Abstract
Lithium-induced nephrogenic diabetes insipidus (NDI) is accompanied by polyuria, downregulation of aquaporin 2 (AQP2), and cellular remodeling of the collecting duct (CD). The amiloride-sensitive epithelial sodium channel (ENaC) is a likely candidate for lithium entry. Here, we subjected transgenic mice lacking αENaC specifically in the CD (knockout [KO] mice) and littermate controls to chronic lithium treatment. In contrast to control mice, KO mice did not markedly increase their water intake. Furthermore, KO mice did not demonstrate the polyuria and reduction in urine osmolality induced by lithium treatment in the control mice. Lithium treatment reduced AQP2 protein levels in the cortex/outer medulla and inner medulla (IM) of control mice but only partially reduced AQP2 levels in the IM of KO mice. Furthermore, lithium induced expression of H+-ATPase in the IM of control mice but not KO mice. In conclusion, the absence of functional ENaC in the CD protects mice from lithium-induced NDI. These data support the hypothesis that ENaC-mediated lithium entry into the CD principal cells contributes to the pathogenesis of lithium-induced NDI.
Nephrogenic diabetes insipidus (NDI) is characterized by the inability of the kidney to concentrate urine in response to vasopressin. The disease is most commonly acquired and often occurs as an adverse effect in humans subjected to various drug treatments (e.g., lithium [Li] therapy). Li, which is a frequently used drug against manic-depressive illness, can cause NDI in up to 20 to 40% of patients who take the medication.1 Long-term Li treatment of rats results in severe downregulation of aquaporin 2 (AQP2) and AQP3 protein levels in parallel with extensive polyuria.2,3 Moreover, Li causes a remodeling of the rat kidney collecting duct (CD), which includes a decreased fraction of principal cells and an increased fraction of intercalated cells.4,5 These deleterious effects on the CD are proposed to be linked to Li accumulation within the principal cells. Several studies suggested that a likely candidate for Li entry is the epithelial sodium channel (ENaC), which is present in the late distal convoluted tubule (DCT) cells, the connecting tubule (CNT) cells, and the CD principal cells.6,7 ENaC is a heteromultimeric protein composed of three subunits: α, β, and γ.8 ENaC has a higher permeability for Li than for Na.9,10 Moreover, amiloride, a specific blocker of ENaC, has been shown to reduce Li uptake in ENaC-expressing renal cells11 and to block reabsorption in the distal nephron of Na-depleted rats.12 Recent studies also showed that amiloride partially prevents development of Li-NDI in rats11,13 and partially restores the urine-concentrating ability in patients who are on Li therapy.14 To provide possibly definitive proof of whether ENaC is involved in the absorption of Li, we took advantage of transgenic mice deficient of αENaC specifically in the CD while leaving ENaC expression in the late DCT and CNT intact.15 Under salt restriction, whole-cell voltage clamp of principal cells of the cortical CD (CCD) showed no detectable ENaC activity, whereas large amiloride-sensitive currents were observed in the CCD of controls15 and in the CNT/DCT of both KO and controls.16 Despite the loss of ENaC activity in CD, the animals survived well and were able to maintain Na and potassium (K) balance, even when challenged by 1 week of salt restriction, 23 hours of water deprivation, or 2 days of K loading.15 We followed these transgenic mice and their littermate controls during long-term Li treatment.
RESULTS
Li Does not Induce Polyuria and Polydipsia in CD-Specific αENaC KO Mice
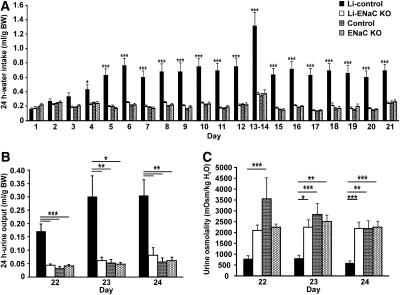
Long-term Li treatment induced increased water intake in the control animals, which was not seen in KO mice (Figure 1A). As early as day 4 after starting Li treatment, water intake increased in the Li-treated controls, whereas it remained at the same level throughout the study in the Li-treated KO mice. After 5 days of Li treatment, there was a highly significant difference in water intake between the Li-treated controls and the three other groups that continued for the remaining days of the diet. In contrast, water intake of the Li-treated KO mice was not significantly different from the untreated control and KO mice (Figure 1A). The urine output of the Li-treated controls was significantly higher compared with the three other groups (Figure 1B). In parallel, the urine osmolality was significantly lower in the Li-treated controls compared with the other groups (Figure 1C). In contrast, there were no significant differences in urine output and urine osmolality between the Li-treated KO mice and the untreated control and KO mice. Thus, Li did not cause polyuria and polydipsia in the Li-treated KO mice that were seen in the Li-treated controls.
