Abstract
Autoantibodies are central to the pathogenesis of several autoimmune diseases including systemic lupus erythematosus. Plasma cells secrete these autoantibodies, but the anatomical sites of these cells are not well defined. Here, we found that although dsDNA-specific plasma cells in NZB/W mice were present in spleen and bone marrow, a large number were in the kidneys and their number correlated with the serum dsDNA-IgG titer. We observed renal plasma cells only in mice with nephritis, where they located mainly to the tubulointerstitium of the cortex and outer medulla. These cells had the phenotypic characteristics of fully differentiated plasma cells and, similar to long-lived bone marrow plasma cells, they were not in cell cycle. In patients with lupus nephritis, plasma cells were often present in the medulla in those with the most severe disease, especially combined proliferative and membranous lupus nephritis. The identification of the kidney as a major site of autoreactive plasma cells has implications for our understanding of the pathogenesis of lupus nephritis and for strategies to deplete autoreactive plasma cells, a long-standing therapeutic aim.
Systemic lupus erythematosus (SLE) is a severe systemic autoimmune disease with multiple clinical manifestations. It is characterized by the production of autoantibodies that recognize a wide range of antigens, prominent among them nuclear components. These autoantibodies are thought to be important in disease pathogenesis, depositing in the form of immune complexes in multiple organs, and subsequently inciting inflammatory reactions that cause tissue damage and clinical disease.1–3
Autoantibodies are made by plasma cells that can be short- or long-lived.4 Short-lived plasmablasts are produced early in response to T-dependent antigens and are found predominantly in the spleen and lymph nodes, have a half-life of 3 days before dying of apoptosis, and make isotype-switched but not affinity-matured immunoglobin (Ig).5,6 Some plasmablasts, arising predominantly from the germinal center and enriched for high-affinity variants, migrate to the bone marrow where they fully differentiate into long-lived plasma cells that can survive for several years 7–10. Long-lived plasma cells secrete up to 80% of total serum antibodies11,12 and are thus likely to play a crucial role in humoral immunity. They are thought to persist in survival niches supported by a specific cellular microenvironment and various soluble factors (BAFF, APRIL, CXCL12, IL6, etc.),13–15 although the exact nature of these niches remains undefined.
A number of abnormalities in the regulation of the B cell immune response have been associated with SLE and are thought to play a role in driving autoantibody production. In SLE-prone mice, such as the NZB/W, NZM 2410/J, MRL.lpr, and BXSB strains, enhanced B cell responses to antigen can be observed, resulting in increased plasma cell production to model antigens 16–19. Dysregulation of a number of genes important in regulating the B cell immune response, such as Lyn,20 FcγRIIb,21 PIRB,22 and TLR7,23 has been shown to be associated with SLE. Abnormalities in PC survival in SLE-prone mice might also contribute to autoantibody production.24 In humans, a number of B cell– and hematopoietic-specific genes have been implicated in pathogenesis by recent genome-wide association studies (e.g., BANK1, BLK, and LYN).25 Moreover, SLE patients have been noted to have an increase in circulating plasmablast number when disease is active,26 something confirmed by microarray analysis of peripheral blood mononuclear cells.27,28
Plasma cells have been described in inflamed tissue in the context of several autoimmune diseases, in particular, the joints of patients with rheumatoid arthritis,29 the thymus of those with myasthenia gravis,30 and the kidney of those with SLE.31 In the SLE-prone NZB/W strain, plasma cells have been described in the kidneys18,32 but their antigen specificity, longevity, and contribution to autoantibody production has not been determined. Moreover, their precise anatomic location, and their association with inflammation and disease severity, has not been described. Given the importance of autoantibodies in many autoimmune diseases, a more detailed knowledge of the nature, survival, and microenvironment of the plasma cells that produce them could improve our understanding of disease pathogenesis and provide novel therapeutic options. We therefore addressed these questions in both the NZB/W mouse and in SLE patients with biopsy-proven lupus nephritis.
RESULTS
Inflamed Kidneys Are a Major Reservoir of Autoreactive Plasma Cells in NZB/W Mice
We first confirmed the presence of PCs in the inflamed kidneys of two related murine models of lupus nephritis, the NZB/W and NZM 2410/J mouse strains (Figure 1A and Supplemental Figure 1). By ELISPOT, we observed an increase in IgG-producing PCs in both the spleen and kidneys of NZB/W mice with significant proteinuria (>3 g/L) (Figure 1A), consistent with the results of Cassese et al.32 In contrast to these results, however, no such PC increase was observed in NZB/W bone marrow, perhaps because of the difference in control strains used (Balb/c and CB20 in Cassese et al. but C57BL/6 in our study) or in the age of the mice (5 to 9 months old in Cassese et al. but 7 to 14 months old in our study). Plasma cell numbers were not significantly above background in C57BL/6 kidneys at any age, and PCs were not observed in the kidneys of NZB/W mice that did not have significant proteinuria (<0.3 g/dl) (Supplemental Figure 2).
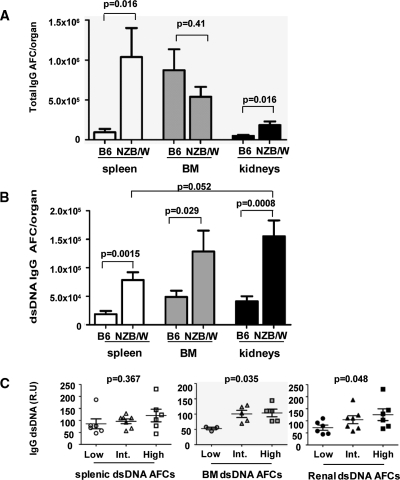
Figure 1.
