Abstract
The effect of chronic kidney disease (CKD) on muscle mass in children, independent of poor growth and delayed maturation, is not well understood. We sought to characterize whole body and regional lean mass (LM) and fat mass (FM) in children and adolescents with CKD and to identify correlates of LM deficits in CKD. We estimated LM and FM from dual energy x-ray absorptiometry scans in 143 children with CKD and 958 controls at two pediatric centers. We expressed whole body, trunk, and leg values of LM and FM as Z-scores relative to height, sitting height, and leg length, respectively, using the controls as the reference. We used multivariable regression models to compare Z-scores in CKD and controls, adjusted for age and maturation, and to identify correlates of LM Z-scores in CKD. Greater CKD severity associated with greater leg LM deficits. Compared with controls, leg LM Z-scores were similar in CKD stages 2 to 3 (difference: 0.02 [95% CI: −0.20, 0.24]; P = 0.8), but were lower in CKD stages 4 to 5 (−0.41 [−0.66, −0.15]; P = 0.002) and dialysis (−1.03 [−1.33, −0.74]; P < 0.0001). Among CKD participants, growth hormone therapy associated with greater leg LM Z-score (0.58 [0.03, 1.13]; P = 0.04), adjusted for CKD severity. Serum albumin, bicarbonate, and markers of inflammation did not associate with LM Z-scores. CKD associated with greater trunk LM and FM, variable whole body LM, and normal leg FM, compared with controls. In conclusion, advanced CKD associates with significant deficits in leg lean mass, indicating skeletal muscle wasting. These data call for prospective studies of interventions to improve muscle mass among children with CKD.
Low body mass index (BMI) has been identified as a predictor of mortality in adults1–3 and children4 with chronic kidney disease (CKD). However, BMI gives little information on body composition. More specific measures of body composition may show stronger associations with outcomes. Protein-energy wasting, characterized by loss of lean body mass, frequently complicates CKD in adults5–8 and is associated with a high risk of morbidity and mortality.9,10 Children with CKD have multiple risk factors for lean mass (LM) wasting, including poor dietary intake, inflammation, growth hormone resistance, and metabolic acidosis. However, body composition has not been well characterized in this population. Prior studies yielded inconsistent results, with some studies demonstrating LM deficits, and others finding no abnormalities.11–18 Most prior studies were limited by a lack of robust reference data in healthy children and adolescents, resulting in an inability to adjust for short stature and delayed sexual maturation, and hampering interpretation of results.
Most body composition studies in children with CKD focused on whole body LM, as measured by dual energy x-ray absorptiometry (DXA).11,12 We hypothesized that the magnitude of LM deficits in children with CKD would be greatest within the skeletal muscle compartment, and that greater muscle deficits would be associated with greater CKD severity. To isolate the skeletal muscle compartment from other lean tissues,19,20 we examined leg LM separately. Our objectives were to characterize whole body and regional LM and fat mass (FM) relative to body size and pubertal stage in children and adolescents with CKD, and to identify correlates of LM deficits in CKD.
RESULTS
Participant Demographic and Disease Characteristics
We evaluated 143 children and adolescents with CKD and 958 healthy controls, ages 5 to 21 years, at two centers: Children's Hospital of Philadelphia (CHOP) and Cincinnati Children's Hospital Medical Center (CCHMC). The characteristics of controls and CKD participants (categorized into three groups according to Kidney Disease Outcomes Quality Initiative [K/DOQI] CKD stage:21,22 CKD 2 to 3 (n = 64), CKD 4 to 5 (n = 45), and maintenance dialysis (n = 34)), are summarized in Table 1. Participants in the CKD groups were older than controls (all P < 0.05). Pubertal maturation was delayed in CKD: within Tanner stages 2, 3, and 4, CKD participants were significantly older (P < 0.001) than controls, adjusted for sex and race. The male predominance was consistent with the demographics of childhood CKD. The disease characteristics in the CKD participants are summarized in Table 2.
Table 1.
