Abstract
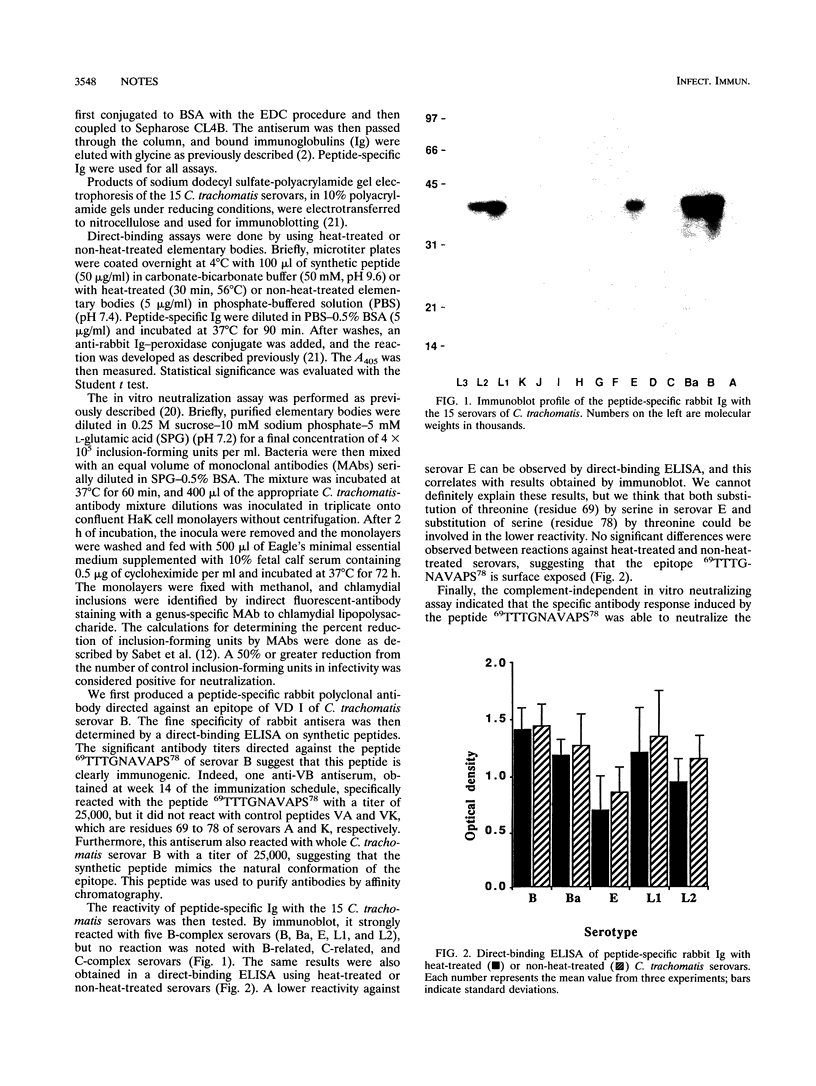
The major outer membrane protein of Chlamydia trachomatis has been extensively studied and is still considered one of the most promising candidates for development of a synthetic vaccine. Neutralizing epitopes in variable domains I, II, and IV have already been reported. In variable domain I, residues 69 to 78 have been identified as a neutralizing epitope for some of the C- and C-related complex serovars (A, C, I, J, L3, and K). It is not known whether epitopes located at the same position in B-complex serovars are neutralizing. To clarify this point, rabbit polyclonal antibodies directed against the peptide 69TTTGNAVAPS78 from the B serovar were produced. Rabbit antisera were further rendered peptide specific by purification on a peptide-bovine serum albumin-Sepharose affinity column. Peptide-specific rabbit immunoglobulin reacted with five of the B-complex serovars (B, Ba, E, L1, and L2) by immunoblot and by direct-binding enzyme-linked immunosorbent assay. Furthermore, this peptide-specific rabbit immunoglobulin neutralized the chlamydial infectivity of both serovars B and E for HaK cells in a complement-independent in vitro assay. The importance of these results stems from the fact that peptide 69TTTGNAVAPS78 was able to induce an antibody response directed against B- and B-related complex serovars, including serovar E, which is responsible for a high proportion of genital infections. This peptide could therefore be considered for the construction of a multivalent synthetic vaccine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossay L., Paradis G., Pépin A., Mourad W., Coté L., Hébert J. Idiotype and anti-anti-idiotype antibodies to Neisseria gonorrhoeae lipooligosaccharides with bactericidal activity but no cross-reactivity with red blood cell antigens. J Immunol. 1993 Jul 1;151(1):234–243. [PubMed] [Google Scholar]
- Brossay L., Villeneuve A., Paradis G., Coté L., Mourad W., Hébert J. Mimicry of a neutralizing epitope of the major outer membrane protein of Chlamydia trachomatis by anti-idiotypic antibodies. Infect Immun. 1994 Feb;62(2):341–347. doi: 10.1128/iai.62.2.341-347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Lucero M. E., Kuo C. C. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect Immun. 1985 Nov;50(2):595–597. doi: 10.1128/iai.50.2.595-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Cheng X., Markoff B. A., Fielder T. J., de la Maza L. M. Functional and structural mapping of Chlamydia trachomatis species-specific major outer membrane protein epitopes by use of neutralizing monoclonal antibodies. Infect Immun. 1991 Nov;59(11):4147–4153. doi: 10.1128/iai.59.11.4147-4153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Cheng X., de la Maza L. M., Peterson E. M. Characterization of a neutralizing monoclonal antibody directed at variable domain I of the major outer membrane protein of Chlamydia trachomatis C-complex serovars. Infect Immun. 1993 Apr;61(4):1365–1370. doi: 10.1128/iai.61.4.1365-1370.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Simmons J., Caldwell H. D. Enhancement of Chlamydia trachomatis infectious progeny by cultivation of HeLa 229 cells treated with DEAE-dextran and cycloheximide. J Clin Microbiol. 1984 Aug;20(2):217–222. doi: 10.1128/jcm.20.2.217-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Mullenbach G., Sanchez-Pescador R., Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986 Dec;168(3):1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. S., Tam M. R., Kuo C. C., Nowinski R. C. Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. J Immunol. 1982 Mar;128(3):1083–1089. [PubMed] [Google Scholar]
- Stephens R. S., Wagar E. A., Schoolnik G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1988 Mar 1;167(3):817–831. doi: 10.1084/jem.167.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992 Jan 1;175(1):227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S. J., Caldwell H. D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989 Feb;57(2):636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- Zhong G. M., Brunham R. C. Immunoaccessible peptide sequences of the major outer membrane protein from Chlamydia trachomatis serovar C. Infect Immun. 1990 Oct;58(10):3438–3441. doi: 10.1128/iai.58.10.3438-3441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]