Abstract
Complement factor H (CfH) is a key regulator of the alternative pathway, and its presence on mouse platelets and podocytes allows the processing of immune complexes. Because of the role of immune complexes in the pathophysiology of lupus nephritis, we studied the role of CfH in the development of nephritis in MRL-lpr mice, an animal model of lupus. At 12 weeks, CfH-deficient MRL-lpr mice had significantly more albuminuria and higher BUN levels than MRL-lpr controls. Cfh-deficient MRL-lpr mice also experienced earlier mortality: at 14 weeks, 6 of 9 CfH-deficient MRL-lpr mice had died of renal failure, whereas all 11 littermate CfH-sufficient MRL-lpr mice were alive (P ≤ 0.001). Histologically, CfH-deficient MRL-lpr mice developed severe diffuse lupus nephritis by 12 weeks (glomerulonephritis scores of 2.6 ± 0.4 versus 0.4 ± 0.2 in littermate controls, P = 0.001). Similar to other CfH-deficient mouse models on nonautoimmune backgrounds, immunofluorescence staining showed extensive linear C3 staining along glomerular capillary walls. IgG was present in the mesangium and peripheral capillary walls along with excessive infiltration of macrophages and neutrophils. Ultrastructurally, there were subendothelial and subepithelial immune deposits and extensive podocyte foot process effacement. In summary, the loss of CfH accelerates the development of lupus nephritis and recapitulates the functional and structural features of the human disease. This illustrates the critical role of complement regulation and metabolism of immune complexes in the pathogenesis of lupus nephritis.
The MRL/Mp-Tnfrsf6lpr/lpr strain (commonly abbreviated as MRL-lpr) is an accurate mouse model of human systemic lupus erythematosus (SLE), which shares many features of human SLE, including the production of autoantibodies leading to the presence of complement-activating immune complexes (ICs) in the circulation and deposited in tissues, consumptive hypocomplementemia, and development of lupus nephritis (LN).1,2 The earliest changes in the kidney, including accumulation of ICs and proliferation within the mesangial area and mild proteinuria, occur by 12 weeks of age.3 Later in the course of the disease, ICs localize in the peripheral capillary loops, and there is accumulation of monocytes and neutrophils and proliferation of both endothelial and mesangial cells, with occasional crescent formation and basement membrane thickening. Ultimately, 50% mortality occurs at 20 to 24 weeks of age.2
The complement system contains >30 plasma and cell-associated proteins, many of which are alike as a consequence of gene duplication events during evolution.4 Activation through classical, alternative, or lectin complement pathways leads to the cleavage of C3 and C5 and generation of C3a, C3b, C5a, and C5b-9. Complement is the first line of defense against some microorganisms and an integral component of innate and adaptive immune responses to many others. Complement proteins are also important to clear ICs.5 To limit complement activation, there are a number of inhibitory proteins, including the regulators of complement activation family, that are highly related within and between even distant species.6–8 The functional activities of these family members are attributable to their binding to C4 and C3 products.9,10
Inhibition of the complement system at various levels has been used to study the roles of complement in the development of LN in MRL-lpr mice, with some unexpected results. Complement inhibition by complement receptor 1-related gene/protein y (Crry) in Crry-transgenic MRL-lpr mice resulted in prolonged survival and significantly less proteinuria and blood urea nitrogen (BUN) levels.11 Comparable effects were observed with the use of soluble recombinant Crry in the same lupus mouse model,12 in which there was reduced production of matrix components such as collagens I, II, and IV, potentially induced by complement-mediated upregulated expression of connective tissue growth factor and TGF-β1.13 Generating mice that lacked a functional complement alternative pathway14,15 or preventing signaling through anaphylatoxin C3a16 or C5a17,18 receptors led to reduced severity of LN in MRL-lpr mice. In contrast, C3a receptor–deficient MRL-lpr mice had higher auto-antibody titers and an earlier onset of renal disease, although long-term renal function and survival were not affected.19 More surprisingly, deficiency of C3, the converging point for all three complement pathways, did not affect the development of LN in MRL-lpr mice, which suggested there were also beneficial effects of complement activation, such as in IC clearance.20
Less is known about the consequences of unrestricted complement activation in the development of LN. The Song group showed that MRL-lpr mice deficient in decay-accelerating factor (CD55) had exacerbated autoimmunity and dermatitis, yet LN was not affected.21,22 Thus, complement regulation by decay-accelerating factor in glomeruli is not critical in LN, which may reflect its localization primarily on rodent podocytes.23,24
Complement factor H (CfH) is a highly abundant plasma complement regulator that inhibits alternative pathway activation by inhibiting the formation and accelerating the decay of C3 convertases and acting as a complement factor I co-factor, which inactivates C3b to iC3b.25 When CfH is absent in CfH−/− mice, animals develop glomerulonephritis (GN) spontaneously, which leads to the late death of some animals of mixed genetic backgrounds.26,27 In glomeruli of affected animals, there is early complement deposition, followed later by progressive IC deposits and glomerular hypercellularity, but without significant renal impairment.27 Consistent with an alternative pathway mediation, CfH−/− mice with a coexistent factor B deficiency are protected from disease.27 The spontaneous disease in CfH−/− mice requires C5 (but not C6) and complement factor I, presumably to generate pro-inflammatory C5a and to create iC3b as a ligand for β2-integrins on inflammatory cells, respectively.26,28,29 Our laboratory has shown that, in an active IC-mediated GN model, CfH on platelets and podocytes is needed for proper systemic and intraglomerular IC processing, respectively, whereas plasma CfH is essential to prevent disease development irrespective of the load of ICs.29–31 Thus, CfH appears to be a critical complement regulatory protein in normal conditions and in IC-mediated diseases. With this as background, we investigated the roles for CfH in LN in MRL-lpr mice and found that CfH−/− MRL-lpr mice had considerably accelerated development of LN relative to littermate CfH+/− MRL-lpr and CfH+/+ MRL-lpr controls, which led to renal failure from which the animals died.
RESULTS
Renal Failure and Early Mortality in CfH−/− MRL-lpr Mice
The natural history of disease in CfH−/− MRL-lpr mice (n = 9) was compared with littermate CfH+/+ and CfH+/− MRL-lpr mice (n = 11 each); the latter group was included to examine whether there was a CfH gene “dose effect.” CfH deficiency led to early mortality in CfH−/− MRL-lpr mice, with six of nine (67%) dying by 14 weeks of age, compared with no mortality in the two control groups (Figure 1A). These deaths appeared to be caused by renal failure, given average premorbid BUN levels of 82.9 ± 13.6 mg/dl. Consistent with the known course of disease in MRL-lpr mice,2 4 of 11 (36.4%) CfH+/− MRL-lpr mice and 6 of 11 (54.5%) CfH+/+ MRL-lpr mice died by 24 weeks; in contrast, the remaining 3 CfH−/− MRL-lpr mice that survived beyond 14 weeks of age died by 21 weeks (P ≤ 0.001; Figure 1A).
Figure 1.