Characteristics of CKD patients and controls
| Healthy Controls | CKD 2 to 3 | CKD 4 to 5 | Dialysis | |
|---|---|---|---|---|
| n | 958 | 64 | 45 | 34 |
| Age (years) | 11.6 | 13.2a | 14.9a | 14.5a |
| (8.5, 14.9) | (10.8, 17.3) | (11.0, 16.6) | (12.2, 17.6) | |
| Men, n (%) | 459 (48) | 35 (55) | 31 (69)a | 21 (62) |
| Race, n (%)a | ||||
| Caucasian | 467 (49) | 47 (73) | 30 (67) | 22 (65) |
| African American | 391 (41) | 14 (22) | 13 (29) | 11 (32) |
| other | 100 (10) | 3 (5) | 2 (4) | 1 (3) |
| Tanner stage 1 to 2, n (%) | 494 (52) | 27 (44) | 14 (31)a | 11 (33) |
| Height (cm) | 151.1 | 151.8 | 155.5 | 154.7 |
| (131.9, 164.0) | (135.9, 165.9) | (135.2, 161.4) | (136.5, 165.3) | |
| Height-for-age Z | 0.25 | −0.19a | −0.74a | −1.14a |
| score | (−0.35, 0.91) | (−1.08, 0.56) | (−1.76, −0.25) | (−2.03, −0.84) |
| BMI (kg/m2) | 18.8 | 18.9a | 20.4a | 19.1 |
| (16.4, 22.0) | (16.7, 23.0) | (17.4, 25.8) | (17.1, 22.6) | |
| BMI-for-age Z score | 0.38 | 0.30 | 0.10 | −0.28a |
| (−0.38, 1.09) | (−0.34, 1.30) | (−0.63, 1.69) | (−0.86, 1.02) | |
| BMI-for-height-age Z | 0.29 | 0.42 | 0.57a | 0.13 |
| score | (−0.46, 0.96) | (−0.10, 1.26) | (−0.23, 1.89) | (−0.60, 0.75) |
| Physical activity (h/d) | 1.81 | 1.91 | 1.41a | 1.52 |
| (1.17, 2.94) | (1.30, 2.87) | (0.53, 2.67) | (0.78, 2.52) |
Continuous variables are presented as medians (interquartile ranges). Physical activity data were collected in 401 controls.
aP < 0.05 for comparison between CKD group and controls.
Table 2.
Disease characteristics in CKD participants
| CKD 2 to 3 | CKD 4 to 5 | Dialysis | |
|---|---|---|---|
| Primary diagnosis, n (%) | |||
| CAKUT | 44 (69) | 27 (60) | 11 (32) |
| FSGS | 4 (6) | 10 (22) | 12 (35) |
| glomerulonephritis | 8 (12.5) | 3 (7) | 10 (30) |
| other | 8 (12.5) | 5 (11) | 1 (3) |
| Interval since CKD diagnosis (years) | 7.1 (4.8, 11.7) | 7.7 (2.4, 12.3) | 7.6 (1.7, 13.6) |
| Dialysis duration (days) | 234 (83, 455) | ||
| Dialysis modality, n (%), hemodialysis | 20 (59) | ||
| Current glucocorticoid use, n (%) | 5 (8) | 1 (2) | 7 (21) |
| Current growth hormone use, n (%) | 3 (5) | 3 (7) | 7 (21) |
| Serum albumin (g/dl) | 4.1 (3.8, 4.3) | 4.0 (3.4, 4.1) | 3.6 (3.1, 3.9) |
| Serum bicarbonate (mmol/L) | 25 (23, 27) | 23 (20, 24) | 24 (21, 27) |
| IL-6 (pg/ml) | 1.04 (0.63, 2.26) | 1.24 (0.59, 2.21) | 2.64 (2.02, 4.81) |
| CRP (mg/L) | 3.0 (2.0 to 6.0) | 6.0 (4.0 to 6.0) | 6.0 (3.0 to 9.5) |
Continuous variables are presented as median (interquartile range).