Figure 1.
Li-treated KO mice do not exhibit higher water intake or urine output or lower urinary osmolality. (A through C) Water intake (A), urine output (B), and urine osmolality (C) are measured in Li-treated control mice (n = 12; black), Li-treated KO mice (n = 11; white), untreated control mice (n = 8; bricks), and untreated KO mice (n = 7; hexagons). After 4 days of Li treatment, water intake is significantly increased in the Li-treated control mice compared with the Li-treated KO mice; after 5 days, there is a highly significant difference between the Li-treated control mice and the three other groups (A). There is no difference in water intake among the Li-treated KO mice, the untreated control mice, and the untreated KO mice during the entire diet (A). At the end of the diet, the urine output is significantly increased and the urine osmolality significantly decreased in the Li-treated control mice compared with the three other groups (B and C), whereas no differences are observed among the Li-treated KO mice, the untreated control mice, and the untreated KO mice (B and C). At day 1, n = 6, 6, 3, and 3; at day 5 to 6 and 18 to 19, n = 9, 8, 8, and 7; at day 8 to 9, n = 11, 11, 8, and 7. *P < 0.05; **P < 0.01; ***P < 0.001.
The urinary Na and K concentrations were significantly lower in the Li-treated controls but not in the Li-treated KO mice (Table 1). The urinary excretion of Na and K, however, was not changed in the two groups after Li exposure, and there were no changes in the fractional excretion of Na (FENa+) among the four groups (Table 1). Furthermore, serum concentrations of Na, K, urea, and creatinine and serum osmolality were unaltered in the two groups after Li exposure (Table 1). The urinary Li concentration was significantly lower in the Li-treated controls compared with the Li-treated KO mice, whereas the urinary Li excretion was not different between the control and KO groups (Table 1). There were no differences in Li concentration in the blood between the two Li-treated groups, and no changes in Li clearance and in fractional excretion of Li were observed (Table 1). There was no significant difference in food intake between the two groups during the diet, and no significant changes in body weight were observed at the end of the diet (Table 1).
Table 1.
Urinary and blood measurements from control and KO mice on a normal diet or a Li diet
| Parameter | Li Diet |
Normal Diet |
||
|---|---|---|---|---|
| Controls | KO Mice | Controls | KO Mice | |
| Body weight, % | 99.0 ± 2.2a | 104.2 ± 1.7 | 107.4 ± 2.3 | 105.8 ± 1.1 |
| (n = 12) | (n = 11) | (n = 8) | (n = 7) | |
| Food intake, g/g body wt | 0.25 ± 0.03 | 0.22 ± 0.02 | 0.26 ± 0.03 | 0.24 ± 0.03 |
| (n = 12) | (n = 11) | (n = 8) | (n = 7) | |
| Serum Na+, mM | 149.7 ± 0.8 | 151.4 ± 1.0 | 147.3 ± 0.7 | 147.0 ± 1.0 |
| (n = 6) | (n = 5) | (n = 3) | (n = 3) | |
| Serum K+, mM | 4.3 ± 0.2 | 4.4 | 4.2 ± 0.4 | 4.5 |
| (n = 6) | (n = 2) | (n = 3) | (n = 1) | |
| Serum osmolality, mosmol/kgH2O | 345 ± 3 | 334 ± 8 | 341 | 344 ± 4 |
| (n = 6) | (n = 3) | (n = 2) | (n = 3) | |
| Serum urea, mM | 7.7 ± 0.4 | 7.6 | 8.2 ± 0.6 | 7.4 |
| (n = 6) | (n = 2) | (n = 3) | (n = 1) | |
