Among premenopausal women who had undergone mammography in the previous 2 years, mammography was more sensitive in the detection of breast cancer in those who underwent mammography during the 1st week of their menstrual cycle than in those who underwent mammography during the 2nd, 3rd, or 4th week of their menstrual cycle.
Abstract
Purpose:
To investigate sensitivity, specificity, and cancer detection rate of screening mammography according to week of menstrual cycle among premenopausal women.
Materials and Methods:
In this institutional review board–approved HIPAA-compliant study, sensitivity, specificity, and cancer detection rate of 387 218 screening mammograms linked to 1283 breast cancers in premenopausal women according to week of menstrual cycle were studied by using prospectively collected information from the Breast Cancer Surveillance Consortium. Logistic regression analysis was used to test for differences in mammography performance according to week of menstrual cycle, adjusting for age and registry.
Results:
Overall, screening mammography performance did not differ according to week of menstrual cycle. However, when analyses were subdivided according to prior mammography, different patterns emerged. For the 66.6% of women who had undergone regular screening (mammography had been performed within the past 2 years), sensitivity was higher in week 1 (79.5%) than in subsequent weeks (week 2, 70.3%; week 3, 67.4%; week 4, 73.0%; P = .041). In the 17.8% of women who underwent mammography for the first time in this study, sensitivity tended to be lower during the follicular phase (week 1, 72.1%; week 2, 80.4%; week 3, 84.6%; week 4, 93.8%; P = .051). Sensitivity did not vary significantly by week in menstrual cycle in women who had undergone mammography more than 3 years earlier. There were no clinically meaningful differences in specificity or cancer detection rate.
Conclusion:
Premenopausal women who undergo regular screening may benefit from higher sensitivity of mammography if they schedule screening mammography during the 1st week of their menstrual cycle.
© RSNA, 2010
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10100974/-/DC1
Introduction
Screening with mammography has been clearly shown to reduce mortality from breast cancer among women aged 40–69 years (1,2). However, on balance, the benefits of screening are less advantageous for women younger than 50 years (1–7), leading to controversy regarding whether women aged 40–49 years should undergo routine mammographic screening (8,9). Screening mammography is less sensitive (4–6) and less specific (5) in women aged 40–49 years than in older women. Improving the interpretive performance of mammography in premenopausal women could increase the net benefit of screening.
One explanation as to why screening mammography is less accurate in younger women is that they have a higher mammographic breast density than do older women (5,10–16). Previously, we reported that increased mammographic density explained 68% of the excess risk of having a false-negative result with mammography for women in their 40s compared with older women (17). Thus, the accuracy of mammography among premenopausal women might improve with screening at a point in their menstrual cycle when breast density is lower; several studies suggest this may occur during the follicular phase, which is the first half of the cycle (18–21). However, recent studies performed with continuous measures of breast density have shown that breast density changes during the menstrual phase are small and may not translate to clinically important improvements in mammography by themselves (20,21). To our knowledge, the only study in which researchers directly examined screening mammography accuracy according to menstrual cycle phase yielded inconclusive results (22). The researchers found that screening mammography performed during the follicular phase was 10% more sensitive and no more specific than screening mammography performed during the luteal phase; however, this difference was not significant because of the small number of women with cancer (n = 84).
Our purpose was to investigate the sensitivity, specificity, and cancer detection rate of screening mammography according to week of menstrual cycle among premenopausal women.
Materials and Methods
Study Population
Information was collected at the following six National Cancer Institute–funded Breast Cancer Surveillance Consortium mammography registries (http://breastscreening.cancer.gov) (23): Carolina Mammography Registry, Colorado Mammography Project, New Hampshire Mammography Network, New Mexico Mammography Project, Vermont Breast Cancer Surveillance System, and Group Health Cooperative in western Washington. At the registries, workers collected demographic and clinical information, including radiologists’ assessments and recommendations based on the mammographic interpretation, as well as patient risk factors, at each mammographic examination. Data were pooled at a central Statistical Coordinating Center (Seattle, Wash) for analysis. Each registry and the Statistical Coordinating Center received institutional review board approval for active or passive consenting processes or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures were compliant with the Health Insurance Portability and Accountability Act, and all registries and the Statistical Coordinating Center received a Federal Certificate of Confidentiality and other protection for the identities of women, physicians, and facilities.