Early mortality and renal failure in CfH-deficient MRL-lpr mice. (A) Early mortality in CfH−/− MRL-lpr mice. Shown are survival rates from the three groups of MRL-lpr mice over 24 weeks. (B) CfH−/− MRL-lpr mice had significantly higher albuminuria than littermate CfH+/− or CfH+/+ MRL-lpr mice at 12 weeks of age. Shown are individual urinary albumin/creatinine ratios (mg/mg) in all mice from each group at 8 and 12 weeks of age. (C) CfH−/− MRL-lpr mice, but not littermate CfH+/− or CfH+/+ MRL-lpr mice, developed renal failure at 12 weeks of age. Shown are individual BUN values in all mice from each group at 12 weeks of age. Horizontal bars depict the means of each group. *P = 0.044, **P = 0.012, and #P ≤ 0.001 versus CfH+/− and CfH+/+ MRL-lpr mice.
A second set of studies was performed to examine functional and histopathologic features of LN in CfH−/− MRL-lpr mice. At 8 weeks of age, there was only mild albuminuria in all three groups of mice, whereas at 12 weeks of age, the CfH−/− MRL-lpr mice developed marked albuminuria (908.4 ± 504.5 mg/mg creatinine) compared with 0.7 ± 0.3 and 0.5 ± 0.1 mg/mg creatinine in control CfH+/− and CfH+/+ MRL-lpr mice, respectively (P = 0.044; Figure 1B).
Mice from each of the three groups had normal BUN levels at 8 weeks of age (data not shown). Comparable to albuminuria data, CfH−/− MRL-lpr mice had a dramatic increase in BUN levels at 12 weeks of age (70.0 ± 9.5 mg/dl), while these remained normal in the control CfH+/− and CfH+/+ MRL-lpr mice (26.9 ± 1.0 and 28.4 ± 2.3 mg/dl, respectively, P = 0.012; Figure 1C). Thus, deficiency of CfH markedly accelerated renal disease in CfH−/− MRL-lpr mice in the 4-week period between 8 and 12 weeks of age.
Accelerated Development of LN in CfH−/− MRL-lpr Mice
At 8 weeks, control CfH+/− MRL-lpr and CfH+/+ MRL-lpr mice did not have renal pathologic changes, whereas CfH−/− MRL-lpr mice had developed mild GN (Table 1). At 12 weeks, mild LN was evident in some CfH+/− MRL-lpr and CfH+/+ MRL-lpr mice (Figure 2A), whereas CfH−/− MRL-lpr mice developed severe diffuse LN characterized by mesangial, endocapillary, and extracapillary (i.e., crescentic) cellular proliferation, and hyalinosis lesions in glomeruli representing large subendothelial IC deposits (so called “wire loops” in human LN; Table 1). Figure 2, B–D, shows these features in three representative CfH−/− MRL-lpr mice.
Table 1.
Histologic features in CfH−/− MRL-lpr mice
| Feature | 8 Weeks |
12 Weeks |
||||
|---|---|---|---|---|---|---|
| CfH+/+ | CfH+/− | CfH−/− | CfH+/+ | CfH+/− | CfH−/− | |
| Glomerular IgG | 0.8 ± 0.2 | 0.5 ± 0.2 | 1.9 ± 0.3a | 1.8 ± 0.5 | 0.8 ± 0.3 | 2.5 ± 0.3b |
| Glomerular C3 | 0.6 ± 0.1 | 0.6 ± 0.1 | 3.5 ± 0.2a | 1.5 ± 0.4 | 0.7 ± 0.2 | 3.3 ± 0.3a |
| GN | 0.1 ± 0.1 | 0 | 0.9 ± 0.2a | 0.4 ± 0.3 | 0 | 2.6 ± 0.4a |
| GS | 0 | 0 | 0.2 ± 0.2 | 0 | 0 | 1.3 ± 0.5c |
| % GS/hyalinosis | 0 | 0 | 0.1 ± 0.1 | 0 | 0 | 13.7 ± 8.9c |
| % crescents | 0 | 0 | 0 | 0.1 ± 0.1 | 0 | 11.0 ± 6.7d |
Shown are scores of histopathologic features in different groups of MRL-lpr mice at 8 and 12 weeks of age. Data are means ± SEM. GS, glomerulosclerosis.
aP < 0.01; cP = 0.035; and dP = 0.023 versus CfH+/+ and CfH+/− MRL-lpr mice.
bP < 0.05 versus CfH+/−/MRL-lpr mice.
Figure 2.
Accelerated development of lupus nephritis in CfH-deficient MRL-lpr mice. (A–D) Representative periodic acid-Schiff–stained kidneys from 12-week-old CfH+/+ MRL-lpr mice (A), showing mild mesangial proliferative GN, and from three individual CfH−/− MRL-lpr mice (B–D), which showed endocapillary and extracapillary proliferative GN. Arrow, wire loop (C); asterisk, cellular crescent (D). (E–I) Merged representative immunofluorescence photomicrographs for IgG (red) and C3 (green), and ultrastructural features of disease in 12-week-old CfH+/+ MRL-lpr mice (E and F), 8-week-old CfH−/− MRL-lpr mice (G), and 12-week-old CfH−/− MRL-lpr mice (H and I). (E and F) Twelve-week-old CfH+/+ MRL-lpr mice had mesangial immune deposits composed of IgG and C3 (arrowheads). (G) Eight-week-old CfH−/− MRL-lpr mice had linear staining for C3 along glomerular capillary walls, with mesangial and moderate peripheral capillary IgG staining that was largely independent from C3. (H) By 12 weeks of age, CfH−/− MRL-lpr mice had extensive IgG deposition in the mesangium and peripheral capillary walls, which colocalized with C3 in some regions of the capillary wall (white underline). (I) By electron microscopy, immune deposits were present in the mesangium, subendothelial (arrows) and subepithelial regions (arrowheads) (underlined region, podocyte effacement). Original magnifications, ×600 (A–E, G, and H), ×10,000 (F and I).
As true in CfH−/− mice on nonautoimmune backgrounds,27,30 immunofluorescence microscopy showed extensive linear C3 staining along glomerular capillary walls in CfH−/− MRL-lpr mice by 8 weeks of age (Figure 2G). IgG was initially present in mesangial regions but progressively accumulated in capillary walls, such that by 12 weeks, in some instances, it appeared to replace C3 (Figure 2H). Littermate CfH+/− MRL-lpr and CfH+/+ MRL-lpr mice had considerably less C3 and IgG within the mesangium at both ages, with no apparent extension to the peripheral capillary walls (Figure 2E; Table 1).