| Serum creatinine, μM | 27.0 ± 1.4 | 27.0 ± 2.2 | 28.0 ± 1.0 | 27.0 ± 1.1 |
| (n = 9) | (n = 8) | (n = 6) | (n = 6) | |
| Plasma/serum Li+, mM | 0.61 ± 0.16 | 0.38 ± 0.07 | 0.75 ± 0.04 × 10−3 | 0.84 ± 0.06 × 10−3 |
| (n = 7) | (n = 8) | (n = 3) | (n = 3) | |
| Urinary Na+, mmol/24 h | 0.16 ± 0.03 | 0.15 ± 0.03 | 0.13 ± 0.02 | 0.14 ± 0.01 |
| (n = 11) | (n = 11) | (n = 7) | (n = 7) | |
| Urinary Na+, mM | 23 ± 4b | 97 ± 13 | 91 ± 13 | 108 ± 20 |
| (n = 11) | (n = 11) | (n = 7) | (n = 7) | |
| FENa+, % | 0.47 ± 0.06 | 0.97 ± 0.19 | 0.77 ± 0.18 | 1.04 ± 0.22 |
| (n = 5) | (n = 5) | (n = 3) | (n = 3) | |
| Urinary K+, mmol/24 h | 0.54 ± 0.07 | 0.48 ± 0.07 | 0.40 ± 0.03 | 0.46 ± 0.03 |
| (n = 11) | (n = 11) | (n = 7) | (n = 7) | |
| U-K+, mM | 79 ± 16b | 328 ± 50 | 324 ± 70 | 349 ± 62 |
| (n = 11) | (n = 11) | (n = 7) | (n = 7) | |
| C-creatinine, μl/min per g | 9.7 ± 2.4 | 8.3 ± 3.7 | 6.0 ± 1.5 | 7.7 ± 2.3 |
| (n = 9) | (n = 8) | (n = 6) | (n = 6) | |
| Urinary creatinine, μM | 1819 ± 541 | 4260 ± 732 | 3577 ± 500 | 4207 ± 935 |
| (n = 12) | (n = 11) | (n = 7) | (n = 7) | |
| Urinary Li+, mmol/24 h | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.20 ± 0.02 × 10−3 | 0.17 ± 0.005 × 10−3 |
| (n = 11) | (n = 12) | (n = 3) | (n = 3) | |
| Urinary Li+, mM | 13.4 ± 2.5c | 35.5 ± 6.1 | 93.0 ± 25.0 × 10−3 | 105.0 ± 15.0 × 10−3 |
| (n = 11) | (n = 12) | (n = 3) | (n = 3) | |
| Li+ clearance, μl/min per g | 5.2 ± 0.9 | 4.8 ± 1.0 | 6.2 ± 0.8 | 6.3 ± 0.8 |
| (n = 7) | (n = 3) | (n = 3) | (n = 3) | |
| FELi+, % | 70 ± 23 | 197 ± 105 | 69 ± 1 | 51 ± 10 |
| (n = 7) | (n = 8) | (n = 3) | (n = 3) | |
All measurements are from day 24 except the body weight, which is measured at day 21. Body weight in percentage is calculated as a fraction of day 0 (beginning of the experiment). FELi+, fractional excretion of lithium.
aP < 0.05 (controls on Li-diet versus controls on normal diet).
bP < 0.01 (controls on a Li-diet versus three other groups).
cP < 0.01 (controls on a Li-diet versus KO on a Li-diet).
Effect of Li Treatment on AQP2 and H+-ATPase Abundance in CD-Specific αENaC KO Mice
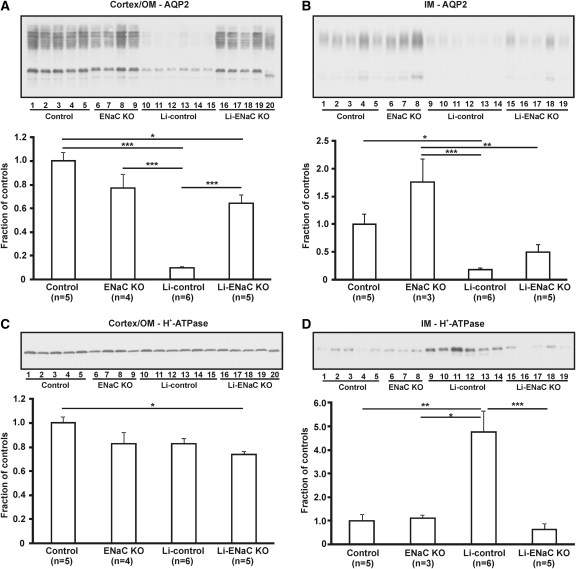
It was previously shown that Li treatment of rats causes a dramatic decrease in AQP2 abundance.2,4 To investigate whether the effect was abolished in the Li-treated KO mice, we performed Western blotting of samples from cortex/outer medulla (OM) and inner medulla (IM; Figure 2). Consistent with the previous results, long-term Li treatment resulted in a severe and significant downregulation of AQP2 in both IM (Figure 2A) and cortex/OM (Figure 2B). In the cortex/OM of Li-treated KO mice, AQP2 was significantly higher compared with the Li-treated controls, and no significant difference was observed when compared with the untreated KO mice. Thus, Li treatment did not affect total cortical and outer medullary AQP2 expression in the KO mice. In IM, AQP2 abundance was higher in the Li-treated KO mice compared with the Li-treated controls, although not statistically significant. A significantly lower AQP2 expression was seen in the Li-treated KO mice compared with the untreated KO mice. Thus, Li treatment seems to have some effect on AQP2 expression in the IM of KO mice.