We included both screen-film and digital screening mammograms obtained between 1996 and 2007 in premenopausal women aged 35–54 years with no history of breast cancer, mastectomy, or breast augmentation; these images had been interpreted by more than 770 radiologists. Women were considered premenopausal if they reported that their last menstrual period began no more than 35 days before the date of mammography and that they were not currently using hormone therapy. We excluded mammograms in women who reported oral contraceptive use at the time of the examination (n = 42 214, 9.8%). We excluded women whose last menstrual period occurred more than 35 days before mammography.
Data Collection and Definitions
Demographic and risk factor information, including birth date, race, ethnicity, menopausal status, hormone therapy use, oral contraceptive use, height, weight, and time since last mammography were collected with a questionnaire at each mammographic examination. For date of last menstrual period, one registry asked women to provide the date of their last menstrual period; two registries asked women to provide the date of the 1st day or the beginning of their last menstrual period; two registries asked in either of these ways in different study years; and one registry asked for the 1st day of the last menstrual period either as a date or as 1–7, 8–14, 15–21, 22–35, or more than 35 days ago depending on the study year (24). We used the self-reported time since last menstrual period to calculate each woman’s week in her menstrual cycle on the day of mammography, as follows: week 1, 0–7 days since last menstrual period; week 2, 8–14 days since last menstrual period; week 3, 15–21 days since last menstrual period; and week 4, 22–35 days since last menstrual period. The follicular phase was defined as week 1 or 2. The luteal phase was defined as week 3 or 4.
Mammography was considered a screening examination if the radiologist or technician indicated the examination was performed for routine screening and if he or she obtained bilateral routine images (25). To avoid misclassifying diagnostic mammography as a screening examination, we excluded mammograms obtained in women who had undergone a breast imaging examination within the prior 9 months.
Mammograms were classified as first mammograms when the Breast Cancer Surveillance Consortium database contained no prior mammograms, no indication of comparison images, and no self-report of prior mammography. Mammograms were classified as subsequent mammograms if they were obtained in a patient who (a) had undergone mammography within the prior 2 years or (b) had undergone mammography more than 3 years previously. Classification was based on a combination of mammograms in the database and on the self-reported date of last mammography, as recorded on the questionnaire.
The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) four-category terminology was used to classify mammographic breast density as almost entirely fat, scattered fibroglandular tissue, heterogeneously dense, or extremely dense (26). Mammographic assessments were collected by using the BI-RADS lexicon (26). We considered a mammogram positive if it was given a BI-RADS score of 0 (additional imaging evaluation needed), 4 (suspicious abnormality), or 5 (highly suggestive of malignancy) or if it was given a score of 3 (probably benign finding), with a recommendation for immediate follow-up (25). We considered a mammogram negative if it was given a BI-RADS score of 1 (negative) or 2 (benign finding) or if it was given a score of 3 (probably benign finding), with either no recommendation for follow-up or a recommendation for short-interval or routine follow-up (25).
To determine cancer status after mammography, each mammography registry linked to a state cancer registry or regional Surveillance, Epidemiology, and End Results program. Five of the six sites also linked to pathology databases. A woman was considered to have breast cancer if she received a diagnosis of invasive carcinoma or ductal carcinoma in situ within 12 months after screening mammography and before her next mammographic screening (25).
We calculated the sensitivity of mammography as the proportion of positive mammograms among women in whom breast cancer was diagnosed within 1 year after their examination (25,26). Specificity was defined as the proportion of negative mammograms among women without cancer during 1-year follow-up (25,26). The cancer detection rate was calculated as the proportion of mammograms with a positive assessment and that resulted in the diagnosis of cancer within 12 months after the examination. Data are presented as a rate per 10 000 mammograms (25,26).
Statistical Analysis
We calculated the distributions of population characteristics and mammogram outcomes by week of menstrual cycle. We used a χ2 test to compare the age distribution at first mammography versus that at subsequent mammography.
