Abstract
A half million Americans have ESRD, which puts them at high risk for cardiovascular disease and poor outcomes. Little is known about the epidemiology of atrial fibrillation among patients with ESRD. We analyzed data from annual cohorts (1992 to 2006) of prevalent hemodialysis patients from the United States Renal Data System. In each cohort, we searched 1 year of medical claims for relevant diagnosis codes to determine the prevalence of atrial fibrillation. Among 2.5 million patient observations, 7.7% had atrial fibrillation, with the prevalence increasing 3-fold from 3.5% (1992) to 10.7% (2006). The number of affected patients increased from 3620 to 23,893 (6.6-fold) during this period. Older age, male gender, and several comorbid conditions were associated with increased risk for atrial fibrillation. Compared with otherwise similar Caucasians, the prevalence of atrial fibrillation rates was substantially lower for blacks, Asians, and Native Americans. One-year mortality was twice as high among hemodialysis patients with atrial fibrillation compared with those without (39% versus 19%), and this increased risk was constant during the 15 years of the study. In conclusion, the prevalence of diagnosed atrial fibrillation among patients receiving hemodialysis in the United States is increasing, varies by race, and remains associated with substantially increased mortality. Identifying potentially modifiable risk factors for incident atrial fibrillation requires further investigation.
More than 530,000 patients receive treatment for end-stage renal disease (ESRD) in the United States, with >370,000 undergoing chronic dialysis treatment.1 It appears, although it has not been directly studied, that dialysis patients are particularly prone to developing atrial fibrillation (AF). In an abstract presented at the 2002 annual meeting of the American Society of Nephrology, Herzog et al. from the United States Renal Data System (USRDS) Cardiovascular Special Studies Center showed that approximately 10% (127,318) of the 1,285,177 unique patients in the national registry between 1977 and 2000 were hospitalized at least once with a diagnosis code indicating AF.2 The 2005 USRDS annual data report dedicated a brief section to AF in ESRD patients: Those with the rhythm disorder were predominantly older and white.3 The prevalence of AF was highest in the northern Midwest and the Northeast and lowest in the South.3 Other smaller studies found AF to be present in 7% to 27% of prevalent dialysis patients, with substantial age differences among studies.4–7
The USRDS annual data report called AF a “neglected cardiovascular problem in dialysis patients” and added that although “AF is a common clinical problem in dialysis patients, [yet] it has not generated the same degree of research interest as issues relating to ischemic heart disease in ESRD patients.”3 We propose to fill this void by studying the burden of AF in patients undergoing hemodialysis in the United States. We specifically sought to define (1) the prevalence of AF over a period of 15 years, (2) the factors associated with AF, and (3) mortality risk in ESRD patients with AF.
RESULTS
Study Patients
Using the USRDS registry from 1989 to 2006, we established annual cross-sectional cohorts of all patients who underwent maintenance hemodialysis by December 31 in the years 1992 to 2006 and whose primary payor was Medicare. The 1992 cohort included 103,883 prevalent patients and the patient count reached 223,477 in 2006, reflecting the dramatic increase in the U.S. ESRD population. Overall, 2,483,199 observations were recorded across all 15 years.
Detailed characterizations of selected annual cohorts, by presence versus absence of AF (defined as presence of two diagnosis codes in the same year), are shown in Table 1 (data for all years are available in Supplemental Appendix 1): On average, patients with AF were older by approximately 10 years compared with patients without AF (e.g., 70.9 versus 61.3 years in 2006). The proportion of Caucasian patients was much larger among patients with AF and the other races were correspondingly under-represented compared with patients who did not have this arrhythmia. The prevalence of all comorbidities increased over time and all comorbidities were more prevalent among patients with AF compared with those without it (Table 1).
Table 1.