Participant Growth Characteristics
Height-for-age Z-scores were significantly lower in all CKD groups, compared with controls (all P < 0.0001). In contrast, mean BMI-for-age Z-scores were significantly lower than controls in dialysis participants only (P = 0.02). BMI-for-height-age Z-scores, calculated as recommended in the 2008 K/DOQI nutrition guidelines,23 were higher in CKD 2 to 3 (P = 0.2) and CKD 4 to 5 participants (P = 0.009), compared with controls, but did not differ between dialysis participants and controls (P = 0.6).
Body proportions differed only slightly between controls and CKD participants of the same age, height, sex, race, and pubertal status. Sitting height was 10.1 mm (95% confidence interval [CI]: [16.1, 41.0]; P = 0.001) lower in CKD 4 to 5 and 15.8 mm ([8.8, 22.8]; P < 0.0001) lower in dialysis participants compared with controls. Differences between CKD 2 to 3 and controls (−1.6 [−6.8, 3.6]; P = 0.5) were NS.
Whole Body and Regional Body Composition
DXA measures of whole body, trunk, and leg LM and FM were expressed as sex- and race-specific Z-scores relative to height, sitting height, and leg length, respectively. Arm length was not available, precluding creation of arm LM Z-scores relative to arm length. Calf muscle and fat cross-sectional areas (CSA) were estimated from peripheral quantitative computed tomography (pQCT) scans in the proximal third of the tibia, and expressed as sex- and race-specific Z-scores relative to tibia length. The 891 healthy controls studied at CHOP (459 men) served as the reference. None of the growth or body composition Z-scores differed between controls studied at CHOP versus CCHMC. Mean differences in whole body, trunk, and leg LM and FM Z-scores between CKD groups and controls, adjusted for sex, age, pubertal status, study site (CHOP versus CCHMC), and height (sitting height, leg length), are illustrated in Figure 1 (LM) and Figure 2 (FM). These differences, and differences in calf muscle and fat CSA Z-scores adjusted for tibia length (rather than height, sitting height, or leg length) and the same other variables, are summarized in Table 3. LM and muscle CSA models were also adjusted for the relevant FM Z-score or fat CSA Z-score because greater FM-for-height is associated with significantly greater LM-for-height.24–26
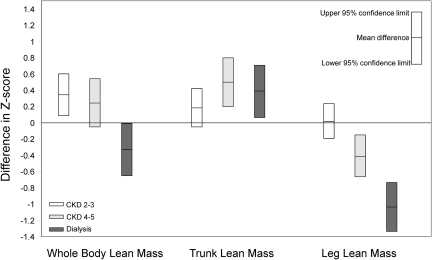
Figure 1.
Whole body lean mass deficits are evident only in dialysis participants because of elevated trunk lean mass in CKD; muscle deficits, represented by leg lean mass deficits proportional to the severity of CKD, are evident in CKD 4 to 5 and dialysis. The figure indicates differences in whole body, trunk, and leg lean mass-for-height Z-scores between CKD participants and controls. Differences in whole body, trunk, and leg lean mass Z-scores adjusted for sex, race, age, height (sitting height or leg length), pubertal status, fat mass Z-score, and study site are presented.
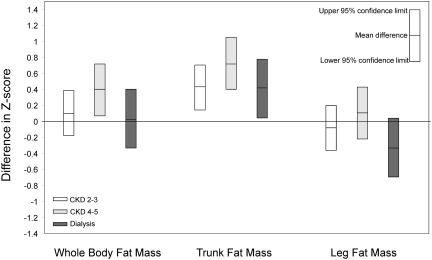
Figure 2.
Whole body and leg fat mass do not differ significantly between CKD participants and controls, but trunk fat mass is significantly higher among all CKD participants. The figure shows differences in whole body, trunk, and leg fat mass-for-height Z-scores between CKD participants and controls. Differences in whole body, trunk, and leg fat mass Z-scores adjusted for sex, race, age, height (sitting height or leg length), pubertal status, and study site are presented. Central distribution of adiposity was evident in all CKD groups.
Table 3.