Since mammography performance and characteristics of screen-detected cancers depend on prior mammography exposure (27), analyses were performed overall and subdivided according to prior mammography. Unadjusted sensitivity, specificity, and cancer detection rate, as well as exact binomial 95% confidence intervals, were calculated according to week of menstrual cycle. We used logistic regression to test for associations between these performance measures and week of menstrual cycle, adjusting for age (as a continuous linear term) and mammography registry. To compare sensitivity (or equivalently, false-negative rates), we modeled the odds of a negative mammogram among women in whom breast cancer was diagnosed. To compare specificity (or equivalently, false-positive rates), we modeled the odds of a positive mammogram among women without a breast cancer diagnosis. Odds ratios are reported with 95% likelihood ratio confidence intervals, with week 4 selected as the reference group because the biologic activity in the breast is greatest in that week. Reported P values were calculated with likelihood ratio tests, testing for an overall association between week of menstrual cycle and each performance measure.
We examined histology and tumor size of cancers detected at first mammography versus these detected at subsequent mammography.
We conducted several sensitivity analyses to assess the potential influence of differences in data collection and our inclusion criteria on study results. We repeated all analyses by excluding data from individual registries one at a time. We restricted analyses to sites that specifically ask for the 1st day or beginning of the last menstrual period. We excluded obese (body mass index ≥30) and underweight (body mass index <18.5) women, because weight can influence the hormonal balance and menstrual cycle. We removed women whose time since last menstrual cycle exceeded 28 days to omit long menstrual cycles that are more likely to be anovulatory. We estimated models separately for women aged 35–44 years and women aged 45–54 years to account for the relation between age and whether the screening was first mammography or subsequent mammography.
We used a two-sided α value of .05 to determine statistical significance. Analyses were performed with statistical software (SAS, version 9.2; SAS Institute, Cary, NC).
Results
We included 387 218 screening mammograms obtained in premenopausal women (Table 1). Women included in the study were primarily aged 40–49 years. Only 17.8% of mammograms were first mammograms, and 66.6% of women had undergone previous mammography within the prior 2 years. Age, prior mammography, breast symptoms, and BI-RADS breast density did not vary by week of menstrual cycle. Women who had not undergone prior mammography were younger than women who had (P < .001): Of the 64 258 women who were undergoing mammography for the first time, 36.1% were aged 35–39 years, 50.8% were aged 40–44 years, 10.7% were aged 45–59 years, and only 2.4% were aged 50–54 years. In contrast, of the 296 706 women who were undergoing subsequent mammography, only 5.9% were aged 35–39 years, 41.0% were aged 40–44 years, 39.4% were aged 45–49 years, and 13.8% were aged 50–54 years.
Table 1.
Distribution of Population Characteristics and Mammography Outcomes by Week in Menstrual Cycle among 387 218 Screening Mammograms in Premenopausal Women between 1996 and 2007
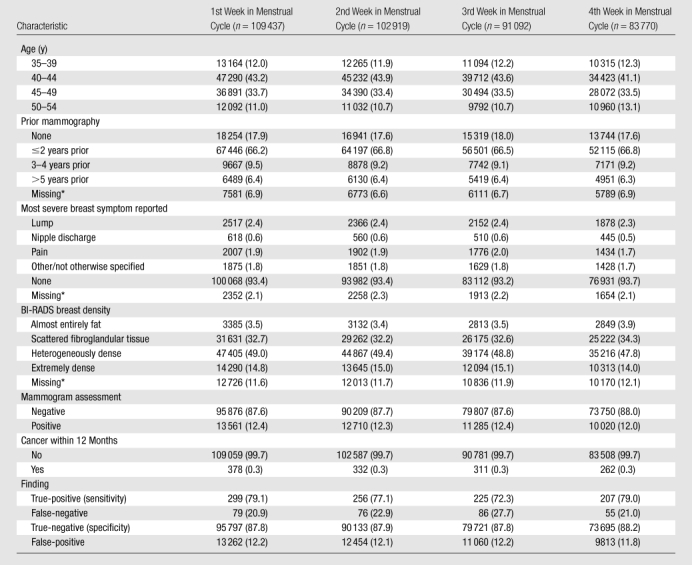
Note.—Data are numbers of women. Data in parentheses are percentages. Unless otherwise indicated, percentages were calculated for women with known values.
Percentages were calculated for the total number of women.