Characteristics of patients undergoing maintenance hemodialysis on each December 31 from selected years between 1992 and 2006 by presence versus absence of AF
| Characteristics | 1992 |
1996 |
2000 |
2003 |
2006 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No AF | AF | No AF | AF | No AF | AF | No AF | AF | No AF | AF | |
| Number (row percent) | 100,263 (96.5) | 3620 (3.5) | 132,269 (94.3) | 7923 (5.7) | 160,206 (92.2) | 13,467 (7.8) | 183,893 (90.9) | 18,410 (9.1) | 199,584 (89.3) | 23,893 (10.7) |
| Age | 59.6 (±15.5) | 70.4 (±10.3) | 60.2 (±15.4) | 70.5 (±10.9) | 60.9 (±15.3) | 70.8 (±11.4) | 61.3 (±15.2) | 71.1 (±11.5) | 61.3 (±15.0) | 70.9 (±11.8) |
| Female | 48,740 (48.6) | 1726 (47.7) | 63,870 (48.3) | 3771 (47.6) | 76,008 (47.4) | 6101 (45.3) | 85,809 (46.7) | 8042 (43.7) | 91,267 (45.7) | 10,513 (44.0) |
| Male | 51,523 (51.4) | 1894 (52.3) | 68,399 (51.7) | 4152 (52.4) | 84,198 (52.6) | 7366 (54.7) | 98,084 (53.3) | 10,368 (56.3) | 108,317 (54.3) | 13,380 (56.0) |
| Race | ||||||||||
| Caucasian | 54,817 (54.7) | 2684 (74.1) | 69,500 (52.5) | 5653 (71.4) | 83,695 (52.2) | 9822 (72.9) | 96,405 (52.4) | 13,425 (72.9) | 104,595 (52.4) | 17,103 (71.6) |
| African American | 41,909 (41.8) | 837 (23.1) | 57,137 (43.2) | 1995 (25.2) | 68,962 (43.1) | 3169 (23.5) | 78,247 (42.6) | 4309 (23.4) | 84,358 (42.3) | 5859 (24.5) |
| Asian American | 2186 (2.2) | 82 (2.3) | 3606 (2.7) | 219 (2.8) | 4911 (3.1) | 377 (2.8) | 6227 (3.4) | 556 (3.0) | 7299 (3.7) | 741 (3.1) |
| Native American | 1351 (1.4) | 17 (0.5) | 2026 (1.5) | 56 (0.7) | 2638 (1.7) | 99 (0.7) | 3014 (1.6) | 120 (0.7) | 3332 (1.7) | 190 (0.8) |
| Medicaid | 35,234 (35.1) | 944 (26.1) | 52,207 (39.5) | 2389 (30.2) | 64,956 (40.6) | 4116 (30.6) | 70,898 (38.6) | 5552 (30.2) | 65,782 (33.0) | 6319 (26.5) |
| Dialysis vintage | 3.9 (±3.9) | 3.6 (±3.7) | 4.1 (±4.1) | 3.9 (±4.0) | 4.2 (±4.3) | 4.0 (±4.0) | 4.4 (±4.4) | 4.0 (±4.3) | 4.5 (±4.6) | 4.3 (±4.4) |
| <1 year | 19,972 (19.9) | 923 (25.5) | 23,970 (18.1) | 1494 (18.9) | 28,636 (17.9) | 2420 (18.0) | 31,049 (16.9) | 3652 (19.8) | 32,758 (16.4) | 4320 (18.1) |
| 1 to 3 years | 36,099 (36.0) | 1274 (35.2) | 46,581 (35.2) | 2885 (36.4) | 53,104 (33.2) | 4783 (35.5) | 59,293 (32.2) | 6729 (36.6) | 62,393 (31.3) | 7933 (33.2) |
| >3 years | 44,192 (44.1) | 1423 (39.3) | 61,718 (46.7) | 3544 (44.7) | 78,466 (49.0) | 6264 (46.5) | 93,551 (50.9) | 8029 (43.6) | 104,433 (52.3) | 11,640 (48.7) |
| Comorbid conditions | ||||||||||
| diabetes | 40,499 (40.4) | 1500 (41.4) | 74,932 (56.7) | 4732 (59.7) | 100,578 (62.8) | 8983 (66.7) | 123,729 (67.3) | 13,174 (71.6) | 142,638 (71.5) | 18,087 (75.7) |
| hypertension | 47,890 (47.8) | 2286 (63.2) | 105,969 (80.1) | 7030 (88.7) | 146,530 (91.5) | 12,734 (94.6) | 174,253 (94.8) | 17,732 (96.3) | 193,016 (96.7) | 23,449 (98.1) |
| heart failure | 37,612 (37.5) | 2690 (74.3) | 72,550 (54.9) | 6706 (84.6) | 93,690 (58.5) | 11,559 (85.8) | 110,781 (60.0) | 16,055 (87.2) | 124,014 (62.1) | 20,959 (87.7) |
| coronary artery disease | 20,922 (20.9) | 1599 (44.2) | 44,655 (33.8) | 4680 (59.1) | 63,493 (39.6) | 8752 (65.0) | 76,444 (41.6) | 12,168 (66.1) | 83,203 (41.7) | 15,251 (63.8) |
| cerebrovascular disease | 9267 (9.2) | 700 (19.3) | 24,094 (18.2) | 2484 (31.4) | 35,064 (21.9) | 4658 (34.6) | 42,779 (23.3) | 6593 (35.8) | 48,775 (24.4) | 8811 (36.9) |
| peripheral artery disease | 18,757 (18.7) | 1168 (32.3) | 42,435 (32.1) | 3942 (49.8) | 58,062 (36.2) | 7373 (54.8) | 70,382 (38.3) | 10,267 (55.8) | 80,989 (40.6) | 13,816 (57.8) |
| COPD | 9919 (9.9) | 845 (23.3) | 22,621 (17.1) | 2635 (33.3) | 33,063 (20.6) | 4884 (36.3) | 43,255 (23.5) | 7490 (40.7) | 52,410 (26.3) | 10,520 (44.0) |
| cancer | 4157 (4.2) | 233 (6.4) | 9110 (6.9) | 862 (10.9) | 13,163 (8.2) | 1690 (12.6) | 16,625 (9.0) | 2621 (14.2) | 19,547 (9.8) | 3621 (15.2) |
| inability to ambulate | NA | NA | 1920 (1.5) | 176 (2.2) | 3394 (2.1) | 367 (2.7) | 4052 (2.2) | 637 (3.5) | 5678 (2.8) | 1065 (4.5) |
| inability to transfer | NA | NA | 555 (0.4) | 68 (0.9) | 925 (0.6) | 123 (0.9) | 1142 (0.6) | 204 (1.1) | 1935 (1.0) | 418 (1.8) |
Data are presented as count (column percentage) or mean (±SD) unless indicated otherwise. Information on inability to ambulate and inability to transfer was not collected on the Medical Evidence Report until the 1995 version of the form (HCFA-2728, later CMS-2728). Data on all years between 1992 and 2006 are presented in Appendix 1. COPD, chronic obstructive pulmonary disease; NA, not available.