Ultrastructural features in glomeruli of 12-week-old CfH−/− MRL-lpr mice included significant mesangial, subendothelial, and subepothelial immune deposits and extensive podocyte foot process effacement (Figure 2I), which were absent in age-matched littermate control CfH+/− MRL-lpr and CfH+/+ MRL-lpr mice (Figure 2F). These morphologic features of this accelerated LN model have considerable similarities to acute human LN.32
Characterization of Cells Infiltrating CfH−/− MRL-lpr Mouse Kidneys
The phenotypes of cells infiltrating glomeruli and the tubulointerstitium (TI) of CfH−/− MRL-lpr mice were evaluated by immunohistochemistry. At 8 weeks of age, a time when CfH−/− MRL-lpr mice had developed features of LN, there was 7/4+ cellular infiltration of glomeruli and the TI and CD11b+ cellular accumulation in glomeruli (Table 2). At 12 weeks of age, when CfH−/− MRL-lpr mice had developed diffuse proliferative LN, there were greater numbers of 7/4+ and CD11b+ cells in glomeruli (Figure 3A, b and d) compared with CfH+/+ MRL-lpr controls (Figure 3A, a and c). F4/80+ and CD4+ cells (Figure 3A, e and f) and B cells (data not shown) were mostly absent from glomeruli of all mice. There was marked infiltration of the TI with F4/80+, 7/4+, CD11b+, and CD4+ cells in 12-week-old CfH−/− MRL-lpr mice, particularly in periglomerular and perivascular regions (Figure 3A, f and h).
Table 2.
Infiltrating cellular phenotypes in CfH−/− MRL-lpr mouse kidneys
| Cells | 8 Weeks |
12 Weeks |
||||||
|---|---|---|---|---|---|---|---|---|
| CfH+/+ | CfH+/− | CfH−/− | Pa | CfH+/+ | CfH+/− | CfH−/− | Pa | |
| G 7/4+ | 1.9 ± 0.5 | 1.1 ± 0.3 | 4.1 ± 0.5 | 0.001 | 3.9 ± 1.3 | 2.8 ± 0.1 | 7.9 ± 1.0 | 0.036 |
| G CD11b+ | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.7 ± 0.2 | 0.017 | 0.4 ± 0.1 | 0.4 ± 0.1 | 1.1 ± 0.2 | 0.025 |
| TI F4/80+ | 77.2 ± 6.8 | 73.9 ± 6.5 | 89.6 ± 6.5 | NS | 118.6 ± 15.3 | 87.3 ± 16.8 | 229.3 ± 34.1 | 0.024 |
| TI CD11b+ | 6.6 ± 1.2 | 5.8 ± 0.9 | 8.5 ± 2.1 | NS | 11.9 ± 2.1 | 7.3 ± 1.3 | 29.3 ± 6.4 | 0.034 |
| TI 7/4+ | 64.1 ± 10.1 | 49.0 ± 9.9 | 82.0 ± 8.0 | 0.05 | 109.9 ± 15.7 | 70.6 ± 2.8 | 175.4 ± 27.2 | 0.034 |
| TI CD4+ | 3.8 ± 0.7 | 3.3 ± 1.1 | 4.2 ± 1.1 | NS | 5.2 ± 1.9 | 4.0 ± 0.2 | 14.3 ± 3.0 | 0.021 |
| TI B220+ | 3.7 ± 0.5 | 4.5 ± 0.5 | 3.1 ± 0.2 | NS | 4.7 ± 0.4 | 5.0 ± 1.5 | 6.3 ± 1.8 | NS |
Shown are quantitative data for different infiltrating cells in glomeruli (G, cells/glomerulus) or in the tubulointerstitium (TI, cells/400× field) in different groups of MRL-lpr mice at 8 and 12 weeks of age. Data are means ± SEM. NS, not significant (P > 0.05).
aAll P values shown are for CfH−/− MRL-lpr mice versus age-matched littermate control CfH+/+ and CfH+/− MRL-lpr mice (Kruskal-Wallis test). There was no significant difference between the two control groups in all comparisons.
Figure 3.
Characterization of cells infiltrating kidneys of CfH−/− MRL-lpr mice. (A) Representative immunohistochemical staining showing marked glomerular and periglomerular accumulation of 7/4+ (b) and CD11b+ (d) cells, as well as periglomerular and perivascular accumulation of CD4+ and F4/80+ cells (f and h) in the kidneys of CfH−/− MRL-lpr mice compared with control CfH+/+ MRL-lpr mice (a, c, e, and g). Original magnifications, ×400. (B) Flow cytometric analyses of CD11b+ cells from kidneys of 12-week-old MRL-lpr mice, showing greater quantities of infiltrating CD11b+ cells in CfH−/− MRL-lpr mice compared with CfH+/+ MRL-lpr mice. CD11b+ cells from the kidneys of CfH−/− MRL-lpr mice were further analyzed and found to consist of three distinct populations of CD11b+Gr1highF4/80−, CD11b+Gr1interF4/80+, and CD11b+Gr1lowF4/80− cells. CD11b+F4/80+ cells also had higher CD11c expression than that of CD11b+Gr1lowF4/80− cells. (C) The activation status of renal infiltrating CD4+ cells was evaluated by the relative quantities of CD44highCD62Llow, CD69+, and CD25+ cells. The numbers in the two-dimensional histograms correspond to the percentage of cells contained within the adjacent red boundary. SSC, side scatter.
To further enumerate and characterize cells infiltrating the TI, flow cytometry was performed on 12-week-old CfH−/− MRL-lpr and control CfH+/+ MRL-lpr mice, at which time sufficient quantities of cells could be isolated from both groups. CD11b and Gr1 were combined to distinguish neutrophils (CD11b+Gr1high) and monocytes (CD11b+Gr1inter),33 whereas F4/80 was used, given its expression on renal dendritic cells.34 Consistent with the immunohistochemical findings, there were more CD11b+ and Gr1+ cells in the TI of CfH−/− MRL-lpr mice compared with control groups. Furthermore, CD11b+ cells could be separated into three distinct subpopulations: Gr1lowF4/80− and Gr1interF4/80+ cells of monocytic/dendritic cell lineage (the latter likely an activated macrophage35) and Gr1highF4/80− neutrophils (Figure 3B).33,34,36 Most Gr1+ cells were also CD11b+ and could be distinguished by the presence or absence of F4/80, with CD11b+F4/80+ cells having higher levels of CD11c expression than CD11b+F4/80− cells. Most (>90%) of CD11c+ cells were also CD11b+. Of note, these subpopulations of CD11b+ cells in the renal TI of CfH−/− MRL-lpr mice were also present in control CfH+/+ MRL-lpr mice, yet, in lesser quantities, indicating CfH deficiency led to an increased influx of these inflammatory cells into the TI.
The T-cell activation markers, CD44, CD62L, CD25, and CD69, were also analyzed in CD4+ cells from the TI from the different groups. Of TI CD4+ cells, 95 to 98% were CD44highCD62Llow, 65 to 68% were CD69+, and 15 to 18% were CD25+ (Figure 3C). Notably, these expression levels were comparable among CfH−/− MRL-lpr and CfH+/+ MRL-lpr mice.
Immunological Features in CfH−/− MRL-lpr Mice
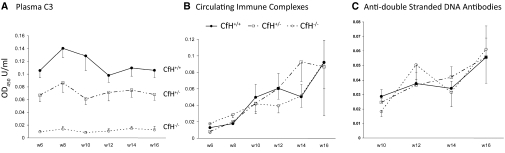
Features of humoral autoimmunity were measured in CfH−/− MRL-lpr mice and controls between 6 and 16 weeks of age, which spanned times before LN had developed until the majority of CfH−/− MRL-lpr mice had died of renal failure. As with the human disease, MRL-lpr mice develop consumptive hypocomplementemia. The deficiency of CfH led to further C3 consumption, with an apparent gene “dose effect” with CfH−/− MRL-lpr and CfH+/− MRL-lpr mice having 6 to 13% and 48 to 73% C3 levels of littermate CfH+/+ MRL-lpr mice over the time period examined (Figure 4A).