We found that 12.3% of mammograms were positive, and 0.3% of mammograms led to a diagnosis of breast cancer. Overall, the percentage of true-positive (sensitivity) and true-negative (specificity) mammograms did not differ significantly (P = .21 and P = .14, respectively) by week in menstrual cycle; however, sensitivity was lower in week 3 (72.3%) than in other weeks (77.1%–79.1%) (Table 1).
When we subdivided the sample according to prior mammography, different patterns emerged within the strata (Tables 2, 3). In women undergoing mammography for the first time, a borderline significant association between week in menstrual cycle and sensitivity was observed after adjusting for age and registry (P = .051), with weeks 1 and 2 having lower sensitivity (72.1% and 80.4%, respectively), and thus higher false-negative rates, than weeks 3 and 4 (84.6% and 93.8%, respectively). The greatest estimated difference in odds of a false-negative finding was between weeks 1 and 4 (odds ratio, 6.04; 95% confidence interval: 1.50, 40.90; P = .009).
Table 2.
Sensitivity, Specificity, and Cancer Detection Rate of Screening Mammography and Adjusted Odds Ratios and 95% Confidence Intervals by Week in Cycle, Subdivided by Time Since Prior Mammography
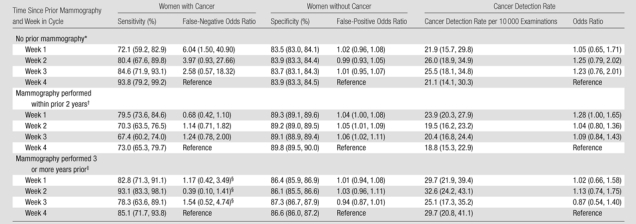
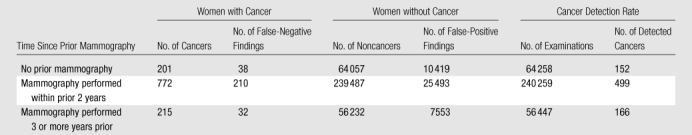
Table 3.
Cancers, Noncancers, and Cancer Detection Rate Subdivided by Time Since Prior Mammography
For subsequent mammography performed within 2 years after the previous examination (Table 2), sensitivity varied significantly with week in menstrual cycle after adjusting for age and registry (P = .041): Week 1 had the highest sensitivity (79.5%) compared with weeks 2, 3, and 4 (sensitivity, 67.4%–73.0%). For women in whom previous mammography had been performed 3 or more years prior, unadjusted sensitivity was highest during the follicular phase (82.8%–93.1% vs 78.3%–85.1% in the luteal phase). For this subgroup, the logistic regression model adjusting for both age and registry did not converge because of sample size limitations across registry sites. When registry was dropped from the model, these observed differences were not significant (P = .13).
For specificity, even though some of the associations were significant because of the large sample sizes, differences according to week in cycle were modest for first and subsequent examinations; in fact, most results differed by less than 1.0% (Table 2). We observed no significant differences in cancer detection rates by week in menstrual cycle.
Results of the sensitivity analyses resembled those presented in Tables E1–E11 (online).
Among women undergoing mammography for the first time, the 163 screen-detected cancers were more likely than ductal carcinoma in situ to be invasive in weeks 3 (n = 35, 79.6%) and 4 (n = 24, 80.0%) than in weeks 1 (n = 27, 61.4%) and 2 (n = 30, 66.7%); this did not hold true in women undergoing subsequent mammography. When we restricted the sample to all 149 invasive cancers (screen-detected cancers or otherwise) among women undergoing mammography for the first time, the association between sensitivity and week in cycle strengthened (week 1, 62.8%; 95% confidence interval: 46.7, 77.0; week 2, 75.0; 95% confidence interval: 58.8, 87.3; week 3, 85.4%; 95% confidence interval: 70.8, 94.4; week 4, 96.0%; 95% confidence interval: 79.6, 99.9; P = .0045). Large invasive tumors (15 mm in diameter or larger) were found more frequently among the 109 screen-detected invasive cancers (with known tumor size) in women undergoing mammography for the first time (n = 63, 57.8%) than in the 483 women undergoing subsequent mammography (n = 208, 43.1%); however, we detected no trends in invasive cancer tumor size according to week in menstrual cycle.