Figure 2.
Li treatment decreases AQP2 expression in inner medulla but not in cortex of KO mice. (A through D) Western blot and corresponding densitometric analysis of AQP2 (A and B) and H+-ATPase expression (C and D) in cortex/OM (A and C) and IM (B and D) of untreated control mice (control), untreated CD-specific αENaC KO mice (ENaC KO), Li-treated control mice (Li-control), and Li-treated CD-specific αENaC KO mice (Li-ENaC KO). In cortex/OM, AQP2 protein is significantly reduced in Li-control compared with the other three groups, whereas AQP2 levels in the Li-ENaC KO is not different from ENaC KO (A). AQP2 protein in IM is significantly lower in Li-control compared with the two untreated groups but not compared with Li-ENaC KO (B). The expression of AQP2 in the IM of Li-ENaC KO is significantly lower compared with ENaC KO (B). No differences in H+-ATPase protein are observed between the Li-control and the other groups in cortex/OM (C). In the IM, H+-ATPase is significantly higher in the Li-control compared with the three other groups, whereas the expression in the Li-ENaC KO is not significantly different from the untreated groups (D). *P < 0.05; **P < 0.01; ***P < 0.001.
Western blot analysis further showed no significant differences in H+-ATPase abundance in the cortex/OM between the Li-treated controls and the other groups (Figure 2C). In contrast, the H+-ATPase expression was significantly increased in the IM of the Li-treated controls compared with the other three groups (Figure 2D). Moreover, no differences between the Li-treated and untreated KO mice were observed. Thus, Li treatment did not cause changes in the expression of H+-ATPase in the IM of KO mice in contrast to the control mice.
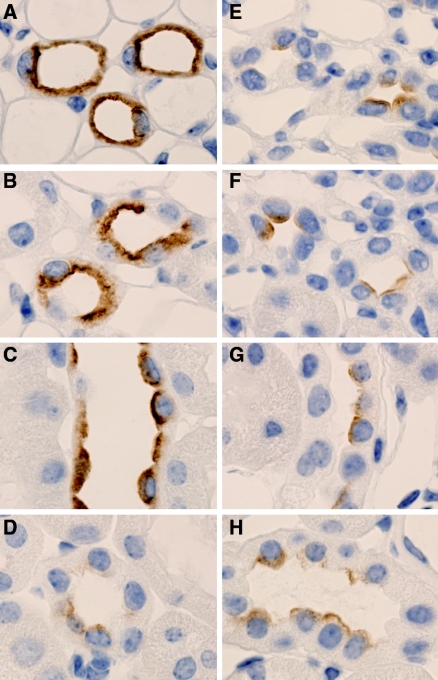
Decreased AQP2 Labeling in the CD of Li-Treated Control Mice Compared with Li-Treated KO Mice
To investigate further the AQP2 labeling in Li-treated control and Li-treated KO mice, we performed immunolabeling on kidney sections from these two groups. In the CD of Li-treated KO mice, a strong AQP2 labeling was seen in IM, inner stripe of OM (ISOM), and cortex (Figure 3, A through C), whereas in the CD of the Li-treated controls, the AQP2 labeling was markedly reduced (Figure 3, E through G). Thus, Li did not cause a downregulation of AQP2 expression in the CD of KO mice. In the CNT, there were no major changes in AQP2 labeling between the two groups (Figure 3, D and H), indicating that Li does not affect AQP2 levels in this segment.
Figure 3.
Decreased AQP2 labeling in the CD of Li-treated control mice compared with Li-treated KO mice. (A through H) Immunohistochemistry using whole kidney sections from Li-treated CD-specific αENaC KO mice (A through D) and Li-treated control mice (E through H). Sections are incubated with anti-AQP2 antibody. In the IM (A and E), ISOM (B and F), and the CCD (C and G), AQP2 labeling is markedly reduced in the Li-treated control mice (E through G) compared with the Li-treated CD-specific αENaC KO mice (A through C). In the CNT, there are no changes in AQP2 labeling between the two groups (D and H).