Temporal Trends in the Prevalence of AF
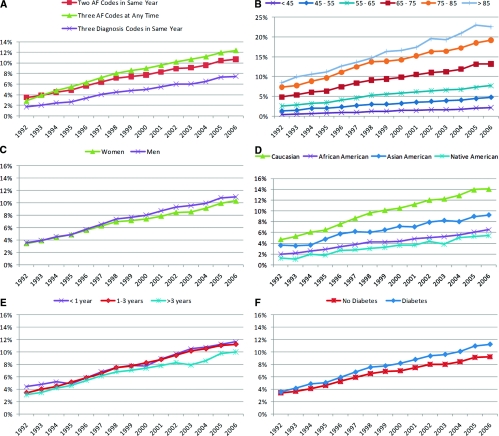
Next, we applied three different algorithms to identify patients with permanent, persistent, or recurrent paroxysmal AF.8 These data are plotted in panel A of Figure 1. We observed a dramatic increase over time in the prevalence of AF for all three definitions. The highest overall prevalence was observed when requiring three AF codes at any point in the past, whereas restricting this requirement to the same year resulted in the lowest prevalence. Using two codes in the same year resulted in an estimate between the other two for most years. To avoid bias across age groups from availability of Medicare claims before the start of dialysis in older patients, we decided to base all analyses on this last algorithm: two AF codes within the same calendar year but on or after the date of the first dialysis treatment that were at least 14 days apart (191,968 observed outcomes).
Figure 1.
Trends in the prevalence of AF in U.S. patients receiving hemodialysis, 1992 to 2006. (A) Prevalence of AF among all prevalent hemodialysis patients on December 31 of 15 consecutive years: comparison of three different algorithms to ascertain AF. Prevalence of AF, defined by more than two diagnosis codes in the same calendar year, among prevalent hemodialysis patients on December 31 of 15 consecutive years by (B) age group, (C) gender, (D) race, (E) dialysis vintage, and (F) diabetes.
Between 1992 and 2006, the prevalence of AF increased more than 3-fold, from 3.5% to 10.7% (Figure 1A). Because of the epidemic of ESRD in the United States, the increase in the absolute numbers of hemodialysis patients with AF was even more dramatic, a 6.6-fold increase from 3620 patients in 1992 to 23,893 patients in 2006 (Table 1).
Although AF has become more prevalent among all age groups, the absolute increase was highest in older age strata: In 2006, the prevalence of AF was 13.2% in patients aged 65 to 75 years, 19.2% in those aged 75 to 85 years, and 22.5% among those >85 years of age (Figure 1B). The risk of AF seems to have increased more rapidly in men compared with women (Figure 1C). Similarly, Caucasians appear to have experienced a disproportionate increase in AF risk compared with any of the other race groups (Figure 1D). It appears that patients who had required renal replacement therapy for >3 years experienced a slower increase (Figure 1E), and patients with diabetes seemed to experience an accelerated AF risk compared with patients without diabetes, at least in more recent years (Figure 1F).
Factors Associated with AF
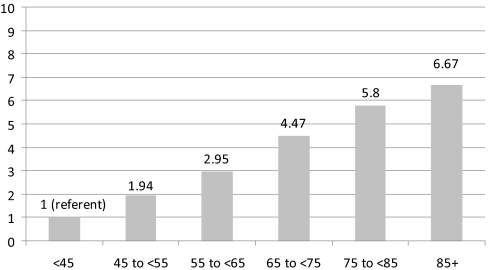
In the 2006 prevalence cohort, older age, male gender, Caucasian race, and the presence of almost all measured comorbidities and frailty indicators were univariately associated with an increased prevalence of AF (Table 2). Multivariate adjustment revealed that several of these estimates were substantially confounded, but most characteristics remained significantly associated with AF. Using information from all years, fully adjusted analyses revealed very similar findings. Age remained an important correlate of AF: Compared with similar patients aged <45 years, AF prevalences were double for patients aged 45 to <55 years (relative risk [RR]: 1.94; 95% confidence interval [CI]: 1.87 to 2.02), triple for those aged 55 to <65 years (RR: 2.95; 95% CI: 2.84 to 3.06), 4.5-fold for those 65 to <75 years (RR: 4.47; 95% CI: 4.32 to 4.64), and almost 6-fold higher for patients in the 75- to <85-year age group (RR: 5.80; 95% CI: 5.60 to 6.02; Figure 2). Maintenance hemodialysis patients who were ≥85 years of age had an almost 7-fold higher prevalence of AF compared with otherwise similar patients <45 of age (RR: 6.67; 95% CI: 6.42 to 6.97).