Discussion
Among premenopausal women who had undergone previous mammography in the prior 2 years, mammography was more sensitive in the detection of breast cancer in women who had udergone mammography during the 1st week of their menstrual cycle compared with those who underwent mammography during the 2nd, 3rd, or 4th week of the cycle. Our results are consistent with the findings of the Canadian National Breast Screening Study (22), which revealed a sensitivity of 59% during the first half of the menstrual cycle and 49% during the second half in 84 women who entered the study aged 40–44 years and developed breast cancer; however, this difference was not significant. Also like the Canadian study, our study showed no difference in specificity according to menstrual cycle phase.
If screening during the follicular phase increases the sensitivity of mammography in women who undergo regular screening, the mechanism may be lower mammographic breast density during that phase. In a cross-sectional study (18), we found that a significantly larger proportion of women were categorized as having extremely dense breasts during the luteal phase (28% for both week 3 and week 4) than during the follicular phase (24% and 23% for weeks 1 and 2, respectively). In another study in which we used paired quantitative measures of breast density obtained 9–18 months apart in 204 premenopausal women aged 40–55 years, we found a small nonsignificant increase in the percentage of breast density during days 22–35 compared with days 9–14 (1.1%, P = .09) (21). Several other studies have reported similarly small borderline significant increases in quantitative measures of breast density during the luteal phase (20); however, Hovhannisyan and colleagues (28) found no evidence of differences in density. In our study of 264 030 women with a BI-RADS density measurement, we saw no difference in density according to phase of the menstrual cycle; this may have been due to measurement error introduced by the fact that breast density was rated by more than 770 community-based radiologists (29–32).
Mammographic breast density is a measure of the nonfat epithelial and stromal components of the breast, both of which are influenced by the menstrual cycle. Studies based on breast magnetic resonance (MR) imaging show that parenchyma volume is lowest immediately before ovulation and increases during the luteal phase (33,34). Recommendations for screening with MR imaging suggest that the examination be performed during the 2nd week of the menstrual cycle (35,36). Pathologic studies of human breast tissue revealed greater epithelial cell proliferation, lobule size, and stromal edema in the luteal phase (37–41). A meta-analysis reported breast epithelial cell mitosis, epithelial volume, and water tend to peak in the middle of week 4 (42).
A second mechanism for increased false-negative results during the luteal phase may be poorer breast compression for mammograms obtained in this phase, when many women experience breast tenderness and engorgement (42). Hovhannisyan et al (28) found a significantly higher compression force was needed to obtained film mammograms during week 4.
Why would there be a benefit of screening before ovulation for subsequent mammography but not first mammography? Mammography is less sensitive in women who undergo regular screening (27) because tumors tend to be smaller in these women. The small changes in breast density that occur during the menstrual cycle may be enough to improve sensitivity for finding small tumors. In contrast, cancers detected at first mammography and those detected on mammograms obtained in women with no prior recent screening are larger on average and more easily detectable; therefore, small fluctuations in breast density or poorer breast compression may have less influence on the sensitivity of mammography in the detection of these established tumors.
Our finding of higher sensitivity after ovulation (weeks 3 and 4) in women who underwent first mammography is more difficult to interpret. Benign breast cysts enlarge in some women during the luteal phase (43); however, the menstrual cycle does not appear to affect breast tumor cell proliferation (44). Among first mammograms, screen-detected cancers were more likely to be invasive during the luteal phase, and the relationship between sensitivity and week in cycle strengthened when restricted to invasive tumors. Perhaps progesterone-dependent stromal edema during the luteal phase affects the stroma surrounding more established (larger) tumors differently than tumor stroma, making tumors easier to detect. Changes in stromal edema may alter normal stroma density more than tumor-induced fibrosis. This potentially heightened contrast between invasive tumors and normal stroma might enhance detection during the luteal phase.
One limitation of our study was the potential for measurement error inherent in estimating time in the menstrual cycle from self reporting and lack of information on actual day of ovulation. However, women tend to estimate their day of last menstrual cycle accurately (45). We assumed ovulation occurred on day 14, independent of a woman’s cycle length, which we did not ascertain. Errors in the estimated week in cycle and the radiologists’ assessments of the mammograms are unlikely to be correlated; thus, under reasonable measurement error scenarios, our results are likely attenuated toward the null (46). Still, our measure of self-reported time in the menstrual cycle reflects measures used in clinical practice, so the relationship presented here reflects the expected increase in sensitivity if mammography was timed on the basis of a woman’s self-reported menstrual cycle.