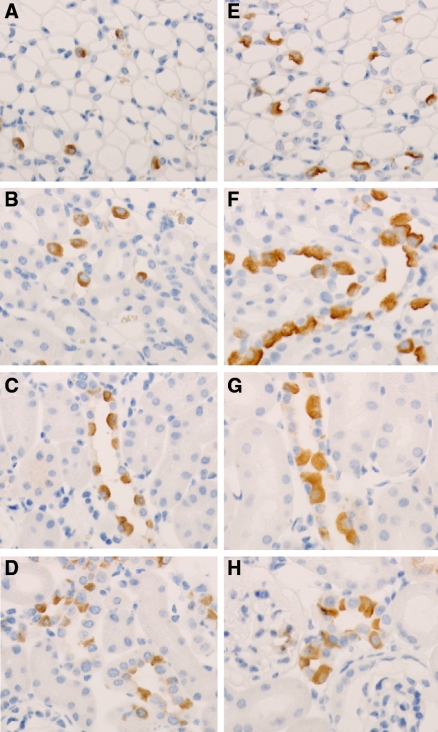
Effect of Li on the Density of H+-ATPase–Positive Cells in Control and KO Mice
Immunohistochemistry revealed a slight increased density of H+-ATPase–positive cells in the proximal part of IM and in the ISOM of the Li-treated controls compared with the Li-treated KO mice (Figure 4, A, B, E, and F). Moreover, H+-ATPase–positive cells localized next to each other were seen in the Li-treated controls. In the CCD of the Li-treated control mice, H+-ATPase–positive cells were only occasionally localized next to each other, and, in general, no major changes in the density of H+-ATPase–positive cells were observed in this segment between the two groups (Figure 4, C and G). In the CNT and DCT, the density of H+-ATPase–positive cells was not different (Figure 4, D and H). Cellular counting of H+-ATPase–positive cells in the CNT and DCT revealed no significant changes in the fraction of H+-ATPase–positive cells between the two groups (36.0 ± 1.4% in control versus 37.0 ± 1.0% in KO; n = 3 in each group; NS). Moreover, multiple H+-ATPase–positive cells next to each other were not observed in these segments. Thus, Li causes a slight increased density of intercalated cells in the IM and ISOM of control mice but not in KO mice. Moreover, Li does not seem to have an effect on the density of intercalated cells in the DCT/CNT.
Figure 4.
No increase in H+-ATPase–positive cells in Li-treated KO mice compared with Li-treated control mice. (A through H) Immunohistochemistry using whole kidney sections from Li-treated CD-specific αENaC KO mice (A through D) and Li-treated control mice (E through H). Sections were incubated with anti–H+-ATPase antibody. The density of H+-ATPase–positive cells was slightly increased in the IM and ISOM of the Li-treated control mice (E and F) compared with the Li-treated CD-specific αENaC KO mice (A and B). In the CCD (C and G) and in the CNT (D through H), there were no major changes in the density of H+-ATPase–positive cells.
DISCUSSION
In this study, we show that the absence of functional ENaC in the CD protects mice from developing Li-induced NDI. The data provide genetic evidence that ENaC is involved in Li entry into CD principal cells and thus plays a crucial role in the pathogenesis of Li-induced NDI. Interestingly, the Li-treated KO mice did not show signs of NDI, suggesting that the CNT is able to escape the Li toxicity.
CD-Specific αENaC KO Mice Are Protected from Development of NDI
In control mice, Li treatment caused severe polyuria, low urine osmolality, and high water intake, consistent with an impairment of urine concentration and thus NDI.2–4,17 In contrast, Li did not have an effect on the urine-concentrating ability in mice that lack αENaC in the CD. Our results are consistent with a recent rat study in which co-treatment with Li and amiloride attenuated the Li-NDI11,13 and with the fact that amiloride administration ameliorates polyuria in patients who are on long-term Li treatment.18 Furthermore, amiloride prevents Li-induced AQP2 downregulation in mCCDc11 cells.11 We also previously used Li protection as a positive control for experiments dealing with the role of ENaC in thiazolidinedione-induced fluid retention.19
The higher AQP2 expression in the CD of the Li-treated KO mice compared with the Li-treated controls is consistent with the absence of a urine-concentrating defect in the CD of the KO mice. However, Western blot analysis showed that Li did have an effect on AQP2 levels in IM of the KO mice as seen by a significantly lower AQP2 abundance in the Li-treated KO mice compared with the untreated KO mice. This suggests that the Li effect on AQP2 levels in the inner medullary CD (IMCD) principal cells is not only via an ENaC-mediated entry. Simultaneous administration of amiloride and Li to rats has also been shown not to restore AQP2 levels fully in the IM.11 This effect may be mediated by prostaglandin E2 (PGE2) because Li increases cyclooxygenase 2 expression in and PGE2 release from medullary interstitial cells,20 and it is known that PGE2 can inhibit the effect of vasopressin on water permeability in the CD.21,22 The decreased AQP2 expression is likely not related to a decrease in interstitial osmolality because furosemide-induced changes in medullary tonicity did not affect AQP2 levels in rat IM.23 Finally we cannot exclude that some residual ENaC activity remains in the IM of the KO mice,19 but this is unlikely to play a physiologically role. The AQP2 downregulation in the IMCD was not correlated with changes in urine output and osmolality in the KO mice. Thus, the water reabsorption ability did not seem to be impaired. A possibility is that Li did not affect AQP2 trafficking and that a substantial amount of AQP2 remained in the apical plasma membrane to sustain water reabsorption. We previously showed that although cytoplasmic AQP2 is markedly reduced in rat IM after long-term Li treatment, some AQP2 still remains in the apical membrane.4
Li administration was associated with a slightly higher density of intercalated cells (H+-ATPase–positive cells) in the IM and ISOM of the control mice. The increased density of intercalated cells was less pronounced, however, compared with previous studies of rats.4,11,17 The phenomenon was not seen in KO mice, showing that Li had no effect on the intercalated cells in these mice. Thus, Li-induced changes of the intercalated cells require the action of Li on the principal cells.