Figure 4.
Deficiency of CfH significantly reduces plasma C3 levels while not affecting circulating immune complexes or anti–double-stranded DNA antibody levels in MRL-lpr mice. (A) Plasma C3 levels measured biweekly from 6 to 12 weeks of age in CfH+/+, CfH+/−, and CfH−/− MRL-lpr mice. P < 0.001, CfH−/− MRL-lpr mice versus CfH+/+ and CfH+/− MRL-lpr mice. P < 0.05 CfH+/− MRL-lpr mice versus CfH+/+ MRL-lpr mice. Littermate CfH+/+, CfH+/−, and CfH−/− MRL-lpr mice had comparable circulating immune complex (B) and anti–double-stranded DNA antibody levels (C) over time.
In contrast, deficiency of CfH did not seem to affect features of underlining humoral autoimmunity in MRL-lpr mice. Before 10 weeks of age, all mice in the three groups had low levels of circulating ICs and anti–double-stranded (ds)DNA antibodies. Over time, as the autoimmune disease progressed, both circulating ICs and anti–dsDNA antibodies increased, yet these were not affected by the presence of absence of CfH (Figures 4, B and C). Moreover, the severity of LN did not correlate with the quantities of circulating ICs or anti-dsDNA antibodies.
The effects of CfH deficiency on cellular autoimmunity in MRL-lpr mice were evaluated at 8 and 12 weeks of age. The progressive splenomegaly that occurs over time in these mice was first evaluated. Although there was a tendency for CfH−/− MRL-lpr mice to have greater spleen weights (relative to total body weight), there was not a statistical difference observed (Table 3). Lymphocytes isolated from spleens and renal lymph nodes were analyzed by flow cytometry. The percentage of CD3+CD4+, CD3+CD8+ and CD3+CD4−CD8− (double negative) T cells were comparable in CfH−/−, CfH+/−, and CfH+/+ MRL-lpr mice (data not shown). The ratios of CD69+, CD25+, and CD62Llow CD44highCD4+ cells, as markers of T-cell activation, were also not significantly different among these three groups of mice (Table 3). Thus, deficiency of CfH appeared to not impact features of cellular autoimmune responses in MRL-lpr mice.
Table 3.
Features of cellular immunity in CfH−/− MRL-lpr mice
| Cells (origin) | Feature | 8 Weeks |
12 Weeks |
||
|---|---|---|---|---|---|
| CfH+/+ | CfH−/− | CfH+/+ | CfH−/− | ||
| Spleen weight/body weight (mg/g) | 4.4 ± 0.4 | 5.4 ± 0.6 | 6.3 ± 1.5 | 8.2 ± 1.3 | |
| CD4+ (spleen) | CD25+ | 16.0 ± 0.5 | 15.7 ± 0.6 | 13.2 ± 3.0 | 16.2 ± 2.2 |
| CD69+ | 29.8 ± 2.1 | 29.4 ± 2.1 | 33.3 ± 1.2 | 33.0 ± 1.7 | |
| CD62LlowCD44high | 45.5 ± 1.8 | 46.7 ± 3.4 | 63.5 ± 4.5 | 63.5 ± 1.3 | |
| CD4+ (renal lymph nodes) | CD25+ | 13.7 ± 1.5 | 12.3 ± 1.7 | 10.7 ± 1.2 | 9.9 ± 0.6 |
| CD69+ | 28.4 ± 1.7 | 26.3 ± 2.1 | 28.5 ± 1.6 | 29.6 ± 2.1 | |
| CD62LlowCD44high | 32.6 ± 1.2 | 34.4 ± 5.3 | 34.4 ± 1.9 | 32.8 ± 2.5 | |
Shown is the percentage of the individual cells within the total CD4+ population. Data are means ± SEM.
DISCUSSION
The complement system has complicated and paradoxical roles in SLE and LN.37 Although deficiencies of early classic pathway complement components (C1 > C4 > C2) are associated with the development of disease features of SLE,38 activation of complement at later stages (i.e., C3 and beyond) is likely to contribute to the pathogenesis of both human and experimental SLE.37 Here we showed that MRL-lpr lupus mice lacking the key complement regulator CfH develop LN at a significantly accelerated rate. These data provide further evidence that activation of complement at the stage of C3 and beyond is pathogenic in LN.
CfH is an important fluid phase complement regulator in humans and mice.39,40 Thus, CfH abnormalities can underlie atypical hemolytic uremic syndrome, membranoproliferative GN type 2/dense deposit disease (DDD), and age-related macular degeneration, with an expanding list of identified mutations.9,41 As a general rule, DDD is attributable to type I mutations, leading to reduced functional plasma CfH and the ensuing unrestricted systemic alternative pathway activation, whereas atypical hemolytic uremic syndrome is attributable to type II mutations clustering in the terminal regions of CFH, leading to impaired binding to anionic sites (e.g., on endothelia or the glomerular capillary wall) and local protection against complement activation.9
CfH deficiency in CfH−/− mice leads to spontaneous complement activation in vivo starting no later than 4 days postnatally.27 This results in consumptive hypocomplementemia, including through C3 deposition in glomeruli soon after birth. However, this dramatic complement activation seems to be functionally inconsequential until later in life, when animals develop GN spontaneously; this can lead to the late death of approximately 25% of mice of mixed 129, C57BL/6, and DBA/2 backgrounds.26,27 Features of this glomerular disease are comparable to human membranoproliferative GN type 2/DDD.27 Despite progressive glomerular deposits and hypercellularity, affected animals do not have significant functional impairment of glomerular filtration.27
Consistent with an alternative pathway mediation, CfH−/− mice with a coexistent factor B deficiency are protected from disease.27 Inflammatory cells (monocytes, neutrophils) bear G-protein–coupled receptors for C3a and C5a (C3aR and C5aR) and the β2-integrins, CD11b/CD18 and CD11c/CD18, which are also termed complement receptors (CR)3 and 4, reflecting their binding to iC3b. The spontaneous disease in CfH−/− mice requires C5 (but not C6) and complement factor I.26,28,29 Our recent studies have also shown that GN does not occur in a chronic serum sickness model in C57BL/6 CfH−/− mice, despite marked accumulation of iC3b-bearing ICs in glomeruli, unless there is ongoing complement activation.29 Taking these data together, there seems to be a necessary role for generation of C5a in glomeruli (prevented in C5 deficiency or absent complement activation) and the presence of iC3b in glomerular ICs (prevented in complement factor I deficiency) to allow C5a/C5aR and iC3b/β2-integrin engagement/activation on inflammatory cells in glomerular inflammation.