Scheduling mammography on the basis of time in the menstrual cycle would have practical limitations and would add complexity to mammography scheduling because women cannot always predict when their cycle will begin. On the other hand, because many women experience breast tenderness during the end of the luteal phase, avoiding this time could also reduce the discomfort of mammography.
In conclusion, our findings suggest that in women who undergo screening at regular intervals, the sensitivity of screening mammography may be higher during the 1st week of the menstrual cycle. This finding is consistent with the findings of Baines et al (22) and is supported by a plausible biologic mechanism through breast density, stromal edema, or both, and its effect on imaging. However, our finding of the opposite effect among first screening mammograms, for which sensitivity was lowest in week 1 and highest in week 4, is unexpected. Future research is needed to understand this finding. In the meantime, for premenopausal women who choose to undergo regular screening, the sensitivity of mammography may be improved by timing subsequent mammography to occur during the 1st week of their menstrual cycle.
Advances in Knowledge.
For subsequent screening mammography performed in patients who had undergone mammography in the past 2 years, sensitivity was highest in week 1 of the menstrual cycle (79.5%) and lower in later weeks (week 2, 70.3%; week 3, 67.4%; week 4, 73.0%).
For first screening mammography, sensitivity tended to be lower during the follicular phase (first half of the menstrual cycle) than during the luteal phase (second half of the menstrual cycle) (week 1, 72.1%; week 2, 80.4%; week 3, 84.6%; week 4, 93.8%).
No clinically meaningful differences in specificity or cancer detection rates according to week of menstrual cycle were detected.
Implication for Patient Care.
Premenopausal women who undergo regular screening mammography may benefit from higher sensitivity if they schedule their examination during the 1st week of their menstrual cycle.
Disclosures of Potential Conflicts of Interest: D.L.M. Financial activities related to the present article: institution received a grant from National Cancer Institute (NCI). Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. R.W. Financial activities related to the present article: institution received a grant from NCI. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. D.L.W. Financial activities related to the present article: institution received a grant from NCI; consultant for and received reimbursement for attending meetings from Group Health. Financial activities not related to the present article: institution received a grant or has a grant pending from NCI. Other relationships: none to disclose. D.S.M.B. Financial activities related to the present article: institution received a grant and support for travel to meetings for the study or for other purposes from National Institutes of Health. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. S.H.T. No potential conflicts of interest to disclose. P.A.C. Financial activities related to the present article: institution received a grant from NCI. Financial activities not related to the present article: institution received a grant or has a grant pending from NCI. Other relationships: none to disclose. R.D.R. Financial activities related to the present article: institution received a grant from American Cancer Society and NCI; received support for travel to a meeting for the study or other purposes from American Cancer Society and NCI. Financial activities not related to the present article: institution received a grant or has a grant pending from NCI. Other relationships: none to disclose. M.B.D. Financial activities related to the present article: institution received a grant from AMC Cancer Research Center. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose. Z.Z. No potential conflicts of interest to disclose. E.W. Financial activities related to the present article: institution received Group Health subcontract from National Institutes of Health/NCI grant; received Group Health consulting fee from National Institutes of Health/NCI grant. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose.
Supplementary Material
Acknowledgments
The authors thank Rebecca Hughes, BA, for manuscript editing and Sarah Lowry, MPH, for help with literature review. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://breastscreening.cancer.gov/work/acknowledgement.html. The authors had full responsibility for the design of the study; collection, analysis, and interpretation of data; decision to submit the manuscript for publication; and writing of the manuscript. We thank the participating women, mammography facilities, and radiologists for the data they have provided. A list of the Breast Cancer Surveillance Consortium investigators and the procedures for requesting Breast Cancer Surveillance Consortium data for research purposes are provided at http://breastscreening.cancer.gov/.
Received May 18, 2010; revision requested June 28; revision received August 12; accepted August 18; final version accepted August 23.
Funding: This research was supported by the National Institutes of Health (grants U01CA63740, U01CA86076, U01CA86082, U01CA63736, U01CA70013, U01CA69976, U01CA63731, and U01CA70040).