Table 2.
Factors associated with prevalent AF in U.S. hemodialysis patients
| Factors | 2006 Univariate |
2006 Multivariate |
All Years Multivariate |
|||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Age (per year) | 1.05 | 1.04 to 1.05 | 1.03 | 1.03 to 1.04 | 1.04 | 1.04 to 1.04 |
| Female (versus male) | 0.94 | 0.92 to 0.96 | 0.89 | 0.87 to 0.91 | 0.89 | 0.88 to 0.90 |
| Race (versus Caucasian) | ||||||
| African American | 0.46 | 0.45 to 0.48 | 0.62 | 0.60 to 0.64 | 0.61 | 0.60 to 0.61 |
| Asian American | 0.66 | 0.61 to 0.70 | 0.80 | 0.75 to 0.86 | 0.82 | 0.79 to 0.85 |
| Native American | 0.38 | 0.33 to 0.44 | 0.55 | 0.48 to 0.63 | 0.53 | 0.50 to 0.57 |
| Medicaid (versus no Medicaid beneficiary) | 0.75 | 0.73 to 0.78 | 0.91 | 0.89 to 0.94 | 0.93 | 0.92 to 0.94 |
| Years since first ESRD treatment | 0.99 | 0.98 to 0.99 | 1.01 | 1.00 to 1.01 | 1.02 | 1.01 to 1.02 |
| Comorbidities (versus absence of comorbidity) | ||||||
| diabetes | 1.55 | 1.51 to 1.60 | 0.95 | 0.93 to 0.98 | 0.98 | 0.97 to 0.99 |
| hypertension | 3.51 | 3.21 to 3.84 | 1.12 | 1.03 to 1.22 | 1.22 | 1.19 to 1.24 |
| heart failure | 5.93 | 5.71 to 6.16 | 2.63 | 2.53 to 2.74 | 2.46 | 2.42 to 2.50 |
| coronary artery disease | 3.07 | 2.99 to 3.17 | 1.27 | 1.24 to 1.30 | 1.40 | 1.38 to 1.41 |
| cerebrovascular disease | 2.24 | 2.18 to 2.30 | 1.18 | 1.16 to 1.21 | 1.21 | 1.20 to 1.23 |
| peripheral artery disease | 2.64 | 2.57 to 2.71 | 1.14 | 1.11 to 1.16 | 1.17 | 1.16 to 1.19 |
| COPD | 2.82 | 2.74 to 2.89 | 1.39 | 1.35 to 1.42 | 1.36 | 1.38 to 1.38 |
| cancer (excluding nonmelanoma skin cancer) | 1.91 | 1.84 to 1.99 | 1.17 | 1.13 to 1.20 | 1.15 | 1.14 to 1.17 |
| unable to ambulate | 0.75 | 0.73 to 0.78 | 1.10 | 1.03 to 1.18 | NA | |
| unable to transfer | 1.67 | 1.53 to 1.83 | 1.18 | 1.07 to 1.31 | NA | |
| Calendar year | NA | NA | 1.04 | 1.04 to 1.04 | ||
Multiyear analyses did not include variables on inability to transfer or inability to ambulate because these variables were not collected until the 1995 version of the Medical Evidence Report.
Figure 2.
Adjusted relative prevalences of AF by age group. Patients aged <45 years constituted the reference group. Values adjusted for age categories, gender, race, dialysis vintage, Medicaid eligibility, and all available indicators of comorbid conditions.
Women had an 11% lower prevalence of AF than men. Compared with Caucasians, African Americans had a 39% lower prevalence of AF, whereas the prevalences were 18% lower for Asians and almost half for Native Americans. Most comorbid conditions had independent associations with prevalent AF, with heart failure being associated most strongly (RR: 2.46). Diabetes was the notable exception with no clinically meaningful association. Dialysis vintage also played a minor role, with an adjusted 2% increase in AF risk per year since reaching ESRD.
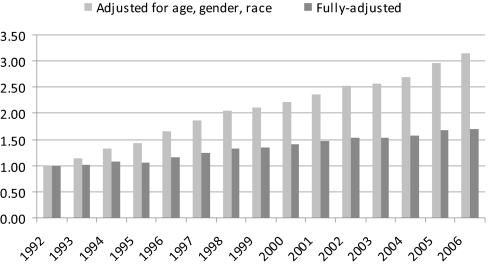
Using the generalized linear model with all patients, we found that the prevalence of AF increased by 4% each year in fully adjusted analyses. In a demographic-adjusted model that included calendar years as categorical variables, the prevalence of AF increased 3-fold from 1992 to 2006 (RR: 3.14; 95% CI: 3.03 to 3.25); additional adjustment for the remaining variables (Medicaid beneficiary, dialysis vintage, comorbid conditions) attenuated this finding, but a 70% (RR: 1.70; 95% CI: 1.64 to 1.75) increase in AF prevalence from 1992 to 2006 remained (Figure 3).
Figure 3.
Adjusted relative prevalences of AF by calendar year. Patients in the 1992 prevalence cohort constituted the reference group. Fully adjusted model included age, gender, race, dialysis vintage, Medicaid eligibility, and all available indicators of comorbid conditions.