Autoreactive plasma cells are found in the inflamed kidneys of NZB/W mice. (A) Total IgG antibody–forming cells (AFCs) present in the spleen, kidneys, and bone marrow of NZB/W and sex- and age-matched C57BL/6 mice were detected by ELISPOT. One independent experiment representative of two is shown (n = 5 mice per group). (B) dsDNA-specific IgG–secreting cells in NZB/W and C57BL/6 mice detected with a modified ELISPOT assay. Pooled results of three experiments are shown (n = 11 mice per group). Absolute numbers were multiplied by 2 for the kidney and by 7.9 for the bone marrow to account for the two kidneys and the whole bone marrow.32 (C) Correlation between serum dsDNA IgG and autoreactive plasma cells in NZB/W kidneys. NZB/W mice were divided into three groups depending on the number of dsDNA-specific AFCs in the different organs: (Low) <2 times, (Intermediate) 2 to 5 times, (High) >5 times above background level detected in C57BL/6 mice). Low, intermediate, and high numbers of AFCs are represented by circles, triangles, and squares, respectively. dsDNA-specific serum IgG titers were determined by ELISA (relative units, R.U.). Error bars represent SEM. P values were determined using the Mann-Whitney unpaired test with a risk of 5% except in (C) where a Spearman correlation test was used.
We then modified the ELISPOT technique to detect plasma cells secreting antibodies specific for dsDNA. Strikingly, most IgG anti-dsDNA–specific PCs were found in the kidneys, with the bone marrow also containing a substantial number (Figure 1B). As different coatings were used in the anti-dsDNA and anti-IgG ELISPOT assays, it is not possible to precisely determine the percentage of autoreactive PCs in the different organs. However, the proportion of autoreactive PCs appeared to be higher in the kidney compared with the other organs (around 50% of total PCs in the kidneys, 20% in the spleen, and 30% in the bone marrow). Finally, we separated mice into three groups according to the number of dsDNA-specific plasma cells in the different organs, and analyzed the titers of anti-dsDNA antibodies in their sera. Mice with more dsDNA-specific renal and bone marrow PCs had significantly higher titers of dsDNA-specific antibodies (Figure 1C), something not true for splenic PCs, and consistent with renal and bone marrow PCs playing a dominant role in systemic autoantibody production. Moreover, the size of renal and bone marrow ELISPOTs was similar, suggesting a comparable rate of antibody production.
Phenotypic Characterization of Renal Plasma Cells
We then compared the phenotype of renal splenic and BM PCs. Many splenic PCs are not fully differentiated, and still express B cell markers such as B220, CD19, CD43, and CXCR4 (Figure 2, A and B, top panels). Most BM PCs are terminally differentiated, downregulate B cell markers, and upregulate CD38 (Figure 2, A and B, middle panels).33 When analyzing renal plasma cells, we observed that they expressed low levels of CXCR4 and CD19 and a very high level of CD38, suggesting that, similar to BM PCs,33 they are fully differentiated (Figure 2B, bottom panels). We confirmed the lower expression of CXCR4 on BM and renal plasma cells compared with the spleen by immunofluorescence (Supplemental Figure 3). Moreover, we were not able to detect IgM+ PCs in the inflamed kidneys by histology, suggesting that most of the PCs in this organ are isotype-switched (data not shown). Interestingly, renal PCs express intermediate levels of B220 and higher CD43 compared with spleen and BM PCs (Figure 2B, left panels), suggesting that they may correspond to a different subpopulation of PCs than those usually observed in lymphoid organs. In addition, we found that renal PCs from NZM 2410/J mice have the same surface phenotype as those from NZB/W mice (Supplemental Figure 1C).
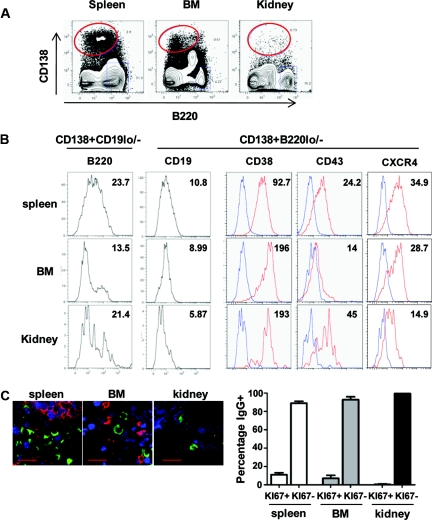
Figure 2.
Renal plasma cells are fully differentiated and long-lived. (A) PCs of NZB/W mice were detected by flow cytometry as CD138+ and B220lo/− in spleen, bone marrow, and kidney. (B) Expression of surface markers on spleen, bone marrow, and kidney PCs gated as CD138+B220lo/− or CD138+/CD19lo/−. Mean fluorescence intensity is indicated for each histogram. Staining for CD38, CD43, and CXCR4 are in red and isotype controls are in blue. One representative dot plot of at least three mice is shown. (C) Percentage of Ki67+ and Ki67− plasma cells in the different organs in NZB/W mice. Frozen sections of spleen (top left), bone marrow (top middle), and kidney (top right) of NZB/W mice were stained for IgG-secreting PCs (green), B cells (B220; red), and the cell cycle marker Ki67 (blue). Scale bars = 20 μm. Spleen, n = 225 cells per four mice; kidney, n = 215 cells per three mice; bone marrow, n = 245 cells per five mice.
It has been suggested that both short- and long-lived PCs might produce autoantibodies,4 with the latter proving resistant to current immunosuppressive therapy.4,34 Long-lived PCs have very low rates of cell division, so we stained tissues from NZB/W mice with antibodies to the cell-cycle marker Ki67 (Figure 2C). Around 15% of the IgG-positive PCs in the spleen were Ki67+, consistent with many of them being newly generated. In the bone marrow, in which most PCs are long-lived and not in cell cycle, only 7% were positive for Ki67. In the kidneys, <1% of the IgG+ PCs were positive for Ki67, suggesting that very few of the PCs in the kidney are dividing (Figure 3B). The same result was observed when analyzing Ki67 expression by flow cytometry on PCs isolated from spleen, BM, and kidneys from NZM mice (Supplemental Figure 1C). Taken together, our data demonstrate that renal PCs are fully differentiated, isotype-switched, and nondividing to at least the same extent as BM PCs.