Western blotting also revealed an upregulation of H+-ATPase protein levels in the IM of the Li-treated control mice as shown in other studies of rats and is likely due to an increase in the density of intercalated cells.11,17 We did not observe changes in H+-ATPase abundance in OM/cortex in contrast to that previously shown in Li-treated rats,11,17 suggesting that the effect of Li on the intercalated cells in mice is not as dramatic as in rats.
There was no significant change in plasma Li concentration between the Li-treated groups, and the plasma Li concentration of 0.6 mM in the Li-treated control mice corresponds to studies of rats using the same protocol.3
ENaC-Mediated Li Entry
Under normal conditions (i.e., in Na-replete conditions), renal Li clearance is used as a measurement of tubular fluid delivery from the proximal tubule. The Li clearance technique assumes that transcellular transport of Li does not occur in the distal tubule and the CD. Previous studies have shown that amiloride-sensitive Li reabsorption takes place only in the distal nephron during conditions with Na depletion (low-Na diet).12,24 In this study, we showed that Li-treated mice receiving a normal Na-containing diet develop NDI and that the absence of ENaC in the CD prevents the development of NDI. Thus, we show that ENaC-mediated Li reabsorption does occur in the CD in mice on a normal-salt diet.
Li treatment is known to be associated with natriuresis, and experiments with rats using the same Li protocol as in this study (with the same Na intake in Li-treated and control rats) have shown that the rate of urinary Na excretion is increased in Li-treated animals compared with controls.3 In this study, we did not observe a difference in urinary Na excretion among the four groups. In a study by Nielsen et al.,25 rats that were on a Li diet and consumed the same amount of Na as the controls also had no differences in urinary Na excretion rates, but these rats had a marked increase in the FENa+. However, no differences in FENa+ were observed in that study either. Thus, there may be differences in the Li-induced Na handling in mice and rats.
Li Escape in the CNT
Mice with ENaC inactivated in the CD did not respond on the Li treatment, suggesting that the CNT is able to escape the deleterious effects of Li. This is despite that the CNT cells contain ENaC and therefore should be expected to respond to Li exposure. The CNT exhibits the same components for water transport as the CD. AQP2 is expressed in both the CNT and the CD, although the abundance is lower along the CNT.26,27 Vasopressin-mediated regulation of AQP2 expression and trafficking occurs in rat CNT, and in mouse and rat, the V2 receptor is equally expressed in the CNT and the CCD.27,28 Vasopressin also increases adenylate cyclase activity in rat CNT.29
In our study, we did not observe differences in AQP2 levels in the CNT of the Li-treated control and KO mice, and the density of intercalated cells did not seem to be increased in the two groups. Moreover, AQP2 protein levels in OM/cortex were decreased only in the Li-treated control mice and not in the Li-treated KO mice. Consistently, Li causes AQP2 downregulation mainly in the CCD and to a lesser extent in CNT of rats.30
Differences in ENaC expression between CNT and CCD were observed in rats subjected to Li diet with fixed Na intake (but not with free access to Na).25 In these animals, β- and γENaC downregulation was observed in CCD and outer medullary CD but not in CNT. In contrast, increased apical labeling of all three ENaC subunits was observed in this segment. This was explained by possible compensatory mechanisms occurring in CNT to limit the renal Na loss observed in these animals.25
The difference in Li sensitivity between the CNT and the CD could be explained by differences in Li delivery to the CNT and the CD, resulting in differences in the luminal Li concentration along the CNT and the CD. It is also possible that the Li intracellular concentration rises to toxic levels only in the CD as a result of a different exit pathway for Li through either the basolateral or the apical plasma membrane in the CNT cells and CD principal cells. Yet another possibility is that the expression of or the sensitivity to an intracellular target for Li is different in the CNT and the CD. A potential intracellular target for Li is glycogen synthase kinase 3β (GSK3β). Li has been shown to inhibit GSK3 activity in the kidney,20 and proteomic studies of IMCD isolated from Li-treated rats revealed an upregulation of the phosphorylated inactive form of GSK3β.31 Moreover, an impaired response to vasopressin was recently shown in CD-specific GSK3β KO mice, suggesting a potential important role of GSK3β in the vasopressin-mediated water reabsorption.32 A recent study showed equal expression of the GSK3β transcript in mouse DCT/CNT and CD.33 However, a potential different sensitivity, for example, to phosphorylation and thus activity of the GSK3β protein may exist in the two segments.