The naturally occurring LN in MRL-lpr mice has many similarities to the varieties of LN seen in humans and has proven to provide a reliable model to study disease pathophysiology.42,43 Morever, therapeutic strategies for human LN, such as the use of mycophenolate mofetil, have been supported by studies in MRL-lpr mice.44 That the complement system is relevant to the IC-mediated GN occurring in MRL-lpr mice has been supported by a number of studies in which disease was reduced.11–18 In contrast, CfH−/− MRL-lpr mice develop a severe inflammatory GN resulting in the death of over two thirds of animals by 14 weeks of age. Thus, CfH deficiency in MRL-lpr mice markedly accelerates development of LN. This is a relatively unique finding in this strain, joining deficiencies of type 1 angiotensin receptor,45 Toll-like receptor 9,46 and P-selectin,47 as disease accelerators. Of note, the early mortality from LN in CfH−/− MRL-lpr mice seems to be the most severe among these. Although neither the absence of Fas nor CFH is typical of human SLE and LN, this model is unique in accelerating LN in murine lupus models. Although MRL-lpr mice have an expanded pool of CD3+CD4−CD8−“double-negative” T cells, of potential relevance to human SLE,48 CfH deficiency did not affect this cellular population.
Histopathologic features of LN occurring in CfH−/− MRL-lpr mice included marked deposition of IgG- and C3-containing ICs in glomerular subendothelial, mesangial, and subepithelial locations, the former generating “wire loops”; glomerular inflammation with neutrophils and macrophages; extracapillary (crescentic) proliferation; podocyte foot process effacement; and TI inflammation with neutrophils, monocyte/macrophages, dendritic cells, and CD4+ T cells. With this, animals developed severe functional renal disease, with marked albuminuria and azotemia, which appeared to lead to their early death. Overall, these disease features recapitulate human LN; by current criteria set forth by the 2003 International Society of Pathology/Renal Pathology Society for human LN,32 this would be classified as diffuse proliferative LN with crescent formation [class IV-G(A)], membranous LN (class V), and TI nephritis. This disease has similarities to the spontaneously occurring LN in CfH-sufficient MRL-lpr mice49 while being considerably different than the spontaneous GN described in nonautoimmune background CfH−/− mice.27 Therefore, the severe proliferative LN seen in CfH−/− MRL-lpr mice likely represents an accelerated version of spontaneous LN in MRL-lpr mice because of CfH deficiency, and its development may have a different mechanism compared with the spontaneous membranoproliferative GN in the CfH−/− mice with nonautoimmune background.
LN is an autoimmune disease in which autoantibodies are produced and form ICs in glomeruli, which can activate complement and interact with Fc receptors on inflammatory cells.50,51 Activation of the classical pathway of complement by IgG- (and IgM-) containing ICs can lead to recruitment of the alternative pathway, which seems to be important in expression of LN, as supported by data with complement factor B- and D-deficient MRL/lpr mice.14,15 Although CfH is a fluid-phase regulator of the complement alternative pathway, it also is important to regulate complement in sites such as the glomerular and retinal capillary walls, where it can deposit to serve as a fixed complement regulator.52 Thus, in the setting of glomerular-bound ICs in MRL-lpr mice, the presence of CfH seems to be a valuable regulator of complement activation; ultimately, this regulation does seem to be overwhelmed later in the disease course. In CfH−/− MRL-lpr mice lacking this key complement regulator, glomerular-bound ICs were able to activate C3, leading to considerably accelerated disease.
We found that CfH on rodent platelets is responsible for immune adherence, which is a role for human erythrocyte CR1.53 Interestingly, a similar CfH for CR1 switch seems to occur in the mouse podocyte as well.31 Hence, in a chronic serum sickness model in C57BL/6 CfH−/− mice, there was greater glomerular deposition of ICs; however, in these circumstances, there was no development of GN unless CfH was absent from plasma, showing the importance of fluid-phase (and, presumably local glomerular) complement regulation by CfH in this model system.29 Moreover, the hypocomplementemia occurring in CfH−/− mice could also affect systemic and intraglomerular IC processing.54 These aspects are relevant in SLE, given that altered IC processing by human erythrocyte and podocyte CR1 is believed to be important in SLE and LN.55–59 In these studies, there were greater glomerular ICs in CfH−/− MRL-lpr mice compared with CfH-sufficient MRL-lpr mice, despite comparable free plasma (circulating) ICs and anti-ds DNA antibody levels. This finding can be attributed to alterations of systemic and intraglomerular IC processing because of absent platelet and podocyte CfH, respectively, as well as hypocomplementemia. The roles for platelet and podocyte CfH to process ICs as a surrogate for CR1 in humans and for plasma CfH to limit complement activation could not be separated in these studies and are the subject of our ongoing investigations.
We identified a variety of cell types infiltrating CfH−/− MRL-lpr mouse kidneys. Neutrophils (7/4+) and monocyte/macrophages (CD11b+) primarily contributed to glomerular hypercellularity, as is true in active human LN.60 Although TI inflammation in LN is often considered a secondary phenomenon, rarely occurring in the absence of glomerular disease,32 there is growing appreciation that the extent of TI disease is highly relevant. The extent of TI disease has a negative predictive value for renal outcome, more so than does glomerular pathology, even when disease seems to be initiated in glomeruli.61,62 Thus, it was significant that CfH−/− MRL-lpr mice had considerable TI nephritis relative to CfH-sufficient MRL-lpr controls. Based on the expression of relevant markers, this TI inflammation was composed of multiple cell types, including neutrophils (CD11b+Gr1highF4/80−), monocyte/macrophages (CD11b+Gr1interF4/80+), dendritic cells (CD11b+CD11c+F4/80+), and activated CD4+ cells (CD44highCD62Llow and CD69+). Monocytic cells can mediate tissue inflammation and injury in LN while also contributing to clearance of apoptotic debris.63,64 Although some of the other TI infiltrating cell types have less been described in MRL-lpr mice, their existence and distinct functions have been studied in other disease models.65 In a peritonitis model, Gr1inter monocytes recruited into the sites of inflammation simultaneously with and independently of the recruitment of Gr1high neutrophils, and matured to F4/80+ macrophages as the inflammation progressed.35 In renal ischemia-reperfusion, F4/80+ dendritic cells are the predominant TNF-α–secreting cell and seem to play a critical pathogenic role in this model.66 Of note, the presence of these infiltrating cells is not strictly dependent on the absence of CfH, because they were also present in diseased MRL-lpr mouse kidneys later in the course of disease. As such, they are a feature of severe LN in MRL-lpr mice, with deficiency of CfH accelerating their infiltration into lupus mouse kidneys. Engagement and signals through cellular receptors for activated C3 and C5 complement products (i.e., C3aR, C5aR, CR1, CR3, and/or CR4) expressed on all of these cellular types likely accounts for their recruitment to glomeruli and within the TI in this LN model system.
Thus, CfH−/− MRL-lpr mice spontaneously develop a disease process that recapitulates many features of human LN. This seems to be similar to the native disease occurring in CfH-sufficient MRL-lpr mice and distinct from the spontaneous disease in older CfH−/− mice on mixed genetic backgrounds.26,27 As such, we have considered CfH deficiency to accelerate LN in MRL-lpr mice; hence, its presence is a protective factor. The lack of systemic CfH led to altered systemic and intraglomerular IC processing and unrestricted complement activation through the alternative pathway. The subsequent generation of activated complement products led to marked inflammation within glomeruli and the TI. The individual roles for abnormal IC processing and activation of the various complement receptors on the different inflammatory cells is a topic that requires further study.