One-Year Mortality
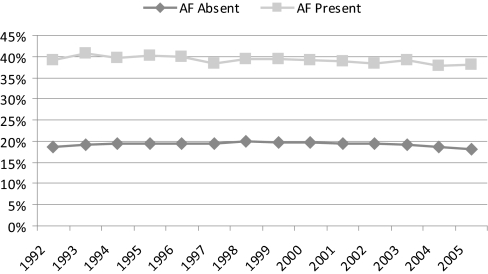
In all study patients, 1-year mortality was 19.3% among patients without AF and 38.9% among those with prevalent AF, with 1-year mortality rates for each annual cohort presented in Figure 4. One-year mortality rates were more than double in patients with AF compared with those without it (hazard ratio [HR]: 2.32; 95% CI: 2.30 to 2.34) and 72% higher after adjustment for demographic information (age, gender, and race-adjusted HR: 1.72; 95% CI: 1.70 to 1.73). Multivariate adjustment for all other variables attenuated this estimate further (HR: 1.45; 1.44 to 1.46). We tested for any changes in the demographic-adjusted HRs associated with AF over time, but the interaction term between prevalent AF and calendar year did not indicate that any temporal trends were present (P = 0.50).
Figure 4.
Crude 1-year mortality in U.S. patients receiving hemodialysis, 1992 to 2005, by absence versus presence of AF. No censoring was applied for receipt of a kidney transplant or for switching to peritoneal dialysis. No data were available for the 2006 cohort because of incomplete follow-up for the year 2007.
DISCUSSION
In this most comprehensive study of the prevalence of AF in the U.S. hemodialysis population to date, we described three landmark findings. The overall prevalence of AF in this patient population was high, exceeding 10% in 2006. Although the prevalence of diagnosed AF has tripled in the past 15 years, the number of affected patients has increased almost 7-fold. Finally, prevalent AF is associated with considerable excess mortality, which has remained quantitatively unchanged over time. Given the ever-increasing number of patients with ESRD in the United States and elsewhere, greater attention should be paid to identifying potentially modifiable risk factors for AF that may be specific to patients undergoing maintenance hemodialysis.
Our findings should be considered in the context of a study by Lakshminaryan et al., who used the 5% Medicare sample and a similar methodology to ours in defining annual prevalences of AF in the general Medicare population.9 As such, we can compare their findings with ours among ESRD patients >65 years of age. In 2002, the prevalence rates of AF in general Medicare beneficiaries aged 65 to <75, 75 to <85, and ≥85 years were approximately 3.5%, 8%, and 11.5%, respectively, whereas the corresponding rates in our ESRD patients were 9.3%, 13.9%, and 17%. For comparison, among members of Kaiser Permanente of Northern California who were hospitalized for heart failure in 1999 to 2000, 36.9% were found to have AF.10
Few published studies have focused on AF in dialysis patients. A study from a single center in Spain reported a 13.6% prevalence of AF among their 190 patients who had been receiving dialysis for at least 3 months in 1998: 9.4% had permanent AF and 4.2% had intermittent AF.6 At the same center, of 225 incident patients without AF, 28 patients developed it over 2 years of follow-up. Age, female gender, and left atrial dimension were associated with incident AF.11 An Italian study of 316 patients receiving maintenance hemodialysis in 1996 reported a 23.4% prevalence of persistent AF (present in at least two electrocardiograms).5 Another single-center study from Italy found a prevalence of 27% among 488 chronic dialysis patients: The arrhythmia was permanent in 13.9%, persistent in 9.6%, and paroxysmal in 3.5% of patients.7 All of these studies reported higher prevalence estimates of AF compared with our population. Although their method of assessment using medical records and electrocardiography is likely accurate, our method of ascertainment has lower sensitivity but excellent specificity. This supports the possibility that our estimates for the prevalence of AF among hemodialysis patients in the United States are conservative and that the true prevalence could be even higher. Similar to the general population,8 age was an important correlate of the presence of AF, but with rather striking increases in the risk across age strata. Men seem to have a slightly higher risk compared with women, a difference that has only appeared in recent years. We were unable to propose a plausible explanation for this discrepancy. One of the more striking results of our study is the difference in AF prevalences among races: Compared with Caucasians, Asians (−18%), African Americans (−39%), and Native Americans (−47%) have drastically lower risks of AF, even after adjustment for imbalances in other characteristics. Our findings of a substantially lower relative prevalence of AF among African Americans compared with Caucasians are consistent with studies of middle-aged adults,12 older adults,13 patients hospitalized with heart failure,10 patients admitted to emergency departments,14 and patients discharged from hospitals.15 Interestingly, the findings of these studies are not just qualitatively consistent but are remarkably similar in the magnitude of the RRs observed. For example, in patients with heart failure, African Americans had half of the AF prevalence of Caucasians. This finding was robust, even after adjustment for many demographic and clinical characteristics that were available in unusually great detail.10
It is well described that African Americans undergoing maintenance hemodialysis experience a unique mortality advantage over Caucasians, even after adjustment for many patient characteristics, and a similar association also exists for South or Southeast Asians.16–20 Studies have shown that African Americans start ESRD treatment with less cardiovascular disease compared with Caucasians, which might explain some of the association between race and AF.17,21,22 The risk of death increased more quickly by degree of kidney function (i.e., by estimated GFR) in Caucasians than in African Americans,23 suggesting that African Americans may be more resistant to the fluid expansion due to heart failure and advanced kidney disease.