CONCISE METHODS
Experimental Protocols
Li chloride was solubilized in water, and the solution was added to food to yield a Li concentration of 40 mmol/kg dry food as described previously.3 CD-specific αENaC KO mice (Scnn1alox/lox//HoxB7:Cre) and the corresponding control mice (Scnn1alox/lox) (2 to 4 and 12 months old) were given a Li-containing diet for 24 to 25 days. In parallel, CD-specific αENaC KO mice and control mice were given a normal diet for 24 days. All mice were placed in individual conventional cages, and water intake was measured daily. From day 22 to day 24 of the experiment, all mice were placed in metabolic cages to measure urine output and osmolality. For the entire experiment, mice had free access to food and water. At the end of the experiment, blood was collected from the eye or from the aorta. The experiment was performed three times, and data were pooled.
Urine and Plasma Analysis
Osmolarity and Na and K concentration were analyzed at the Laboratoire Central de Chimie Clinique, Centre Hospitalier Universitaire Vaudoise (CHUV), Switzerland, or at the Department of Clinical Biochemistry, Skejby University Hospital, Denmark. Li concentration was measured at the Service of Nephrology, CHUV, Switzerland, or at the Department of Anatomy, Aarhus University, Denmark.
Western Blot Analysis
The kidneys were dissected into cortex/OM and IM and homogenized in dissecting buffer as described previously.4 The homogenates were centrifuged for 15 minutes at 4°C. The total protein concentration was measured (Pierce BCA protein assay reagent kit; Pierce, Rockford, IL). For confirmation of equal loading of protein, an initial gel was stained with Coomassie Brilliant blue. SDS-PAGE was performed on 12.5% polyacrylamide gels (Pierce). After transfer of proteins by electroelution to nitrocellulose membranes, blots were blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, and 0.1% Tween 20 [pH 7.5]) for 1 hour and incubated with anti-AQP2 antibody (7661 AP, 1:1000) or anti-H+-ATPase (7659 AP, 1:1000).5 The labeling was visualized with a peroxidase-conjugated secondary antibody using an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Buckinghamshire, UK). The labeling density was quantified using Quantity One software (Bio Rad Laboratories Ltd., Hertfordshire, UK).
Immunohistochemistry
Kidneys from Li-treated KO mice (n = 3) and Li-treated control mice (n = 3) were fixed by intravascular perfusion with 3% paraformaldehyde in 0.1 M phosphate buffer and subjected to paraffin embedding and sectioning (2-μm-thick sections). Sections were incubated overnight at 4°C with rabbit polyclonal AQP2 antibody (7661AP, 1:6000 dilution) or rabbit polyclonal H+-ATPase antibody (H7659AP, 1:1000 and 1:2000 dilution),5 followed by incubation with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody. Labeling was visualized using 3,3′-diaminobenzidine staining.4
Quantification of H+-ATPase–Positive Cells in CNT/DCT
Cellular counting was performed on kidney sections from Li-treated KO and Li-treated control mice labeled with H+-ATPase antibody. Counting was performed on electronic images taken with a ×25 objective. The number of H+-ATPase–positive and –negative cells with a distinct nucleus was counted in the CNT and the DCT of Li-treated KO mice (1751 cells, n = 3) and in the CNT/DCT of Li-treated control mice (1732 cells, n = 3). The fraction of H+-ATPase–positive cells was calculated from the number of H+-ATPase–positive cells divided by the total number of cells counted for each animal.
Statistical Analysis
Results are presented as means ± SEM. Data were analyzed by one-way ANOVA and unpaired t test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
Financial support for this study was provided by the Swiss Kidney Foundation, the Swiss National Science Foundation, the Leducq Foundation, the Danish Medical Research Council, and the Danish National Research Foundation.