CONCISE METHODS
Experimental Protocol in Lupus Mice
CfH−/− C57BL/6 mice27 (generously provided by Dr. Marina Botto, Imperial College, UK) were backcrossed onto the MRL-lpr strain (Jackson Laboratories, Bar Harbor, ME). Ten crosses were initially performed onto MRL/MpJ-Tnfrsf6lpr/2J mice (stock number 006825). Because of a phenotypic drift with this MRL-lpr strain, this was followed by five crosses onto the MRL/MpJ-Tnfrsf6lpr/J mice (stock number 000485), which faithfully maintains the original MRL-lpr phenotype. CfH+/− MRL-lpr mice were intercrossed to generate CfH−/−, CfH+/−, and CfH+/+ MRL-lpr mice for use in these studies, which maintained the Tnfrsf6lpr/lpr genotype in all animals.
Littermate CfH−/−, CfH+/−, and CfH+/+ MRL-lpr mice were studied at 8 (n = 10, 5, and 8 in each group of mice, respectively) and 12 weeks (n = 6, 3, and 5 in each group, respectively) of age. At the time of death, blood, urine, and kidneys were harvested from each mouse. Littermate CfH−/− (n = 9), CfH+/− (n = 11), and CfH+/+ (n = 11) MRL-lpr mice were also studied in a separate survival study. Serum samples from each mouse were collected every 2 weeks from 6 to 16 weeks of age. The survival of each mouse was recorded until 24 weeks of age. These studies were approved by the University of Chicago Animal Care and Use Committee.
Measurements from Blood and Urine
BUN concentrations were detected with a Beckman Autoanalyzer (Beckman Coulter, Fullerton, CA). Urinary albumin concentrations were measured with a mouse albumin ELISA kit (Bethyl Laboratories, Montgomery, TX) and normalized to creatinine concentrations in the same urine (measured with Stanbio Creatinine Procedure No. 0400; Stanbio Laboratory, Boerne, TX).54
Serum C3 levels, anti-dsDNA antibodies, and circulating IC levels were determined by ELISA as described previously.11,12,67 Serum samples obtained from the survival study from 6 to 16 weeks were included.
For serum C3 ELISA, plates were coated with goat anti-mouse C3 (Cappel Laboratories, Durham, NC). Serially diluted serum samples were added followed by horseradish peroxidase-goat anti-mouse C3 (Cappel). Results are expressed as relative OD450 values adjusted for standard wild-type C57BL/6 mouse serum samples included in all. Because of the polyclonal nature of the anti-mouse C3 antibody, native intact C3 is identified by this approach, as are cleavage fragments of C3 (i.e., iC3b and its component C3c) to some extent.68
For serum anti-dsDNA Abs ELISA, 96-well plates were coated with methylated BSA (Sigma-Aldrich), followed by calf thymus dsDNA (Sigma-Aldrich). Serial dilutions of sera were incubated for 2 hours at room temperature, followed by horseradish peroxidase–conjugated goat anti-mouse IgG (Kierkegard & Perry Laboratories, Gaithersburg, MD) and OPD peroxidase substrate (Sigma-Aldrich). The OD450 was measured. Sera from several 24-week-old MRL-lpr mice were pooled and served as control. The anti-dsDNA antibodies are presented as relative U by plotting against the standard curve. Sera from 24-week-old MRL/+ and Balb/c mice were used as negative controls.
To measure circulating IC levels, 96-well plates were coated with human C1q (Quidel, San Diego, CA). Serum samples were serially diluted and incubated for 2 hours at room temperature, followed by horseradish peroxidase-goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and OPD peroxidase substrate (Sigma). Sera from several 24-week-old MRL-lpr mice were pooled and served as controls. Circulating IC levels were quantified by plotting against the standard curve and presented as relative U.
Histopathologic Studies
To evaluate renal pathologic changes, kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Four-micrometer sections were stained with periodic acid-Schiff and examined by a renal pathologist (M.H.) in a blinded manner. For each slide, the severity of GN, glomerulosclerosis, interstitial nephritis, and arteritis was graded in a semiquantitative (0 to 4) manner as described previously.12,49 The number of glomeruli with sclerosis and/or hyalinosis and crescent formation was determined and expressed as a percentage of total glomeruli observed in the entire cortical field.
Ultrastructural features were also examined in glomeruli from several randomly chosen mice from each of the experimental groups. Renal cortex was processed for electron microscopy using standard techniques as described previously.31 Sections were viewed with a JEOL JEM-1010 electron microscope (Tokyo, Japan), and representative photographs were taken.
For immunofluorescence microscopy, kidney sections were snap frozen in 2-methylbutane cooled on dry ice and kept at −80°C until use. Four-micrometer cryostat sections were fixed in ether-ethanol and directly double stained with FITC-conjugated antibodies to mouse C3 (Cappel) and Alexa Fluor 647–conjugated antibodies to mouse IgG (Molecular Probes, Eugene, OR). The staining intensity and distribution was semiquantitatively scored from 0 to 4 in a blinded manner as described previously.54
To evaluate renal cellular infiltrates, immunohistochemistry was performed with paraffin-embedded kidney sections. Endogenous peroxidases and biotin were blocked by 0.3% hydrogen peroxide and the Avidin/Biotin blocking kit (Vector Laboratories, Burlingame, CA), with 10% normal goat serum used as a separate blocking step. The slides were incubated with rat anti-mouse 7/4 (AbD Serotec, Oxford, UK) for neutrophils, anti-B220 (BD Pharmingen, San Jose, CA) for B cells, anti-CD4 (BD Pharmingen) for CD4+ T cells, and anti-F4/80 (AbD Serotec) for monocytic cells, followed by goat anti-rat IgG (BD Pharmingen) and streptavidin-peroxidase (Sigma). Specifically bound antibodies were detected by ImmPACT DAB develop Kit (Vector Laboratories). To quantify each cell type in the glomeruli or tubulointerestitium within renal cortices, at least 20 glomeruli or 400× fields were examined by an observer masked to origin of the slides. To compare the distribution of different inflammatory cell types, double immunohistochemistry staining was performed. Slides were first stained with rat anti-mouse CD4 or anti-mouse B220 as described above. The specific antibody binding was detected by diaminobenzidine development (brown). The peroxidases and biotin in the first staining were blocked by 0.3% hydrogen peroxide and the Avidin/Biotin blocking kit. The slides were incubated with rat-anti F4/80 or anti-7/4 as described above. Specific signal was detected by NovaRED Kit (Vector Laboratories) (red). Finally, the sections were counterstained with hematoxylin (Vector Laboratories).