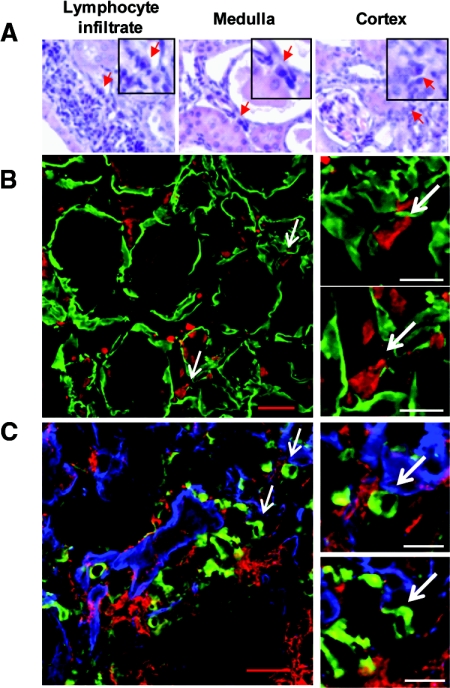
Figure 3.
Most NZB/W renal plasma cells are scattered in the tubulointerstitium. (A) PCs were detected by their characteristic shape and nucleus in formalin-fixed sections stained with hematoxylin and eosin. PCs (red arrows) were present in lymphocyte infiltrates (left) and scattered in the tubulointerstitium of the outer medulla (center) and cortex (right). Magnification, ×40. (B) Localization of PCs in the tubulointerstitium was confirmed by staining PCs with an anti-mouse IgG antibody (red) and the tubular basal membrane with a rabbit anti-mouse laminin (green). Left panel: representative view of the outer medulla. Scale bar = 20 μm. Right panels: Magnified view of PCs. Scale bar = 10 μm. (C) PCs are in the same area as infiltrating myeloid cells. PCs were stained with anti-mouse IgG antibody (green), myeloid cells with anti-CD11b (red), and tubular basement membranes with rabbit anti-mouse laminin (blue). Left panel: Representative view of the tubulointerstitium. Scale bar = 20 μm. Right panels: Magnification view of plasma cells in contact with CD11b+ myeloid cells. Scale bar = 10 μm.
Plasma Cells Are Observed Mainly in the Tubulointerstitium of the Inflamed Kidneys
We then characterized the anatomical localization of PCs in the inflamed kidneys. Hematoxylin and eosin staining showed PCs throughout the kidneys—some were observed in the lymphoid infiltrates commonly seen in lupus nephritis (Figure 3A, left), but most were seen in the outer medulla (center) and cortex (right). Their localization was confirmed by immunofluorescence. PCs were identified by staining for cytoplasmic IgG, and were negative for B220, CD68, and IgM. Although we observed some heterogeneity between mice, the majority of IgG PCs were observed in the tubulointerstitium of the outer medulla and cortex (Figure 3B), suggesting preferential homing to this zone.
Dendritic cells and macrophages are found in this region of the tubulointerstitium in normal and inflamed kidneys.35–37 As PCs in the spleen and lymph nodes appear to colocalize with myeloid cells,38,39 we analyzed the relative localization of PCs and myeloid cells in the inflamed kidneys. We confirmed the substantial myeloid cell infiltration previously described by others,36 and observed that around 60% of the PCs were in contact with CD11b+ cells with a macrophage-like shape (Figure 3C). These cells were negative for CD11c, consistent with them being macrophages. CD11c+ DCs were detected only in lymphoid structures in the inflamed kidneys and thus were not interacting with the tubulointerstitial PCs. We analyzed a potential association between renal PCs and the presence of immune complex (IC) deposits in the kidney. All mice had IC deposits in the glomeruli, whereas 71% had tubular interstitial IC deposits. No correlation was observed between the number of renal PCs and the presence of interstitial IC deposits (Supplemental Table 1).
Plasma Cell Infiltration Is Associated with Increased Severity of Lupus Nephritis in SLE Patients
Anecdotal evidence suggests PC infiltration might be a significant feature of lupus nephritis in humans. To assess this, we studied the medullary areas of renal biopsies of 42 mostly adult patients (4 children <18 years old, 38 adults, 7 men, 35 women) with different classes of lupus nephritis (LN). Ethnicity was known in 30 patients; the female patients included 15 African Americans, 7 Caucasians, and 32 Hispanics and male patients included 5 Caucasians and 1 Asian. All patients had proteinuria with quantitation by 24-hour proteinuria in 15 and a protein:creatinine ratio in 4—other patients showed dipstick proteinuria. Fifteen patients had nephrotic-range proteinuria, mostly observed in patients with membranous lupus nephritis either alone or in combination with focal or diffuse LN (Figure 4, A through C).
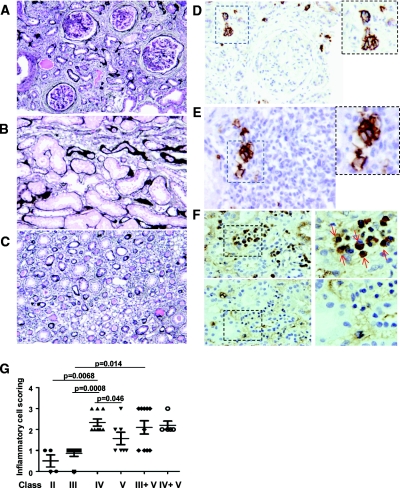
Figure 4.
Plasma cell infiltration in the medulla of kidneys of patients with SLE correlates with inflammation and disease severity. (A) Combined class IV diffuse (A and C) and class V membranous lupus nephritis with segmental endocapillary proliferation and prominent glomerular basement membranes due to widespread glomerular, but not tubular, immune deposits with tubulointerstitial fibrosis and inflammation (Jones' silver stain, ×20). (B) Occasional medullary inflammatory cells in class IV diffuse lupus nephritis (Jones' silver stain, ×40). (C) Fibrosis and interstitial medullary inflammation in combined class IV diffuse and class V membranous lupus nephritis (×20). (D) Occasional cortical interstitial plasma cells in combined class IV diffuse and class V membranous LN (anti-CD138). (E) Scattered medullary interstitial plasma cells in class IV diffuse lupus nephritis (anti-CD138): (A, C, D) from same case; (B and E) from same case. (F) Class IV LN kidney biopsies were stained with an anti-human IgG detection kit (top panels) or with an anti-human IgM antibody conjugated to alkaline phosphatase (bottom panels) and counterstained with eosin. PCs are stained by the anti-IgG (brown, red arrows) and are positive for eosin (blue). (G) H&E scoring of the amount of inflammatory cells in the interstitium depending on the class of LN. 0 = no inflammatory cells, 1 = rare inflammatory cells, 2 = moderate infiltration, and 3 = severe infiltration. ●, LN class II; ■, class III; ▴, class IV; ▾, class V; ♦, class III+V; and ○, class IV+V.
