Abstract
Essential thrombocythemia (ET) is a chronic myeloproliferative neoplasm which can cause thrombohaemorrhagic complications usually involving microvasculature.
Medium-sized arterial thrombosis has been reported, but coronary occlusion usually occurs with additional risk factors, for example, smoking, hyperlipidaemia and so on.
We present a case of acute myocardial infarction (AMI) in a young man (29 years) with ET but without any coronary artery associated risk factors. He was successfully treated for his AMI and ET with cytoreductive treatment and has recovered well.
Due to automated platelet counting, ET is being increasingly identified; early detection can prevent long-term complications, and patients can have normal life span.
Background
Although acute myocardial infarction (AMI) remains one of the most common medical emergencies, the front-line staff can put too much emphasis on age and risk factors alone.
This case was very good example in which the coronary care staff were quite reluctant and resistant for the thrombolytic treatment, as this young man had no cardiovascular risk factors (although had ECG changes).
He received the treatment appropriately after all. The repeated blood count examination confirmed that he had essential thrombocythemia (ET) which was the underlying cause of his AMI. This was confirmed by a haematologist and cardiologists.
This dilemma can delay the life-saving treatment which is crucial in saving the myocardium.
Case presentation
A 29-year-old physically active young man and previously in good health presented to coronary care unit with a history of severe precordial pain associated with sweating for 30 min while at rest.
He was non-smoker with no family history of hyperlipidaemia, diabetes mellitus and hypertension or coronary artery disease.
On examination he was in pain and was tachycardic (100/min). His blood pressure was 130/80 mm of Hg, and the general physical and systemic examination was normal. ECG showed significant ST-wave elevation in the inferior leads suggestive of myocardial infarction (ECG). Serial cardiac enzymes confirmed this. He was started on aspirin along with thrombolysis with streptokinase.
He responded well to the treatment and has remained asymptomatic since then.
Investigations
A stress test performed 6 weeks later and a subsequent coronary angiography proved to be normal. Left ventricular angiogram confirmed inferior myocardial infarction. In the absence of any risk factors, and considering his young age, he was evaluated to find out underlying cause.
Subsequently available blood tests showed normal glucose and lipid profile. However, full blood counts consistently showed raised platelet count of more than a million (1.2–1.4 million/mm3) confirmed by blood film examination.
His haemoglobin (Hb; 13.5 g/dl), white cell count (WCC; 10.6 × 103 µl, with normal differential count), mean cell volume (89 fl) and haematocrit (40) were normal.
Other investigations, including erythrocyte sedimentation rate, plasma viscosity, C-reactive protein, iron profile, chromosomal analysis and splenic ultrasonography, were normal.
Bone marrow was hyper cellular, with a marked increase of megakaryocytes and many of the large ones showing nuclear hyperlobularity. There was minimal increase in reticulin. Bone marrow karyotype was normal.
These negative tests excluded the diagnosis of reactive thrombocytosis (RT) and polycythemia vera (PRV). A diagnosis of ET was made as per the WHO and Polycythemia Vera Study Group (PVSG) criteria, and we concluded that coronary thrombosis in this young man was a result of this.1–3
Differential diagnosis
The differential diagnoses of ET are RT and PRV, myeloproliferative neoplasms and myelodysplastic syndromes. The causes of RT includes iron deficiency, asplenia, metastatic malignancies, infection or inflammatory process. These are ruled out as per WHO and PVSG criteria.2 3
Treatment
His platelet count was normalised initially with hydroxyurea. This was later changed to interferon, to avoid any possible side effects on fertility and as he failed to respond to anagrelide. He is also on aspirin.
Outcome and follow-up
He has remained asymptomatic with no further complications for 13 years of follow-up. His latest blood results are Hb 12.3 g/dl, WCC 7.0 × 103/µl and platelets 286/mm3.