Flow Cytometry
Renal infiltrating cells were isolated as described previously.69 In brief, mouse kidneys were minced and digested at 37°C for 45 minutes with gentle agitation with collagenase I (2 mg/ml) and DNAse I (100 μg/ml) in HBSS/1% (vol/vol) BSA (all from Sigma). Erythrocytes were lysed with NH4Cl, and the cell suspension was passed through a 40-μm cell strainer (BD Bioscience). Isolated cells (approximately 106) from each mouse were stained with Alexa 488–conjugated anti-mouse CD11b (Serotec), allophycocyanin (APC)-conjugated anti-mouse F4/80 (Serotec), PECy7-conjugated anti-mouse CD11c (BD Pharmingen), phycoerythrin (PE)-conjugated anti-mouse Gr-1(Ly-6G) (Serotec), PerCPCy5.5-conjugated anti-mouse B220 (BD Pharmingen), and/or Alexa Fluo 700-conjugated anti-mouse CD8 (BD Pharmingen). Flow cytometry was performed with an LSRII (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowJo software (Tree Star, Ashland, OR).
The subsets and the activation status of lymphocytes from spleen, renal lymph nodes, and kidneys were determined. Spleens and renal lymph nodes were minced, erythrocytes were lysed with NH4Cl, and the cell suspension was passed through a 40-μm cell strainer (BD Bioscience). Renal infiltrating cells were isolated as described above. Approximately 106 cells from each mouse were stained with PerCP-Cy5.5–labeled anti-mouse B220, APCCy7-labeled anti-mouse CD3, Pacific Blue-labeled anti-mouse CD4, AlexaFluo700-labeled anti-mouse CD8, PECy7-labeled anti-mouse CD44, APC-labeled anti-mouse CD62L (all from BD Pharmingen), FITC-labeled anti-mouse CD69, and/or PE-labeled anti-mouse CD25 (Serotec). Flow cytometry was then performed and analyzed as described above.
Statistical Analysis
Numeric data from all experiments were first analyzed using the “graphical summary” function in Minitab 15 (State College, PA) to determine data normality (Anderson-Darling test) and 95% confidence intervals for mean, median, and SD. Parametric and nonparametric data were analyzed by one-way ANOVA and Kruskal-Wallis tests, respectively. When data sets were significantly different by these measures, subsequent comparisons were made by Tukey's testing and sign confidence intervals for parametric and nonparametric data, respectively; appropriate corrections for multiple comparisons were always incorporated in these analyses. Survival data from the three groups were analyzed using nonparametric distribution analysis with Kaplan-Meier estimates. To analyze potential correlations among data sets, Pearson product moment correlation coefficients and associated P values were calculated.
DISCLOSURES
None.
Acknowledgments
We thank Dr. Marina Botto (Imperial College, UK) for providing CfH-deficient mice. This work was supported by a grant from the Arthritis National Research Foundation and a grant from the Arthritis Foundation Chicago Chapter (to L.B.) and NIH R01 Grants DK041873 and DK055357 (to R.J.Q.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ: Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med 148: 1198–1215, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theofilopoulos AN, Dixon FJ: Models of systemic lupus erythematosus. Adv Immunol 37: 269–390, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Kelley VE, Sneve S, Musinski S: Increased renal thromboxane production in murine lupus nephritis. J Clin Invest 77: 252–259, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walport MJ: Advances in immunology: Complement (first of two parts). N Engl J Med 344: 1058–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Hess C, Schifferli JA: Immune adherence revisited: Novel players in an old game. News Physiol Sci 18: 104–108, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Barlow PN, Baron M, Norman DG, Day AJ, Willis AC, Sim RB, Campbell ID: Secondary structure of a complement control protein module by two-dimensional 1H NMR. Biochemistry 30: 997–1004, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Barlow PN, Norman DG, Steinkasserer A, Horne TJ, Pearce J, Driscoll PC, Sim RB, Campbell ID: Solution structure of the fifth repeat of factor H: A second example of the complement control protein module. Biochemistry 31: 3626–3634, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Aslam M, Guthridge JM, Hack BK, Quigg RJ, Holers VM, Perkins SJ: The extended multidomain solution structures of the complement protein Crry and its chimeric conjugate Crry-Ig by scattering, analytical ultracentrifugation and constrained modelling: Implications for function and therapy. J Mol Biol 329: 525–550, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Zipfel PF, Skerka C: Complement regulators and inhibitory proteins. Nat Rev Immunol 9: 729–740, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Morgan BP, Harris CL: Regulation in the activation pathways. In: Complement Regulatory Proteins, Academic Press, San Diego, CA, 1999, pp 41–136 [Google Scholar]
- 11. Bao L, Haas M, Boackle SA, Kraus DM, Cunningham PN, Park P, Alexander JJ, Anderson RA, Culhane C, Holers VM, Quigg RJ: Transgenic expression of a soluble complement inhibitor protects against renal disease and promotes survival in MRL/lpr mice. J Immunol 168: 3601–3607, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Bao L, Haas M, Kraus DM, Hack BK, Rakstang JK, Holers VM, Quigg RJ: Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. J Am Soc Nephrol 14: 670–679, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Bao L, Zhou J, Holers VM, Quigg RJ: Excessive matrix accumulation in the kidneys of MRL/lpr lupus mice is dependent on complement activation. J Am Soc Nephrol 14: 2516–2525, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe H, Garnier G, Circolo A, Wetsel RA, Ruiz P, Holers VM, Boackle SA, Colten HR, Gilkeson GS: Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol 164: 786–794, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Elliott MK, Jarmi T, Ruiz P, Xu Y, Holers VM, Gilkeson GS: Effects of complement factor D deficiency on the renal disease of MRL/lpr mice. Kidney Int 65: 129–138, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Bao L, Osawe I, Haas M, Quigg RJ: Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol 175: 1602–1610, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Bao L, Osawe I, Puri T, Lambris JD, Haas M, Quigg RJ: C5a promotes development of experimental lupus nephritis which can be blocked with a specific receptor antagonist. Eur J Immunol 35: 3012–3020, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Wenderfer SE, Ke B, Hollmann TJ, Wetsel RA, Lan HY, Braun MC: C5a receptor deficiency attenuates T cell function and renal disease in MRLlpr mice. J Am Soc Nephrol 16: 3572–3582, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Wenderfer SE, Wang H, Ke B, Wetsel RA, Braun MC: C3a receptor deficiency accelerates the onset of renal injury in the MRL/lpr mouse. Mol Immunol 46: 1397–1404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sekine H, Reilly CM, Molano ID, Garnier G, Circolo A, Ruiz P, Holers VM, Boackle SA, Gilkeson GS: Complement component C3 is not required for full expression of immune complex glomerulonephritis in MRL/lpr mice. J Immunol 166: 6444–6451, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Miwa T, Maldonado MA, Zhou L, Sun X, Luo HY, Cai D, Werth VP, Madaio MP, Eisenberg RA, Song WC: Deletion of decay-accelerating factor (CD55) exacerbates autoimmune disease development in MRL/lpr mice. Am J Pathol 161: 1077–1086, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miwa T, Maldonado MA, Zhou L, Yamada K, Gilkeson GS, Eisenberg RA, Song WC: Decay-accelerating factor ameliorates systemic autoimmune disease in MRL/lpr mice via both complement-dependent and -independent mechanisms. Am J Pathol 170: 1258–1266, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bao L, Spiller OB, St JP, Haas M, Hack BK, Ren G, Cunningham PN, Doshi M, Abrahamson DR, Morgan BP, Quigg RJ: Decay-accelerating factor expression in the rat kidney is restricted to the apical surface of podocytes. Kidney Int 62: 2010–2021, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Lin F, Emancipator SN, Salant DJ, Medof ME: Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest 82: 563–569, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Pickering MC, Cook HT: Translational mini-review series on complement factor H: renal diseases associated with complement