Abbreviations:
- BI-RADS
- Breast Imaging Reporting and Data System
References
- 1.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151(10):727–737, W237–W242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 part 1):347–360 [DOI] [PubMed] [Google Scholar]
- 3.Mandelblatt JS, Cronin KA, Bailey S, et al. Effects of mammography screening under different screening schedules: model estimates of potential benefits and harms. Ann Intern Med 2009;151(10):738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography: a meta-analysis. JAMA 1995;273(2):149–154 [PubMed] [Google Scholar]
- 5.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 2003;138(3):168–175 [DOI] [PubMed] [Google Scholar]
- 6.Peer PG, Verbeek AL, Straatman H, Hendriks JH, Holland R. Age-specific sensitivities of mammographic screening for breast cancer. Breast Cancer Res Treat 1996;38(2):153–160 [DOI] [PubMed] [Google Scholar]
- 7.Yankaskas BC, Haneuse S, Kapp JM, et al. Performance of first mammography examination in women younger than 40 years. J Natl Cancer Inst 2010;102(10):692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernster VL. Mammography screening for women aged 40 through 49: a guidelines saga and a clarion call for informed decision making. Am J Public Health 1997;87(7):1103–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Preventive Services Task Force Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151(10):716–726, W-236 [Published correction appears in Ann Intern Med 2010;152(3):199–200.] [DOI] [PubMed] [Google Scholar]
- 10.Oza AM, Boyd NF. Mammographic parenchymal patterns: a marker of breast cancer risk. Epidemiol Rev 1993;15(1):196–208 [DOI] [PubMed] [Google Scholar]
- 11.El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States). Cancer Causes Control 2000;11(10):955–963 [DOI] [PubMed] [Google Scholar]
- 12.Ciatto S, Visioli C, Paci E, Zappa M. Breast density as a determinant of interval cancer at mammographic screening. Br J Cancer 2004;90(2):393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA 1996;276(1):33–38 [PubMed] [Google Scholar]
- 14.Ma L, Fishell E, Wright B, Hanna W, Allan S, Boyd NF. Case-control study of factors associated with failure to detect breast cancer by mammography. J Natl Cancer Inst 1992;84(10):781–785 [DOI] [PubMed] [Google Scholar]
- 15.Bird RE, Wallace TW, Yankaskas BC. Analysis of cancers missed at screening mammography. Radiology 1992;184(3):613–617 [DOI] [PubMed] [Google Scholar]
- 16.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92(13):1081–1087 [DOI] [PubMed] [Google Scholar]
- 17.Buist DS, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40–49 years. J Natl Cancer Inst 2004;96(19):1432–1440 [DOI] [PubMed] [Google Scholar]
- 18.White E, Velentgas P, Mandelson MT, et al. Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 years. J Natl Cancer Inst 1998;90(12):906–910 [DOI] [PubMed] [Google Scholar]
- 19.Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev 2001;10(2):141–142 [PubMed] [Google Scholar]
- 20.Morrow M, Chatterton RT, Jr, Rademaker AW, et al. A prospective study of variability in mammographic density during the menstrual cycle. Breast Cancer Res Treat 2010;121(3):565–574 [DOI] [PubMed] [Google Scholar]
- 21.Buist DS, Aiello EJ, Miglioretti DL, White E. Mammographic breast density, dense area, and breast area differences by phase in the menstrual cycle. Cancer Epidemiol Biomarkers Prev 2006;15(11):2303–2306 [DOI] [PubMed] [Google Scholar]
- 22.Baines CJ, Vidmar M, McKeown-Eyssen G, Tibshirani R. Impact of menstrual phase on false-negative mammograms in the Canadian National Breast Screening Study. Cancer 1997;80(4):720–724 [PubMed] [Google Scholar]
- 23.Ballard-Barbash R, Taplin SH, Yankaskas BC, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol 1997;169(4):1001–1008 [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Breast Cancer Surveillance Consortium Standardized Questionnaires. National Cancer Institute Web site. http://breastscreening.cancer.gov/data/elements.html#questionnaires. Accessed May 13, 2010