Differences in body composition Z-scores between CKD groups and healthy controls
| Adjusted Difference in Z-Score versus Controls |
|||
|---|---|---|---|
| Mean Difference [95% CI] | |||
| CKD 2 to 3 | CKD 4 to 5 | Dialysis | |
| DXA lean mass and pQCT calf muscle CSA | |||
| whole body LM-for-height | 0.36 [0.12, 0.60], P = 0.003 | 0.24 [−0.04, 0.52], P = 0.1 | −0.32 [−0.65, −0.002], P = 0.05 |
| trunk LM-for-sitting height | 0.18 [−0.06, 0.42], P = 0.15 | 0.50 [0.22, 0.79], P = 0.001 | 0.39 [0.06, 0.71], P = 0.02 |
| leg LM-for-leg length | 0.02 [−0.20, 0.24], P = 0.8 | −0.41 [−0.66, −0.15], P = 0.002 | −1.03 [−1.32, −0.74], P < 0.0001 |
| calf muscle CSA-for-tibia length | 0.007 [−0.25, 0.26], P = 0.9 | −0.10 [−0.38, 0.18], P = 0.5 | −0.74 [−1.06, −0.42], P < 0.0001 |
| DXA fat mass and pQCT calf fat CSA | |||
| whole body FM-for-height | 0.12 [−0.16, 0.40], P = 0.4 | 0.41 [0.08, 0.73], P = 0.01 | 0.04 [−0.33, 0.42], P = 0.8 |
| trunk FM-for-sitting height | 0.44 [0.17, 0.72], P = 0.002 | 0.73 [0.41, 1.06], P < 0.0001 | 0.43 [0.05, 0.80], P = 0.03 |
| leg FM-for-leg length | −0.07 [−0.35, 0.21], P = 0.6 | 0.11 [−0.21, 0.44], P = 0.5 | −0.32 [−0.69, 0.06], P = 0.1 |
| calf Fat CSA-for-tibia length | −0.06 [−0.37, 0.24], P = 0.7 | 0.11 [−0.23, 0.45], P = 0.5 | −0.37 [−0.75, 0.008], P = 0.06 |
Differences are adjusted for age, sex, pubertal status, study site (CHOP versus CCHMC), and height (height for whole body, sitting height for trunk, and leg length for leg).
Lean Mass Z-Scores
Compared with controls, mean adjusted whole body LM Z-scores were significantly greater in CKD 2 to 3 (P = 0.003), greater in CKD 4 to 5 (P = 0.1), and lower in dialysis participants (P = 0.05). Adjusted trunk LM Z-scores were greater in all CKD groups compared with controls, significantly for CKD 4 to 5 (P = 0.001) and dialysis participants (P = 0.02). The pattern in dialysis patients persisted when peritoneal dialysis patients were excluded (P = 0.004). No leg LM deficits were apparent among CKD 2 to 3 participants. However, moderate (−0.41 SD; P = 0.001) and substantial (−1.03 SD; P < 0.0001) deficits were evident among CKD 4 to 5 and dialysis participants, respectively. A test for trend indicated significant progressive deficits with greater CKD severity (P < 0.0001). When DXA-derived arm LM relative to height was compared between CKD participants and controls, the pattern of deficits was similar to that observed for leg LM. Significant deficits in pQCT measures of calf muscle CSA were evident only in dialysis participants (−0.74 SD; P < 0.0001). Calf muscle CSA Z-scores for CKD 2 to 3 and 4 to 5 participants did not differ significantly from controls.
Fat Mass Z-scores
Compared with controls, adjusted whole body DXA FM Z-scores were higher in all CKD groups, but only significantly higher in CKD 4 to 5 (P = 0.01). Adjusted trunk FM Z-scores were significantly higher in all CKD groups. Adjusted DXA leg FM Z-scores did not differ significantly between controls and any CKD group. pQCT-derived calf fat CSA z-scores did not differ between any CKD group and controls.