factor H: Novel insights from humans and animals. Clin Exp Immunol 151: 210–230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pickering MC, Warren J, Rose KL, Carlucci F, Wang Y, Walport MJ, Cook HT, Botto M: Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci USA 103: 9649–9654, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M: Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet 31: 424–428, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT, Botto M, Pickering MC: Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest 118: 608–618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexander JJ, Aneziokoro OGB, Chang A, Hack BK, Markaryan A, Jacob A, Luo R, Thirman M, Haas M, Quigg RJ: Distinct and separable roles of the complement system in factor H-deficient bone marrow chimeric mice with immune complex disease. J Am Soc Nephrol 17: 1354–1361, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Alexander JJ, Pickering MC, Haas M, Osawe I, Quigg RJ: Complement factor H limits immune complex deposition and prevents inflammation and scarring in glomeruli of mice with chronic serum sickness. J Am Soc Nephrol 16: 52–57, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Alexander JJ, Wang Y, Chang A, Jacob A, Minto AW, Karmegam M, Haas M, Quigg RJ: Mouse podocyte complement factor H: The functional analogue to human complement receptor 1. J Am Soc Nephrol 18: 1157–1166, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Markowitz GS, D'Agati VD: Classification of lupus nephritis. Curr Opin Nephrol Hypertens 18: 220–225, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Lagasse E, Weissman IL: Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods 197: 139–150, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Muller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Henderson RB, Hobbs JA, Mathies M, Hogg N: Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood 102: 328–335, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Bao L, Quigg RJ: Complement in lupus nephritis: The good, the bad, and the unknown. Sem Nephrol 27: 69–80, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Manderson AP, Botto M, Walport MJ: The role of complement in the development of systemic lupus erythematosus. Annu Rev Immunol 22: 431–456, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Whaley K, Ruddy S: Modulation of the alternative complement pathway by β1H globulin. J Exp Med 144: 1147–1163, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiler JM, Daha MR, Austen KF, Fearon DT: Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci USA 73: 3268–3272, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saunders RE, barrategui-Garrido C, Fremeaux-Bacchi V, Goicoechea de JE, Goodship TH, Lopez TM, Noris M, Ponce C, I, Remuzzi G, Rodriguez de CS, Sanchez-Corral P, Skerka C, Zipfel PF, Perkins SJ: The interactive Factor H-atypical hemolytic uremic syndrome mutation database and website: Update and integration of membrane cofactor protein and Factor I mutations with structural models. Hum Mutat 28: 222–234, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Cohen PL, Eisenberg RA: Lpr and gld: Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 9: 243–269, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Davidson A, Aranow C: Lupus nephritis: Lessons from murine models. Nat Rev Rheumatol 6: 13–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van Bruggen MC, Walgreen B, Rijke TP, Berden JH: Attenuation of murine lupus nephritis by mycophenolate mofetil. J Am Soc Nephrol 9: 1407–1415, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Crowley SD, Vasievich MP, Ruiz P, Gould SK, Parsons KK, Pazmino AK, Facemire C, Chen BJ, Kim HS, Tran TT, Pisetsky DS, Barisoni L, Prieto-Carrasquero MC, Jeansson M, Foster MH, Coffman TM: Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J Clin Invest 119: 943–953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ: Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25: 417–428, 2006 [DOI] [PubMed] [Google Scholar]
- 47. He X, Schoeb TR, Panoskaltsis-Mortari A, Zinn KR, Kesterson RA, Zhang J, Samuel S, Hicks MJ, Hickey MJ, Bullard DC: Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J Immunol 177: 8748–8756, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC: Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181: 8761–8766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Passwell J, Schreiner GF, Nonaka M, Beuscher HU, Colten HR: Local extrahepatic expression of complement genes C3, factor B, C2 and C4 is increased in murine lupus nephritis. J Clin Invest 82: 1676–1684, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Waldman M, Madaio MP: Pathogenic autoantibodies in lupus nephritis. Lupus 14: 19–24, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Foster MH: T cells and B cells in lupus nephritis. Semin Nephrol 27: 47–58, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alexander JJ, Quigg RJ: The simple design of complement factor H: Looks can be deceiving. Mol Immunol 44: 123–132, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Alexander JJ, Hack BK, Cunningham PN, Quigg RJ: A protein with characteristics of factor H is present on rodent platelets and functions as the immune adherence receptor. J Biol Chem 276: 32129–32135, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Quigg RJ, Lim A, Haas M, Alexander JJ, He C, Carroll MC: Immune complex glomerulonephritis in C4- and C3-deficient mice. Kidney Int 53: 320–330, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Moll S, Miot S, Sadallah S, Gudat F, Mihatsch MJ, Schifferli JA: No complement receptor 1 stumps on podocytes in human glomerulopathies. Kidney Int 59: 160–168, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Kavai M: Immune complex clearance by complement receptor type 1 in SLE. Autoimmun Rev 8: 160–164, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Khera R, Das N: Complement receptor 1: Disease associations and therapeutic implications. Mol Immunol 46: 761–772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Iida K, Mornaghi R, Nussenzweig V: Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med 155: 1427–1438, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walport MJ, Ross GD, kworth-Young C, Watson JV, Hogg N, Lachmann PJ: Family studies of erythrocyte complement receptor type 1 levels: Reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol 59: 547–554, 1985 [PMC free article] [PubMed] [Google Scholar]
- 60. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M: The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15: 241–250, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Risdon RA, Sloper JC, De Wardener HE: Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968 [DOI] [PubMed] [Google Scholar]
- 62. Gandhi M, Olson JL, Meyer TW: Contribution of tubular injury to loss of remnant kidney function. Kidney Int 54: 1157–1165, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, Kern P, Herrmann M: Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 28: 114–121, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR: Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol 20: 2581–2592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rogers NM, Matthews TJ, Kausman JY, Kitching AR, Coates PT: Review article: Kidney dendritic cells: their role in homeostasis, inflammation and transplantation. Nephrology (Carlton) 14: 625–635, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD: Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int 71: 619–628, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Bao L, Wang Y, Chen P, Sarav M, Haas M, Minto AW, Petkova M, Quigg RJ: Mesangial cell complement receptor 1-related protein y limits complement-dependent neutrophil accumulation in immune complex glomerulonephritis. Immunology 128: e895–e904, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paixao-Cavalcante D, Hanson S, Botto M, Cook HT, Pickering MC: Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol 46: 1942–1950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bao L, Haas M, Pippin J, Wang Y, Miwa T, Chang A, Minto AW, Petkova M, Qiao G, Song WC, Alpers CE, Zhang J, Shankland SJ, Quigg RJ: Focal and segmental glomerulosclerosis induced in mice lacking decay-accelerating factor in T cells. J Clin Invest 119: 1264–1274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]