Most comorbid conditions prevalent in the study population were associated with the presence of AF. These findings are not surprising given that arteriosclerotic disease predisposes to various manifestations including ischemic heart disease and, subsequently, heart failure. The strongest association was found with heart failure. Clearly, heart failure can lead to AF and, conversely, AF can manifest or aggravate existing heart failure. Our finding that diabetes was inversely (although weakly) associated with AF in multivariate analyses was surprising because fasting glucose is an established and important risk factor for atherosclerotic disease and has also been shown to be a risk factor of incident AF.13 However, it is possible that patients with AF and coexisting diabetes experience disproportionately high mortality, which then removes these patients from the cohort and yields this counterintuitive inverse or lack of association (competing risks). A similar argument can be constructed for the lack of a stronger association between dialysis vintage and AF. It is well known that dialysis patients have a high risk of vascular and valvular calcification, which should put them at increasing risk of valvular or nonvalvular AF.24 Again, it is possible that these patients are at a high mortality risk and may be removed from the risk set in an accelerated fashion.
Similar to patients with AF in the general population,8 hemodialysis patients with AF had higher 1-year mortality compared with those without AF. In the general population, anticoagulation is recommended for all patients with nontransient AF (including recurrent paroxysmal or persistent AF) who have features indicating increased risk of stroke.8 Unfortunately, the relationship between benefit and risk of oral anticoagulation in patients undergoing hemodialysis is unknown because these patients have substantially increased risks of ischemic stroke and bleeding events, including hemorrhagic stroke.25 A recent study from a large U.S. dialysis provider indicated that warfarin use among incident hemodialysis patients with pre-existing AF was associated with increased risk of incident stroke, whereas acetylsalicylic acid or clopidogrel were not associated with such risk.26 Clearly, the risk-benefit relationship of anticoagulation in hemodialysis patients with AF will need to be assessed in randomized trials. Even if anticoagulation were to be found to be net beneficial and cost-effective, its priority among the multiple treatments necessary for the several comorbid conditions usually affecting the dialysis population would also need to be defined. The prevailing clinical uncertainty of whether anticoagulation should be used in hemodialysis patients with AF is reflected in a representative survey of hemodialysis practice patterns in 12 countries. Substantial variability of warfarin use in patients with prevalent AF existed, with treatment rates ranging from 2% (Germany) to 37% (Canada) and 26% of U.S. hemodialysis patients with AF receiving such oral anticoagulation.27
The study presented here has several limitations. Its reliance on medical claims does not permit direct ascertainment of AF from electrocardiograms or medical records. It is also unclear whether the increasing prevalence of AF over time is partly a consequence of more aggressive coding practices in more recent years. Although this might be the case, other studies that did not rely on medical claims and were therefore not susceptible to possible ascertainment bias found temporal trends toward increasing prevalence of AF over time.28,29 We may also have underestimated the true prevalence of AF because we applied a relatively rigorous algorithm that required at least two AF diagnosis codes during each calendar year. Finally, although it is longitudinal, the study presented here assessed prevalence of AF using cross-sectional cohorts over multiple years to understand the burden of AF in this population.
We conclude from this first comprehensive study of the subject that AF is highly prevalent in U.S. hemodialysis patients, especially in older patients. Having AF is associated with increased morbidity and higher mortality, independent of measurable characteristics. In addition, the excess risk of death associated with prevalent AF has not declined in 15 years. It is clear that research is sorely needed to understand potentially modifiable risk factors of AF in this vulnerable population.
CONCISE METHODS
Data Sources and Study Population
We used data from the USRDS spanning the years 1989 to mid-2007 for this study.30
We generated annual cross-sectional populations of all patients undergoing maintenance hemodialysis as of December 31 of each year from 1992 to 2006. Analyses were restricted to patients whose primary payor was Medicare on each index date.
Measurements
In general, only claims dated on or after the first date of ESRD treatment were used for the ascertainment of any outcome or covariate. This was applied to avoid any bias across age groups because patients achieve eligibility for Medicare when they reach 65 years of age; thus, older patients may have Medicare claims in the USRDS that predate their first dialysis treatment.
Outcomes
We considered three different algorithms to identify AF. A single International Classification of Diseases, Ninth Revision (ICD-9) code indicating AF as a primary or secondary diagnosis (ICD-9: 427.3#, where # can be any number or missing) has been used successfully to identify Medicare patients for the National Registry of Atrial Fibrillation31 and in several other claims-based research studies of AF.32–34 Such ICD codes have a specificity of 99% and a positive predictive value of 97% for the diagnosis of AF.35,36 Because it was our intention to identify patients with permanent, persistent, or recurrent paroxysmal AF,8 the three algorithms we developed were more stringent: three claims indicating AF during any time in the past, two claims in the same calendar year, and three claims in the same calendar year. The latter two algorithms require each patient to requalify for AF in each annual cohort, whereas the first algorithm implicitly does not have that requirement. For all algorithms, claims with qualifying AF codes each had to be at least 14 days apart to clearly reflect distinct occurrences.