Discussion
ET is a chronic myeloproliferative neoplasm, a heterogeneous group of diseases involving clonal hematopoietic stem cells that also includes polycythemia vera (PV), idiopathic myelofibrosis and chronic myelogenous leukaemia.4 5
Both PV and ET are characterised by increased sensitivity of haematopoietic cells to their respective primary humoral growth factors: erythroid precursors to erythropoietin in PV and megakaryocytes to thrombopoietin in ET.4
ET is increasingly recognised condition due to introduction of automated platelet counting. Although median age of patients with ET is 60 years, 10–25% of patients are less than 40 years of age and one-third of patients are asymptomatic.1
Janus kinase (JAK) signal transducers and activators of transcription pathway play a central role in initiating signal transduction from haematopoietic growth factor receptors.4 5
ET patients with JAK2 V617F mutation-positive are more sensitive to hydroxyurea.5
The principal causes of morbidity and mortality in ET are thrombosis, haemorrhage and progression to myelofibrosis or acute myelogenous leukaemia.4 5
Patients with ET may present with vasomotor symptoms, for example, TIA, visual disturbances, erythromelalgia, cerebral and myocardial infarction.1
DVT of lower extremities occurs particularly in younger patients with PV or ET.4
Haemostatic complications in ET appear to be multifactorial due to combination of: thrombocytosis, structural and functional abnormalities of platelets and their enhanced interaction with leucocytes and endothelial cells. Additional risk factors for thrombosis are, for example, advanced age, prior history of thrombosis, smoking and hyperlipidaemia.4–6
High-risk patients who should be considered for cytoreductive treatment include: 60 years of age, history of vasomotor symptoms, serious bleeding or thrombosis, and platelet count of 600/mm3 or more.5
Young patients without symptoms with platelet count of less than 600/mm3are considered low risk. Haemorrhagic complications are characteristically of ‘platelet type’, involving spontaneous bleeding at superficial sites, for example, skin, mucous membranes and so on. The risk of bleeding increases with extreme thrombocytosis possibly due to the acquired form of von Willebrand.4
The differential diagnoses of ET are RT and PRV, myeloproliferative neoplasms and myelodysplastic syndromes. The causes of RT includes iron deficiency, asplenia, metastatic malignancies, infection or inflammatory process.1 7 These are ruled out as per WHO and PVSG criteria.1–3
Due to potential therapeutic implications Philadelphia chromosome should be tested in ET patients.4
Recent studies in ET patients suggested that, compared to hydroxyurea plus aspirin, anagrelide plus aspirin was associated with an excess rate of arterial thrombosis, major bleeding and myelofibrotic transformation.4 5 8
Aspirin may be highly effective with cytoreductive therapy in PV and ET patients who have thrombotic and vascular complications. However, antiplatelet therapy must be used with caution in ET patients due to risk of serious bleeding when platelet count is more than 1000 × 10/litre.4 8
As patients with ET have near-normal life span4 and the myelosuppressive therapy itself increases risk of transformation to myelofibrosis or acute myelogenous leukaemia, the treatment has to be carefully planned, balancing risks and benefits.
Patients should be advised to stop smoking; avoid using non-steroidal anti-inflammatory drugs, contraceptives; and terminate any hormone replacement therapy.
Various cytoreductive drugs used in ET are hydroxyurea, anagrelide and interferon .4 5 8
We present a case of ET-related AMI in a healthy young man without any coronary artery-associated risk factors.
Figure 1.
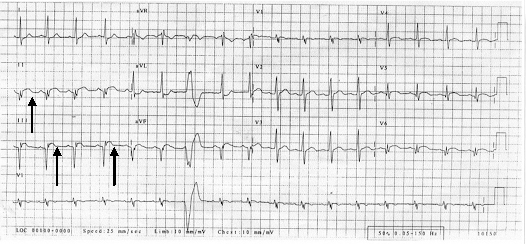
ECG: ST wave elevation in inferior leads, ventricular ectopic.
Learning points.
Young patients with no risk factors for coronary events should be assessed thoroughly on the basis of on their clinical history and presentation before ruling out the diagnosis (of AMI).
Patients with unexplained thrombohaemorrhagic events should have thrombophilia investigation.
ET is associated with low risk of leukaemic transformation and life-threatening complications; life expectancy is minimally affected. It is important to evaluate the risk factors for thrombohaemorrhagic complications, for example, age, thrombotic event, platelet count and cardiovascular risk factors. Treatment is started, balancing risk and benefits in light of the complications associated with the treatment.
In patients with established ET, search for congenital or acquired prothrombotic states should be undertaken as they increase the risk of thrombosis among these patients.
Aspirin should be used cautiously as it may increase risk of bleeding, especially when platelet count is high.
Acknowledgments
Dr Griffiths, Dr Diggory, Dr Thomas
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Murphy S. Diagnostic criteria and prognosis in polycythemia vera and essential thrombocythemia. Semin Hematol 1999;36(1 Suppl 2):9–13 [PubMed] [Google Scholar]
- 2.Murphy S, Peterson P, Iland H, et al. Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol 1997;34:29–39 [PubMed] [Google Scholar]
- 3.Tefferi A, Thiele J, Orazi A, et al. Proposals and rationale for revision of the world health organization diagnostic criteria for polycythemia vera, essential thrombocythemia and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood 2007;4:1092–97 [DOI] [PubMed] [Google Scholar]
- 4.Tiziano B, Guido F. When and how to treat essential thrombocythemia. N Engl J Med 2005;353:85–6 [DOI] [PubMed] [Google Scholar]
- 5.Alessandro MV, Guglielmelli P, Tefferi A. Advances in understanding and management of myeloproliferative neoplasms. CA Cancer J Clin 2009;59:171–91 [DOI] [PubMed] [Google Scholar]
- 6.Pick RA, Glover MU, Nanfro JJ, et al. Acute myocardial infarction with essential thrombocythemia in a young man. Am Heart J 1983;106:406–7 [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A. Essential thrombocythemia and agnogenic myeloid metaplasia. Haematology 1999;172–7 [PubMed] [Google Scholar]
- 8.Claire NH, Peter JC. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N Engl J Med 2005;353:33–45 [DOI] [PubMed] [Google Scholar]