We next examined biopsies for medullary PC infiltration. Semiquantitative assessment of medullary interstitial mononuclear inflammatory infiltration was initially performed on periodic acid–Schiff stained sections. Inflammation was more severe in biopsies from patients with class IV, class III + V, and class IV + V LN (Figure 4G). We next immunophenotyped the cells to distinguish infiltrating medullary PCs from other mononuclear cells. Thirteen of the 42 patients (30.9%) had medullary PC infiltration as detected by CD138 staining (Figure 4, D and E). Most of the PCs in the human kidneys were positive for IgG and not IgM (Figure 4F), suggesting that they are isotype-switched PCs. We then correlated the frequency of medullary PC infiltration with the severity and type of LN. As shown in Table 1, none of the patients with class II LN demonstrated PC infiltration, but PCs were observed in some of the biopsies from all other class of LN. Only one patient with class II LN had medullary PCs. PCs were observed in membranous LN, either alone or in combination with diffuse or focal proliferative LN, and in diffuse proliferative LN (14.28% in class III, 22.2% in class IV, and 28.57% in class V). Thus, patients with class III or IV combined with class V LN present PC infiltration in 40% and 80% of the cases, respectively. The number of infiltrating PCs was also higher in patients with class IV (5.21 ± 2.5 PCs/HPF) and class IV + V (15.69 ± 17.7 PCs/HPF) (Table 1).
Table 1.
Medullary PC infiltration in SLE patients
| Class II | Class III | Class IV | Class V | Class III + V | Class IV + V | |
|---|---|---|---|---|---|---|
| Percentage of patients with PC infiltration | 0% | 14.28% | 22.2% | 28.6% | 40% | 80% |
| n = 4 | n = 7 | n = 9 | n = 7 | n = 10 | n = 5 | |
| No. PCs/HPF in patients with PC infiltration | 3.11 | 5.21 | 0.37 | 3.88 | 15.69 | |
| n = 1 | n = 2 | n = 2 | n = 4 | n = 4 |
We next assessed whether PC infiltration and number correlated with tubular basement membrane (TBM) immune complexes as detected by immunofluorescence. TBM immune complexes were present in 16 of the patients. In patients with combined class IV and V LN, three had TBM deposits, including three of the four patients with PC infiltrate; however, the patient with most marked PC infiltration had no TBM deposits. In class III and V LN, 3 of the 10 patients had TBM deposits, including one who also had medullary PCs. In the seven class V lupus nephritis patients, only one had TBM deposits, without PC infiltration. In the nine class IV patients without combined class V, five had TBM deposits, one with and one without PC infiltration. Among seven class III and four class II lupus nephritis patients, none of the three and one, respectively, with TBM deposits had PC infiltration (Table 2). Thus, medullary PCs are not tightly linked to TBM immune complex deposits, as was also the case in NZB/W mice (Supplemental Table 1). Association between PC infiltration and activity and chronicity indices was also determined. Activity was scored from 0 to 3 where 0 = no activity, 1 = mild (i.e., <25% of glomeruli involved with active lesions including endocapillary proliferation/necrosis/crescents), 2 = moderate (25% to 50% involved), and 3 = severe (>50% involved). Chronicity was scored similarly from 0 to 3 based on composite of degrees of sclerosis and interstitial fibrosis. As shown in Supplemental Table 2, no association was observed between the percentage of patients with PC infiltration and the level of activity. However, patients with high activity (score 1.5 to 3) had on average more PCs (17.5/HPF, n = 4) than those with weak activity (score 0 to 1, 2.49/HPF, n = 9). Patients were also separated into two groups based on their chronicity score. Of the 29 patients with a score comprised of 0 or 1, only 6 (20.7%) had PC infiltration (average number of PC/HPF = 2.9). In contrast, 7 of the 13 patients with a chronicity score of 1.5 to 3 had PC infiltration (average number of PC/HPF = 10.7) (Supplemental Table 3). These results suggest that PC infiltration in the medulla, an area not contiguous with destructive glomerular lesions that could elicit nonspecific inflammatory responses, is associated with increased disease severity in patients with LN, but not specifically with tubulointerstitial IC deposition.
Table 2.
Lack of association between PC infiltration and TBM immune complex (IC) deposits in SLE patients
| Class II | Class III | Class IV | Class V | Class III + V | Class IV + V | |
|---|---|---|---|---|---|---|
| Percentage of patients with | 0% | 42.8% | 55.5% | 14.3% | 30% | 60% |
| TBM ICs | n = 4 | n = 7 | n = 9 | n = 7 | n = 10 | n = 5 |
| Percentage of patients with | 0% | 11.1% | 0% | 10% | 60% | |
| TBM ICs and PC infiltration | n = 0 | n = 1 | n = 0 | n = 1 | n = 3 |
DISCUSSION
Our results show that in lupus nephritis the kidneys are a major source of autoantibody-producing plasma cells. That these PCs are not found in kidneys of SLE patients with class II LN, nor in NZB/W kidneys from mice that have not yet developed overt nephritis, suggests that they do not play a major part in the initiation of disease, but that as it progresses they become an important source of autoantibody, potentially acting as an amplifying mechanism which might drive clinically apparent disease. Although it is not possible to assess the relative role of pathogenic autoreactive renal PCs relative to those in the bone marrow, our results suggest that they contribute to systemic autoantibody titer. In mice with LN, the absolute number of autoreactive PCs is roughly equivalent in the kidney and BM, renal PC number correlates with anti-dsDNA antibody titer, and ex vivo renal and bone marrow PCs secrete antibody at similar rates. Moreover, increased PC number is associated with increased severity and high activity and chronicity indices in patients with SLE. There is no evidence of association between tubulointerstitial immune complexes and PCs in mice, nor humans, arguing against a direct contribution by renal PC antibody production to local inflammation. This major contribution to autoreactive PC number in patients with LN is consistent with renal PCs making a significant contribution to autoantibody titer and thus disease progression.