For each annual cohort, we also tracked their date of death and assessed 1-year all-cause mortality from December 31 of the respective year to December 30 of the following year in patients with and without AF. Date of death is available in the USRDS database.30
Variables
For each patient, we defined several potential predictors and correlates of prevalent AF, including gender and race (Caucasian, African American, Asian American, Native American). We also assessed age, Medicaid coverage as a crude indicator of socioeconomic status, and dialysis vintage (i.e., time since first ESRD treatment) on December 31 of the respective annual cohort. We also ascertained many comorbid conditions using validated algorithms where available, including diabetes mellitus, hypertension, heart failure, coronary artery disease, cerebrovascular disease, peripheral artery disease, chronic obstructive pulmonary disease, and cancer (excluding nonmelanoma skin cancer). These were considered to be present if any two outpatient or one inpatient claims included a corresponding diagnosis code or if the specific condition was recorded on the patient's Medical Evidence Report. Certain procedure codes also qualified for presence of some conditions (e.g., a patient was considered to have had coronary artery disease if they had undergone coronary artery bypass surgery before the respective index date). We also used two frailty indicators from the Medical Evidence Report that were available starting with the 1995 version of the form—inability to transfer and inability to ambulate.
Statistical Analysis
We first depicted the annual prevalence of AF from 1992 to 2006 using the three different algorithms detailed above. For each annual cohort, we tabulated all characteristics by presence of AF, with continuous variables being described by their means and SD and categorical variables using counts and percentages.
To assess what factors are associated with prevalent AF, we first used the most recent cohort from the calendar year of 2006 and conducted univariate and fully adjusted modified Poisson regression, which permits direct estimation of risk ratios even for frequent (nonrare) outcomes.37 Next, we used all study observations (1992 to 2006) to assess whether there was a time trend in the prevalence of AF after adjusting for multiple factors. Inability to ambulate and inability to transfer were not included as covariates in these models because this information was not available for all years. Because adjustment for repeated measures did not change any of the estimated 95% confidence limits (identical to the second decimal digit), the risk ratios and 95% CI reported are derived from models that assumed interpatient independence among all observations.
Finally, we estimated the crude, demographic-adjusted, and fully adjusted HR of 1-year mortality among patients with versus without AF. Because available data covered only a fraction of 2007, the 2006 cohort was excluded. No censoring for modality switches or receipt of a kidney transplant was applied. A test for interaction between year and AF was conducted in a demographic-adjusted model to determine whether the relative mortality risk from AF changed over time.
The study was approved by institutional review boards at Brigham and Women's Hospital and Stanford University School of Medicine and active Data Use Agreements with the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) were in place. This paper was reviewed and approved for privacy content by the NIDDK. The SAS for Windows (version 9.2) statistical software was used for all analyses (www.sas.com).
DISCLOSURES
This work was supported by grant 1R21DK077336 to Dr. Winkelmayer from NIDDK. Dr. Winkelmayer's other recent support includes a Scientist Development Grant from the American Heart Association, a Norman S. Coplon Extramural Research Program Award from Satellite Healthcare, Inc., and an investigator-initiated grant from Fibrogen. In the past 2 years he has participated on advisory boards for AMAG Pharmaceuticals, Amgen, Astellas, Fresenius, GlaxoSmithKline, and Sandoz. Dr. Brookhart has received investigator-initiated research support from Amgen and Rockwell Medical. He has participated on advisory boards for Amgen. Ms. Patrick, Dr. Liu, and Dr. Setoguchi have no conflict of interest to disclose.