We are very grateful to Sebastian Frische (Department of Anatomy, Aarhus University, Aarhus, Denmark) and Marc Maillard (Service of Nephrology, CHUV, Lausanne, Switzerland) for measuring Li concentrations. We also thank Sandrine Egli, Inger-Merete Paulsen, and Helle Høyer for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Stone KA: Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 12: 43–47, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S: Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95: 1838–1845, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kwon TH, Laursen UH, Marples D, Maunsbach AB, Knepper MA, Frokiaer J, Nielsen S: Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 279: F552–F564, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Christensen BM, Marples D, Kim YH, Wang W, Frokiaer J, Nielsen S: Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am J Physiol Cell Physiol 286: C952–C964, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Christensen BM, Kim YH, Kwon TH, Nielsen S: Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am J Physiol Renal Physiol 291: F39–F48, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Loffing J, Pietri L, Aregger F, Bloch-Faure M, Ziegler U, Meneton P, Rossier BC, Kaissling B: Differential subcellular localization of ENaC subunits in mouse kidney in response to high- and low-Na diets. Am J Physiol Renal Physiol 279: F252–F258, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B: Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281: F1021–F1027, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC: Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Palmer LG, Frindt G: Conductance and gating of epithelial Na channels from rat cortical collecting tubule: Effects of luminal Na and Li. J Gen Physiol 92: 121–138, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Shalmi M, Jonassen T, Thomsen K, Kibble JD, Bie P, Christensen S: Model explaining the relation between distal nephron Li+ reabsorption and urinary Na+ excretion in rats. Am J Physiol 274: F445–F452, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Bedford JJ, Leader JP, Jing R, Walker LJ, Klein JD, Sands JM, Walker RJ: Amiloride restores renal medullary osmolytes in lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol 294: F812–F820, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, Leader JP, Walker RJ: Lithium-induced nephrogenic diabetes insipidus: Renal effects of amiloride. Clin J Am Soc Nephrol 3: 1324–1331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC: Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bens M, Vandewalle A: Cell models for studying renal physiology. Pflugers Arch 457: 1–15, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kim YH, Kwon TH, Christensen BM, Nielsen J, Wall SM, Madsen KM, Frokiaer J, Nielsen S: Altered expression of renal acid-base transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1244–F1257, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Batlle DC, von Riotte AB, Gaviria M, Grupp M: Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med 312: 408–414, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Vallon V, Hummler E, Rieg T, Pochynyuk O, Bugaj V, Schroth J, Dechenes G, Rossier B, Cunard R, Stockand J: Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol 20: 721–729, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rao R, Zhang MZ, Zhao M, Cai H, Harris RC, Breyer MD, Hao CM: Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am J Physiol Renal Physiol 288: F642–F649, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hebert RL, Jacobson HR, Fredin D, Breyer MD: Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol 265: F643–F650, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Nadler SP, Zimpelmann JA, Hebert RL: PGE2 inhibits water permeability at a post-cAMP site in rat terminal inner medullary collecting duct. Am J Physiol 262: F229–F235, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Marples D, Christensen BM, Frokiaer J, Knepper MA, Nielsen S: Dehydration reverses vasopressin antagonist-induced diuresis and aquaporin-2 downregulation in rats. Am J Physiol 275: F400–F409, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Thomsen K, Shirley DG: A hypothesis linking sodium and lithium reabsorption in the distal nephron. Nephrol Dial Transplant 21: 869–880, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Nielsen J, Kwon TH, Praetorius J, Kim YH, Frokiaer J, Knepper MA, Nielsen S: Segment-specific ENaC downregulation in kidney of rats with lithium-induced NDI. Am J Physiol Renal Physiol 285: F1198–F1209, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Loffing J, Kaissling B: Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Renal Physiol 284: F628–F643, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Christensen BM, Wang W, Frokiaer J, Nielsen S: Axial heterogeneity in basolateral AQP2 localization in rat kidney: effect of vasopressin. Am J Physiol Renal Physiol 284: F701–F717, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Mutig K, Paliege A, Kahl T, Jons T, Muller-Esterl W, Bachmann S: Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Morel F, Doucet A: Hormonal control of kidney functions at the cell level. Physiol Rev 66: 377–468, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Nielsen J, Kwon TH, Praetorius J, Frokiaer J, Knepper MA, Nielsen S: Aldosterone increases urine production and decreases apical AQP2 expression in rats with diabetes insipidus. Am J Physiol Renal Physiol 290: F438–F449, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA: Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci U S A 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao R, Patel S, Hao C, Woodgett J, Harris R: GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21: 428–437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pradervand S, Zuber MA, Centeno G, Bonny O, Firsov D: A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch August 5, 2010. [epub ahead of print] [DOI] [PubMed] [Google Scholar]