Measuring PCs in the kidney in SLE almost certainly substantially underestimates those associated with inflammation, as many other organs can be involved in disease. In support of this, PCs are prominent in skin biopsies from patients with SLE.40 Further underestimation is likely as we measured only anti-dsDNA antibody–producing PCs, and of course other autoantigens also drive antibody responses in SLE. Autoreactive PCs home to the inflamed kidney—those specific for ovalbumin have been shown to do so after immunization32—and thus the antigen specificity of PCs in the kidney presumably reflect the dominant role played by autoantigen in driving immune responses in SLE patients.
It might have been expected that PCs homing to inflamed kidneys in NZB/W mice and SLE LN patients might localize in areas of maximal inflammation. This did not appear to be the case, and PCs were not seen in the glomeruli despite evidence of both immune complex deposition and proliferative glomerulonephritis. Instead, most were scattered as single cells in the interstitium of the cortex and outer medulla, and others were associated with the inflammatory lymphoid infiltrates that are often seen in lupus nephritis. This localization broadly parallels that of macrophages and DCs in the kidney,35–37 and could be due to local changes in the expression of chemotactic molecules in the context of inflammation—either by renal tissue itself or by infiltrating inflammatory cells such as T cells or myeloid cells. Whether plasma cells share some mechanisms used by myeloid cells in their homing in inflamed kidneys is not known.41 Chemokine expression in inflamed kidneys has been investigated both in humans and in mice. CXCL12, the ligand of CXCR4, has been shown to be upregulated in the inflamed kidneys of NZB/W mice42 and in mouse kidneys after ischemic injury.43 It is also expressed in normal and inflamed kidneys in humans.44,45 The expression of CXCL12 and the similarities in CXCR4 expression between bone marrow and renal PCs suggest that they could have a similar homing mechanism based on the interaction between CXCL12 and CXCR4. Consistent with this, treatment of NZB/W mice with an anti-CXCL12 antibody inhibited autoantibody formation and reversed inflammatory changes in the kidney.42 It has been proposed that homing of plasma cells to inflamed tissues was also dependent on the interaction between CXCR3 and its ligands CXCL9, CXCL10, and CXCL11.46 We observed that CXCR3 is weakly expressed on plasma cells from old NZB/W mice (data not shown); however, we cannot rule out that CXCR3 expression is downregulated once PCs reach inflamed kidneys, as is CXCR4 expression (Figure 2B).33 Germinal centers are thought to be the major source of long-lived PCs.7 We did not observe germinal centers in the inflamed kidneys of NZB/W mice, consistent with previous observations.18,32 Although this does not completely exclude the possibility of generation of PCs directly in the kidneys, it suggests that they are likely to be generated in secondary lymphoid organs before migrating to the inflamed kidneys, as they do to the BM.47
Plasma cell localization needs also to be considered in the context of the specific anatomical features of the renal vasculature. To access the interstitium of the cortex and outer medulla, the plasma cells will have had to negotiate the arcuate artery, the afferent arteriole, and then the glomerulus, before leaving the circulation via the efferent arteriole, capillaries, or perhaps vasa recta. Understanding how inflammation alters the accessibility of plasma cells, and other immune cells, to the interstitium via the vasculature will be important in understanding renal inflammation, and given the findings presented here, perhaps also systemic autoimmunity. It is tempting to speculate on the local influence that the medullary counter-current exchange mechanism may have on this process, perhaps concentrating relevant soluble molecules.
It is striking that the PCs of the NZB/W kidney are overwhelmingly isotype-switched and not in cell cycle. This, together with their surface marker expression, provides strong evidence that they are fully differentiated long-lived PCs. We also observed that renal PCs have a bimodal expression of CD43 with around 20% to 30% of them expressing higher level of CD43 than spleen or BM PCs. CD43 is an adhesion molecule that is usually highly expressed on B1 cells. It is possible that renal PCs use CD43 for their adhesion and maintenance in the inflamed kidneys. Another explanation would be that a proportion of the renal PCs we observed in NZB/W mice differentiated from isotype-switched B1 cells. Indeed, the B1 cell population is expanded in aged NZB/W mice but is found in the spleen and inflamed organs (kidney, lung, and thymus) rather than in the peritoneal cavity.48,49 In addition, a recent paper has shown that in aged NZB/W mice with nephritis, most B1 cells found in the spleen and inflamed organs have switched to IgG.49
As they are not in cycle, renal plasma cells may depend for their survival on soluble factors secreted by supporting cells, as appears to be the case for long-lived PCs in the bone marrow. The inflammatory cytokine IL6 supports long-lived plasma cell survival in vitro and it is increased in the serum of patients with SLE14,50 and in monocytes from SLE patients.51 Increased expression of the prosurvival cytokine BAFF has also been associated with abnormal renal function,52 and in patients with active SLE, BAFF is expressed by CD4 and CD8 T cells but also by plasma cells from both the bone marrow and kidneys,31,53 raising the possibility that autocrine secretion of BAFF might promote PC survival in lupus kidneys. Further characterization of the factors that regulate survival of these cells will be crucial to devise methods for their control.
Long-lived PCs are resistant to conventional immunosuppressive therapy, such as cyclophosphamide or azathioprine, which targets dividing cells. As these PCs do not express CD20, they are not depleted by rituximab, now commonly used in the treatment of a number of autoimmune conditions.54 Despite this, immunosuppressive therapy does often reduce autoantibody titers, although it is interesting to note that after treatment with rituximab autoantibody levels often fall after clinical improvement has occurred (for example, see reference 55), and in many patients autoantibodies fall whereas those against previously experienced infection-related antigens do not.56 The most likely explanation for these observations is that therapy, and in particular B cell depletion, is controlling inflammation, and that this in turn is reducing the inflammation-related PC survival niches in organs such as the kidney. The degree of fall in autoantibody titers may reflect the proportion of PCs in inflamed tissue, as those in the bone marrow should not be as influenced by a reduction in inflammation. Thus, despite the fact that many autoreactive PCs are in the inflamed tissues, the population of PCs that are resistant to conventional therapy may in fact be in the BM. Further studies will be necessary to assess if reduction of inflammation is sufficient to eliminate PCs from organs such as the kidney. If it is not the case, further characterization of the survival microenvironment of such PCs may allow their targeting, which could be crucial for next generation therapies.