- 25.National Cancer Institute Breast Cancer Surveillance C. BCSC Glossary of Terms. National Cancer Institute Web site. http://breastscreening.cancer.gov/data/bcsc_data_definitions.pdf. Accessed May 13, 2010
- 26.American College of Radiology American College of Radiology (ACR) Breast Imaging Reporting and Data System Atlas (BI-RADS Atlas). 4th ed. Reston, Va: American College of Radiology, 2003 [Google Scholar]
- 27.Yankaskas BC, Taplin SH, Ichikawa L, et al. Association between mammography timing and measures of screening performance in the United States. Radiology 2005;234(2):363–373 [DOI] [PubMed] [Google Scholar]
- 28.Hovhannisyan G, Chow L, Schlosser A, Yaffe MJ, Boyd NF, Martin LJ. Differences in measured mammographic density in the menstrual cycle. Cancer Epidemiol Biomarkers Prev 2009;18(7):1993–1999 [DOI] [PubMed] [Google Scholar]
- 29.Kerlikowske K, Grady D, Barclay J, et al. Variability and accuracy in mammographic interpretation using the American College of Radiology Breast Imaging Reporting and Data System. J Natl Cancer Inst 1998;90(23):1801–1809 [DOI] [PubMed] [Google Scholar]
- 30.Berg WA, Campassi C, Langenberg P, Sexton MJ. Breast Imaging Reporting and Data System: inter- and intraobserver variability in feature analysis and final assessment. AJR Am J Roentgenol 2000;174(6):1769–1777 [DOI] [PubMed] [Google Scholar]
- 31.Ooms EA, Zonderland HM, Eijkemans MJ, et al. Mammography: interobserver variability in breast density assessment. Breast 2007;16(6):568–576 [DOI] [PubMed] [Google Scholar]
- 32.Ciatto S, Houssami N, Apruzzese A, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast 2005;14(4):269–275 [DOI] [PubMed] [Google Scholar]
- 33.Fowler PA, Casey CE, Cameron GG, Foster MA, Knight CH. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol 1990;97(7):595–602 [DOI] [PubMed] [Google Scholar]
- 34.Graham SJ, Stanchev PL, Lloyd-Smith JO, Bronskill MJ, Plewes DB. Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging 1995;5(6):695–701 [DOI] [PubMed] [Google Scholar]
- 35.Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997;203(1):145–149 [DOI] [PubMed] [Google Scholar]
- 36.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997;203(1):137–144 [DOI] [PubMed] [Google Scholar]
- 37.Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS., Jr The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol 1981;104(1):23–34 [PMC free article] [PubMed] [Google Scholar]
- 38.Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol 1986;10(6):382–393 [DOI] [PubMed] [Google Scholar]
- 39.Going JJ, Anderson TJ, Battersby S, MacIntyre CC. Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol 1988;130(1):193–204 [PMC free article] [PubMed] [Google Scholar]
- 40.Feuerhake F, Sigg W, Höfter EA, Unterberger P, Welsch U. Cell proliferation, apoptosis, and expression of Bcl-2 and Bax in non-lactating human breast epithelium in relation to the menstrual cycle and reproductive history. Breast Cancer Res Treat 2003;77(1):37–48 [DOI] [PubMed] [Google Scholar]
- 41.Potten CS, Watson RJ, Williams GT, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer 1988;58(2):163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson HW, Cornélissen G, Katinas G, Halberg F. Meta-analysis of sequential luteal-cycle-associated changes in human breast tissue. Breast Cancer Res Treat 2000;63(2):171–173 [DOI] [PubMed] [Google Scholar]
- 43.Morrow M. The evaluation of common breast problems. Am Fam Physician 2000;61(8):2371–2378, 2385 [PubMed] [Google Scholar]
- 44.Silvestrini R, Luisi A, Daidone MG, Di Mauro MG. Effect of menstrual phase on cell proliferative rate of breast cancer. Breast Cancer Res Treat 1998;48(1):93–94 [DOI] [PubMed] [Google Scholar]
- 45.Wegienka G, Baird DD. A comparison of recalled date of last menstrual period with prospectively recorded dates. J Womens Health (Larchmt) 2005;14(3):248–252 [DOI] [PubMed] [Google Scholar]
- 46.Weinberg CR, Umbach DM, Greenland S. When will nondifferential misclassification of an exposure preserve the direction of a trend? Am J Epidemiol 1994;140(6):565–571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


