., Mandelbaum S., Fox M. J., Forster R. K. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986 Apr 15;101(4):429–433. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991 Mar;59(3):822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J. A., Schiemann D. A. HeLa cell infection by Yersinia enterocolitica: evidence for lack of intracellular multiplication and development of a new procedure for quantitative expression of infectivity. Infect Immun. 1981 Apr;32(1):48–55. doi: 10.1128/iai.32.1.48-55.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Fleiszig S. M., Efron N., Pier G. B. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci. 1992 Sep;33(10):2908–2916. [PubMed] [Google Scholar]
- Fleiszig S. M., Zaidi T. S., Ramphal R., Pier G. B. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994 May;62(5):1799–1804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring L. C., Heffron F., Finlay B. B., Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990 Feb;58(2):443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke J. R., Magliocco M. V. Experimental Pseudomonas aeruginosa Infection of the Mouse Cornea. Infect Immun. 1971 Feb;3(2):209–216. doi: 10.1128/iai.3.2.209-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano H., Hori M. Effect of contact lens wear on the mitoses of corneal epithelial cells: preliminary report. CLAO J. 1983 Apr-Jun;9(2):133–136. [PubMed] [Google Scholar]
- Hazlett L. D., Berk R. S. Heightened resistance of athymic, nude (nu/nu) mice to experimental Pseudomonas aeruginosa ocular infection. Infect Immun. 1978 Dec;22(3):926–933. doi: 10.1128/iai.22.3.926-933.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M. M., Singh A., Berk R. S., Rudner X. L. Analysis of adhesion, piliation, protease production and ocular infectivity of several P. aeruginosa strains. Curr Eye Res. 1991 Apr;10(4):351–362. doi: 10.3109/02713689108996341. [DOI] [PubMed] [Google Scholar]
- Jumblatt M. M., Neufeld A. H. Beta-adrenergic and serotonergic responsiveness of rabbit corneal epithelial cells in culture. Invest Ophthalmol Vis Sci. 1983 Aug;24(8):1139–1143. [PubMed] [Google Scholar]
- Lawin-Brüssel C. A., Refojo M. F., Leong F. L., Hanninen L., Kenyon K. R. Time course of experimental Pseudomonas aeruginosa keratitis in contact lens overwear. Arch Ophthalmol. 1990 Jul;108(7):1012–1019. doi: 10.1001/archopht.1990.01070090114052. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Rest R. F. Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5512–5516. doi: 10.1073/pnas.89.12.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern G. A., Lubniewski A., Allen C. The interaction between Pseudomonas aeruginosa and the corneal epithelium. An electron microscopic study. Arch Ophthalmol. 1985 Aug;103(8):1221–1225. doi: 10.1001/archopht.1985.01050080133033. [DOI] [PubMed] [Google Scholar]
- Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967 Jan;50(1):109–136. [PMC free article] [PubMed] [Google Scholar]
- Tümmler B. Unusual mechanism of pathogenicity of Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Infection. 1987 Jul-Aug;15(4):311–312. doi: 10.1007/BF01644144. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979 Dec;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoutman D. E., Hulbert W. C., Pasloske B. L., Joffe A. M., Volpel K., Trebilcock M. K., Paranchych W. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphologic study. Scanning Microsc. 1991 Mar;5(1):109–126. [PubMed] [Google Scholar]