CONCISE METHODS
Animals
NZB/W F1 mice and C57BL/6 mice were bred and maintained under specific pathogen-free conditions. In most studies, mice had proteinuria (>3 g/L) and were positive for serum antinuclear antibodies. Both female and male mice were used in these experiments. Female mice developed disease between 7 and 9 months of age, whereas male mice developed disease between 10 and 14 months. In all experiments age- and sex-matched C57BL/6 mice were used as controls. All experiments were performed according to the regulations of the UK Home Office Scientific Procedures Act (1986).
Human Lupus Nephritis Biopsies
Studies were performed under Institutional Review Board (IRB) approval for exemption with deidentified archival tissue samples and data analyzed. Patient biopsies were selected based on a minimum of 10 glomeruli available by light microscopy, and immunofluorescence and electron microscopy, with a diagnosis of lupus nephritis. From these biopsies, we selected those that included medulla in the sample and had remaining tissue available for immunostaining, to include the spectrum of lupus nephritis. We did not have sufficient patients with class I lupus nephritis to include. Clinical parameters and demographics at time of biopsy were assessed.
Immunohistochemical staining was done after heat-induced antigen retrieval (DAKO # S 1700; Carpenteria, CA). Slides were incubated in primary antibody for 30 minutes (CD138, 1:50, DAKO #7228) and staining was visualized with EnVision +HRP kit (DAKO #4004). Biopsies were semiquantitatively assessed on periodic acid–Schiff stained sections for medullary inflammatory infiltrates, with a score of 0 for no inflammation, 1+ for rare, scattered mononuclear cell infiltrate, 2+ for patchy moderate infiltrate, and 3+ for multifocal severe inflammatory infiltrate. Staining was quantitated by counting positive cells per medullary high-power field (×40) for CD138.
For IgG and IgM staining, human kidney biopsies were processed and stained at the department of histopathology, Addenbrooke's Hospital, Cambridge, UK. Briefly, biopsies were embedded in paraffin and sectioned, and 2-μm sections were routinely stained with hematoxilin and eosin or with anti-human IgG and anti-human IgM (DAKO). The envision enzyme detection system (DAKO) was used.
ANAs and ELISA
Antinuclear antibodies (ANAs) were detected by immunofluorescence using Hep-2 slides (Cambridge Bioscience, UK). Hep-2 slides were first incubated for 30 minutes with serial dilutions of serum (from 1/50 to 1/200) and then washed and ANAs were detected with a rabbit anti-mouse Ig (H + L) secondary antibody conjugated with Alexa Fluor 488. Nuclei were counterstained with 4,6-diamidino-2-phenylindole.
Serum dsDNA-specific IgG was detected by ELISA. Briefly, ELISA plates were precoated with poly-l-lysine (Sigma-Aldrich) at 20 μg/ml for 3 hours at room temperature before being coated with 20 μg/ml of calf thymus dsDNA (Sigma-Aldrich) overnight at 4°C. Plates were saturated in PBS/2% BSA at 37°C for 2 hours. Sera were then added at a starting concentration of 1/100, serially diluted, and incubated for 2 hours at 37°C. Bound immunoglobulins were detected with a goat anti-mouse IgG Fc–specific conjugated with HRP (Jackson Immunoresearch) and revealed with TMB (BD Biosciences). Pooled serum from six NZB/W mice was used as a standard.
ELISPOT
For detection of total IgG-secreting plasma cells, ELISPOT plates (Millipore) were coated with a mix of goat anti-mouse IgG1 + IgG2b + IgG2a + IgG3 antibodies (all at 2 μg/ml) (Southern Biotech) overnight at 4°C.
For detection of dsDNA-specific antibody–secreting cells, ELISPOT plates were precoated with 20 μg/ml poly-l-lysine (Sigma-Aldrich). The precoated plates were subsequently coated with calf thymus dsDNA (20 μg/ml) (Sigma-Aldrich) and incubated overnight at 4°C.
Kidneys were first digested 30 minutes with collagenase (1 mg/ml) and DNase (100 ng/ml). Single-cell suspensions of kidneys and spleens were then obtained by homogenization on 70-μm cell strainers (BD Bioscience) with cell culture medium (DMEM with 10% [vol/vol] FCS, penicillin [100 units per ml], and streptomycin [100 μg/ml]; all from Life Technologies). Bone marrow was flushed from the bone and passed through a 70-μm cell strainer. Cells were added to saturated ELISPOT plates at 105 cells per ml and 106 cells per ml in quadruplicate and incubated overnight at 37°C in 5% CO2 in a humidified incubator with culture medium. Antibody-forming cells were detected with a goat anti-mouse IgG antibody adsorbed against other species and conjugated to horseradish peroxidase (Southern Biotech). Plates were developed using 3-amino-9-ethylcarbazole (Sigma-Aldrich). Plates were read using an AID ELISPOT reader according to the manufacturer's instructions. Absolute numbers were multiplied by 2 for the kidneys and by 7.9 for the bone marrow to extrapolate the total number of plasma cells in both kidneys and in total bone marrow.32
Histology of Mouse Tissues
Mouse kidneys and spleen where either fixed in formalin or embedded in OCT (RA Lamb, U.K.) and snap-frozen in isopentane on dry ice. For frozen organs, 6-μm sections were cut using a cryostat (Leica CM1900) and fixed in ice-cold acetone for 5 minutes. Sections were blocked with 20% horse serum in PBS before staining with the relevant antibodies. Formalin-fixed kidneys were embedded in paraffin and sectioned 5 μm using a microtome (Leica RM2165). Paraffin sections were stained with hematoxylin and eosin (RA Lamb, U.K.).