Acknowledgments
Data reported herein were supplied by USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the U.S. government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Atrial Fibrillation in Dialysis Patients: A Neglected Comorbidity,” on pages 203–205.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. U.S. Renal Data System: USRDS 2009 Annual Data Report: Volume Three: Reference Tables on End-Stage Renal Disease, Section D: Treatment Modalities, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2009, p 510 [Google Scholar]
- 2. Herzog CA, Ma JZ, Collins AJ: Long-term outcome of dialysis patients in the United States hospitalized with atrial fibrillation [Abstract]. J Am Soc Nephrol 13: 433A, 2002 [Google Scholar]
- 3. U.S. Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Chapter 9: Cardiovascular Special Studies, Bethesda, MD, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease, 2005, pp 173–184 [Google Scholar]
- 4. Abe S, Yoshizawa M, Nakanishi N, Yazawa T, Yokota K, Honda M, Sloman G: Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J 131: 1137–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Fabbian F, Catalano C, Lambertini D, Tarroni G, Bordin V, Squerzanti R, Gilli P, Di Landro D, Cavagna R: Clinical characteristics associated to atrial fibrillation in chronic hemodialysis patients. Clin Nephrol 54: 234–239, 2000 [PubMed] [Google Scholar]
- 6. Vazquez E, Sanchez-Perales C, Borrego F, Garcia-Cortes MJ, Lozano C, Guzman M, Gil JM, Borrego MJ, Perez V: Influence of atrial fibrillation on the morbido-mortality of patients on hemodialysis. Am Heart J 140: 886–890, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Genovesi S, Pogliani D, Faini A, Valsecchi MG, Riva A, Stefani F, Acquistapace I, Stella A, Bonforte G, DeVecchi A, DeCristofaro V, Buccianti G, Vincenti A: Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 46: 897–902, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL: ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e257–e354, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Lakshminarayan K, Solid CA, Collins AJ, Anderson DC, Herzog CA: Atrial fibrillation and stroke in the general Medicare population: A 10-year perspective (1992 to 2002). Stroke 37: 1969–1974, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Ruo B, Capra AM, Jensvold NG, Go AS: Racial variation in the prevalence of atrial fibrillation among patients with heart failure: The Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol 43: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Vazquez E, Sanchez-Perales C, Garcia-Garcia F, Castellano P, Garcia-Cortes MJ, Liebana A, Lozano C: Atrial fibrillation in incident dialysis patients. Kidney Int 76: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR: Incidence of atrial fibrillation in whites and African-Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 158: 111–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM: Incidence of and risk factors for atrial fibrillation in older adults. Circulation 96: 2455–2461, 1997 [DOI] [PubMed] [Google Scholar]
- 14. McDonald AJ, Pelletier AJ, Ellinor PT, Camargo CA, Jr: Increasing US emergency department visit rates and subsequent hospital admissions for atrial fibrillation from 1993 to 2004. Ann Emerg Med 51: 58–65, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Khairallah F, Ezzedine R, Ganz LI, London B, Saba S: Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol 94: 500–504, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Bleyer AJ, Tell GS, Evans GW, Ettinger WH, Jr, Burkart JM: Survival of patients undergoing renal replacement therapy in one center with special emphasis on racial differences. Am J Kidney Dis 28: 72–81, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Mesler DE, McCarthy EP, Byrne-Logan S, Ash AS, Moskowitz MA: Does the survival advantage of nonwhite dialysis patients persist after case mix adjustment? Am J Med 106: 300–306, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Pei YP, Greenwood CM, Chery AL, Wu GG: Racial differences in survival of patients on dialysis. Kidney Int 58: 1293–1299, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Robinson BM, Joffe MM, Pisoni RL, Port FK, Feldman HI: Revisiting survival differences by race and ethnicity among hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910–2918, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Agodoa L, Eggers P: Racial and ethnic disparities in end-stage kidney failure—Survival paradoxes in African-Americans. Semin Dial 20: 577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Trivedi H, Xiang Q, Klein JP: Risk factors for non-fatal myocardial infarction and cardiac death in incident dialysis patients. Nephrol Dial Transplant 24: 258–266, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Smith GL, Shlipak MG, Havranek EP, Masoudi FA, McClellan WM, Foody JM, Rathore SS, Krumholz HM: Race and renal impairment in heart failure: Mortality in blacks versus whites. Circulation 111: 1270–1277, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Bellasi A, Veledar E, Ferramosca E, Ratti C, Block G, Raggi P: Markers of vascular disease do not differ in black and white hemodialysis patients despite a different risk profile. Atherosclerosis 197: 242–249, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Reinecke H, Brand E, Mesters R, Schabitz WR, Fisher M, Pavenstadt H, Breithardt G: Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 20: 705–711, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Chan KE, Lazarus JM, Thadhani R, Hakim RM: Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 20: 2223–2233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, Kerr PG, Young EW, Robinson BM: Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int 77: 1098–1106, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB: Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J 131: 790–795, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Tsang TS, Petty GW, Barnes ME, O'Fallon WM, Bailey KR, Wiebers DO, Sicks JD, Christianson TJ, Seward JB, Gersh BJ: The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: Changes over three decades. J Am Coll Cardiol 42: 93–100, 2003 [DOI] [PubMed] [Google Scholar]
- 30. U.S. Renal Data System: Researcher's Guide to the USRDS Database, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005 [Google Scholar]
- 31. Yan Y, Birman-Deych E, Radford MJ, Nilasena DS, Gage BF: Comorbidity indices to predict mortality from Medicare data: Results from the national registry of atrial fibrillation. Med Care 43: 1073–1077, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Choudhry NK, Soumerai SB, Normand SL, Ross-Degnan D, Laupacis A, Anderson GM: Warfarin prescribing in atrial fibrillation: The impact of physician, patient, and hospital characteristics. Am J Med 119: 607–615, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Choudhry NK, Anderson GM, Laupacis A, Ross-Degnan D, Normand SL, Soumerai SB: Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: Matched pair analysis. BMJ 332: 141–145, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lentine KL, Schnitzler MA, Abbott KC, Li L, Xiao H, Burroughs TE, Takemoto SK, Willoughby LM, Gavard JA, Brennan DC: Incidence, predictors, and associated outcomes of atrial fibrillation after kidney transplantation. Clin J Am Soc Nephrol 1: 288–296, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Kokotailo RA, Hill MD: Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke 36: 1776–1781, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Frost L, Vestergaard P: Caffeine and risk of atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Clin Nutr 81: 578–582, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159: 702–706, 2004 [DOI] [PubMed] [Google Scholar]