Femurs were first fixed in formalin for 48 hours and then decalcified in PBS/10% EDTA for 20 days. After decalcification, bones were dehydrated in PBS/30% sucrose for 48 hours, then embedded in OCT, and snap-frozen in isopentane on dry ice before 20-μm sections were cut using a cryostat. Sections were fixed and permeabilized in saturation buffer (2% BSA, 1% horse serum, 0.05% Tween 20, 0.1% Triton X-100 in PBS) for 1 hour at room temperature. Sections were stained in PBS with the appropriate antibodies (see Supplemental Table 4) for 1 hour at room temperature or overnight at 4°C.
Stainings were analyzed on a LSM510 confocal microscope (ZEISS).
Flow Cytometry
Single-cell suspensions of bone marrow, kidneys, and spleens were obtained as described in the ELISPOT section. Peripheral blood mononuclear cells were further purified on lympholyte M gradient (Cedarlane) and saturated with Fc Block (eBioscience) before staining. Stainings were performed with the following antibodies: anti-CD138 APC (281-2), anti-CD19 FITC (eBio1D3), anti-B220 Pacific Blue (RA3-6B2), anti-CD38 PE (90), anti-CD43 biotin (ebioR2-60), anti-CXCR4 PE (2B11), anti-CXCR3 biotin (CXCR3-173), and streptavidin Pe-Cy7 (all from eBioscience).
FACS analysis was performed on a Cyan analyzer (DAKO) and data were analyzed with the Flowjo software (TreeStar, Ashland, OR).
DISCLOSURES
None.
Acknowledgments
We gratefully thank Mark Bowen and Sarah Harford for technical help with histology, Dr. Tim Rayner for assistance with the statistical analysis of our data, Dr. Lorna B. Jarvis for assistance in animal husbandry, and Prof. Fiona Karet for providing reagents. This work was funded by the Wellcome Trust (Programme Grant Number 083650/Z/07/Z) and the National Institute for Health Research Cambridge Biomedical Research Centre. M.E. was supported by a FEBS Long-Term Fellowship, and K.G.C.S. is a Lister Prize fellow.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Fairhurst AM, Wandstrat AE, Wakeland EK: Systemic lupus erythematosus: Multiple immunological phenotypes in a complex genetic disease. Adv Immunol 92: 1–69, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Shlomchik MJ, Craft JE, Mamula MJ: From T to B and back again: Positive feedback in systemic autoimmune disease. Nat Rev Immunol 1: 147–153, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Jacobi AM, Diamond B: Balancing diversity and tolerance: Lessons from patients with systemic lupus erythematosus. J Exp Med 202: 341–344, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA: Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med 199: 1577–1584, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dal Porto JM, Haberman AM, Shlomchik MJ, Kelsoe G: Antigen drives very low affinity B cells to become plasmacytes and enter germinal centers. J Immunol 161: 5373–5381, 1998 [PubMed] [Google Scholar]
- 6. Smith KGC, Hewitson TD, Nossal GJV, Tarlinton DM: The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol 26: 444–448, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Smith KGC, Light A, Nossal GJV, Tarlinton DM: The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J 16: 2996–3006, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R: High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 203: 2419–2424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manz RA, Thiel A, Radbruch A: Lifetime of plasma cells in the bone marrow. Nature 388: 133–134, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dorner T, Hiepe F: Competence and competition: The challenge of becoming a long-lived plasma cell. Nat Rev Immunol 6: 741–750, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Slifka MK, Antia R, Whitmire JK, Ahmed R: Humoral immunity due to long-lived plasma cells. Immunity 8: 363–372, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Benner R, Hijmans W, Haaijman JJ: The bone marrow: The major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol 46: 1–8, 1981 [PMC free article] [PubMed] [Google Scholar]
- 13. Minges Wols HA, Underhill GH, Kansas GS, Witte PL: The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol 169: 4213–4221, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA: Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 171: 1684–1690, 2003 [DOI] [PubMed] [Google Scholar]
- 15. O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ: BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199: 91–98, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jongstra-Bilen J, Vukusic B, Boras K, Wither JE: Resting B cells from autoimmune lupus-prone New Zealand Black and (New Zealand Black x New Zealand White)F1 mice are hyper-responsive to T cell-derived stimuli. J Immunol 159: 5810–5820, 1997 [PubMed] [Google Scholar]
- 17. Theofilopoulos AN, Shawler DL, Eisenberg RA, Dixon FJ: Splenic immunoglobulin-secreting cells and their regulation in autoimmune mice. J Exp Med 151: 446–466, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holmes MC, Burnet FM: The natural history of autoimmune disease in Nzb mice. A comparison with the pattern of human autoimmune manifestations. Ann Intern Med 59: 265–276, 1963 [DOI] [PubMed] [Google Scholar]
- 19. Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE: Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest 68: 419–426, 1993 [PubMed] [Google Scholar]
- 20. Lu R, Vidal GS, Kelly JA, Delgado-Vega AM, Howard XK, Macwana SR, Dominguez N, Klein W, Burrell C, Harley IT, Kaufman KM, Bruner GR, Moser KL, Gaffney PM, Gilkeson GS, Wakeland EK, Li QZ, Langefeld CD, Marion MC, Divers J, Alarcon GS, Brown EE, Kimberly RP, Edberg JC, Ramsey-Goldman R, Reveille JD, McGwin G, Jr., Vila LM, Petri MA, Bae SC, Cho SK, Bang SY, Kim I, Choi CB, Martin J, Vyse TJ, Merrill JT, Harley JB, Alarcon-Riquelme ME, Nath SK, James JA, Guthridge JM: Genetic associations of LYN with systemic lupus erythematosus. Genes Immun 10: 397–403, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kyogoku C, Dijstelbloem HM, Tsuchiya N, Hatta Y, Kato H, Yamaguchi A, Fukazawa T, Jansen MD, Hashimoto H, van de Winkel JG, Kallenberg CG, Tokunaga K: Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: Contribution of FCGR2B to genetic susceptibility. Arthritis Rheum 46: 1242–1254, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kubo T, Uchida Y, Watanabe Y, Abe M, Nakamura A, Ono M, Akira S, Takai T: Augmented TLR9-induced Btk activation in PIR-B-deficient B-1 cells provokes excessive autoantibody production and autoimmunity. J Exp Med 206: 1971–1982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S: Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312: 1669–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KGC: FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol 8: 419–429, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Moser KL, Kelly JA, Lessard CJ, Harley JB: Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun 10: 373–379, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V: Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol 167: 2361–2369, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V: Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 197: 711–723, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lyons PA, McKinney EF, Rayner TF, Hatton A, Woffendin HB, Koukoulaki M, Freeman TC, Jayne DR, Chaudhry AN, Smith KGC: Novel expression signatures identified by transcriptional analysis of separated leukocyte subsets in SLE and vasculitis. Ann Rheum Dis 69: 1208–1213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishikawa H, Ziff M: Electron microscopic observations of immunoreactive cells in the rheumatoid synovial membrane. Arthritis Rheum 19: 1–14, 1976 [DOI] [PubMed] [Google Scholar]
- 30. Hill ME, Shiono H, Newsom-Davis J, Willcox N: The myasthenia gravis thymus: A rare source of human autoantibody-secreting plasma cells for testing potential therapeutics. J Neuroimmunol 202: 50–56, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Chu VT, Enghard P, Schurer S, Steinhauser G, Rudolph B, Riemekasten G, Berek C: Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum 60: 2083–2093, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, Berek C, Hiepe F, Krenn V, Radbruch A, Manz RA: Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur J Immunol 31: 2726–2732, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL: Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 200: 967–977, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller JJ, 3rd, Cole LJ: Resistance of long-lived lymphocytes and plasma cells in rat lymph nodes to treatment with prednisone, cyclophosphamide, 6-mercaptopurine, and actinomycin D. J Exp Med 126: 109–125, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Kruger T, Benke D, Eitner F, Lang A, Wirtz M, Hamilton-Williams EE, Engel D, Giese B, Muller-Newen G, Floege J, Kurts C: Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 15: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, Hammerich L, Panzer U, Kaden S, Quaggin SE, Floege J, Grone HJ, Kurts C: Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 119: 1286–1297, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC: Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol 21: 2951–2962, 1991 [DOI] [PubMed] [Google Scholar]
- 39. Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC: Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol 182: 2113–2123, 2009 [DOI] [PubMed] [Google Scholar]
- 40. McKee P: ECaSG. In: Pathology of the skin, New York, Elsevier, 2005 [Google Scholar]
- 41. Li L, Huang L, Sung SS, Vergis AL, Rosin DL, Rose CE, Jr., Lobo PI, Okusa MD: The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int 74: 1526–1537, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Balabanian K, Couderc J, Bouchet-Delbos L, Amara A, Berrebi D, Foussat A, Baleux F, Portier A, Durand-Gasselin I, Coffman RL, Galanaud P, Peuchmaur M, Emilie D: Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol 170: 3392–3400, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C: Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Lotan D, Sheinberg N, Kopolovic J, Dekel B: Expression of SDF-1/CXCR4 in injured human kidneys. Pediatr Nephrol 23: 71–77, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Hoffmann U, Banas B, Kruger B, Banas M, Bergler T, Boger C, Kammerl M, Obed A, Rummele P, Segerer S, Riegger GA, Kramer BK: SDF-1 expression is elevated in chronic human renal allograft rejection. Clin Transplant 20: 712–718, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Tsubaki T, Takegawa S, Hanamoto H, Arita N, Kamogawa J, Yamamoto H, Takubo N, Nakata S, Yamada K, Yamamoto S, Yoshie O, Nose M: Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol 141: 363–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blink EJ, Light A, Kallies A, Nutt SL, Hodgkin PD, Tarlinton DM: Early appearance of germinal center-derived memory B cells and plasma cells in blood after primary immunization. J Exp Med 201: 545–554, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ito T, Ishikawa S, Sato T, Akadegawa K, Yurino H, Kitabatake M, Hontsu S, Ezaki T, Kimura H, Matsushima K: Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J Immunol 172: 3628–3634, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Enghard P, Humrich JY, Chu VT, Grussie E, Hiepe F, Burmester GR, Radbruch A, Berek C, Riemekasten G: Class switching and consecutive loss of dsDNA reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur J Immunol 40: 1809–1818, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Jones BM, Liu T, Wong RW: Reduced in vitro production of interferon-gamma, interleukin-4 and interleukin-12 and increased production of interleukin-6, interleukin-10 and tumour necrosis factor-alpha in systemic lupus erythematosus. Weak correlations of cytokine production with disease activity. Autoimmunity 31: 117–124, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Mellor-Pita S, Citores MJ, Castejon R, Yebra-Bango M, Tutor-Ureta P, Rosado S, Andreu JL, Vargas JA: Monocytes and T lymphocytes contribute to a predominance of interleukin 6 and interleukin 10 in systemic lupus erythematosus. Cytometry B Clin Cytom 76B: 261–270, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Xu H, He X, Liu Q, Chen Y, Zhu Y, Shi D, Zhang X: The abnormal high expression of B cell activating factor belonging to TNF superfamily (BAFF) and its potential role in kidney transplant recipients. Cell Mol Immunol 5: 465–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morimoto S, Nakano S, Watanabe T, Tamayama Y, Mitsuo A, Nakiri Y, Suzuki J, Nozawa K, Amano H, Tokano Y, Kobata T, Takasaki Y: Expression of B-cell activating factor of the tumour necrosis factor family (BAFF) in T cells in active systemic lupus erythematosus: The role of BAFF in T cell-dependent B cell pathogenic autoantibody production. Rheumatology (Oxford) 46: 1083–1086, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Gurcan HM, Keskin DB, Stern JN, Nitzberg MA, Shekhani H, Ahmed AR: A review of the current use of rituximab in autoimmune diseases. Int Immunopharmacol 9: 10–25, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Smith KGC, Jones RB, Burns SM, Jayne DR: Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse, and re-treatment. Arthritis Rheum 54: 2970–2982, 2006 [DOI] [PubMed] [Google Scholar]
- 56. Ferraro AJ, Drayson MT, Savage CO, MacLennan IC: Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with Rituximab. Eur J Immunol 38: 292–298, 2008 [DOI] [PubMed] [Google Scholar]