Physical Activity
Compared with controls, the mean average daily duration of physical activity was similar for CKD 2 to 3 (P = 0.3), significantly lower for CKD 4 to 5 (P = 0.03), and lower, but not significantly, for dialysis participants (P = 0.2) (Table 1). When the body composition Z-score models comparing CKD to controls were adjusted for differences in physical activity, the results were virtually identical to those presented above. In these physical activity adjusted models, greater amounts of physical activity were associated with slightly higher LM Z-scores and slightly lower FM Z-scores: Whole body, trunk, and leg LM Z-scores were 0.05 [0.001, 0.10] (P = 0.06), 0.07 [0.02, 0.12] (P = 0.008), and 0.04 [−0.004, 0.09] (P = 0.07) SD higher respectively per 1 h/d greater activity. Whole body, trunk, and leg FM Z-scores were lower by −0.05 [−0.11, 0.008] (P = 0.09), −0.05 [−0.11, 0.01] (P = 0.1), and −0.06 [−0.13, −0.002] (P = 0.04) SD respectively per 1 h/d greater activity.
Inflammatory Markers, Albumin, and Bicarbonate Levels
As shown in Table 2, compared with CKD 2 to 3 participants, CRP levels were significantly higher among CKD 4 to 5 (P = 0.003) and dialysis participants (P = 0.003), whereas IL-6 levels were significantly higher in dialysis (P < 0.001) participants only. Serum albumin levels were significantly lower in dialysis (P < 0.001) participants compared with CKD 2 to 3 participants. Bicarbonate levels were significantly lower in CKD 4 to 5 (P ≤ 0.0003) compared with CKD 2 to 3 participants.
Correlates of Leg Lean Mass Deficits among CKD Participants
Table 4 summarizes the multivariable analysis of leg LM deficits. Compared with CKD 2 to 3 participants, leg LM Z-scores were independently, significantly, and progressively lower in the CKD 4 to 5 and dialysis groups. Girls had greater leg LM deficits than boys (P = 0.003), and non-African Americans had greater leg LM deficits than African Americans (P = 0.04). Higher leg FM Z-score was associated with higher leg LM Z-score (P < 0.0001). Compared with participants with congenital anomalies of the kidney and urinary tract (CAKUT) as their primary kidney disease, the mean leg LM Z-scores were greater in participants with glomerulonephritis and focal segmental glomerulosclerosis (FSGS). Current recombinant human growth hormone (rhGH) therapy was associated with greater leg LM Z-scores. Children evaluated at CHOP had significantly lower leg LM Z-scores than those evaluated at CCHMC (P = 0.02). The interval since CKD diagnosis, time on dialysis, age, current glucocorticoid use, physical activity, and serum levels of CRP, IL-6, albumin, and bicarbonate did not have a clinically or statistically significant association with leg LM Z-score.
Table 4.
Determinants of leg lean mass Z-scores among CKD participants
| Difference in Leg LM Z-scores [95% CI] | P | |
|---|---|---|
| CKD category, versus CKD 2 to 3 | ||
| CKD 4 to 5 | −0.56 [−0.92, −0.21] | 0.002 |
| dialysis (versus CKD 2 to 3) | −1.31 [−1.73, −0.89] | <0.0001 |
| Leg length (per cm) | −0.06 [−0.09, −0.03] | <.0001 |
| Age (per year) | 0.04 [−0.03, 0.12] | 0.3 |
| Women (versus men) | −0.51 [−0.84, −0.18] | 0.003 |
| African American (versus non–African American) | 0.37 [0.02, 0.73] | 0.04 |
| Prepubertal (versus pubertal) | −0.51 [−1.03, 0.02] | 0.06 |
| Leg FM Z-score (per 1 SD increment) | 0.43 [0.31, 0.56] | <0.0001 |
| Renal diagnosis, versus CAKUT | ||
| glomerulonephritis | 0.75 [0.21, 1.29] | 0.007 |
| FSGS | 0.58 [0.13, 1.04] | 0.01 |
| other | −0.10 [−0.60, 0.39] | 0.7 |
| Current growth hormone use (versus no use) | 0.58 [0.03, 1.13] | 0.04 |
| Current glucocorticoid use (versus no use) | −0.42 [−1.06, 0.21] | 0.2 |
| CHOP (versus CCHMC) | −0.39 [−0.70, −0.07] | 0.02 |
We also considered the effect of interval since diagnosis of CKD, time on dialysis, physical activity (h/d), and serum levels of CRP, IL-6, albumin, and bicarbonate; however, none of these contributed significantly to the model.
We examined the same potential factors as determinants of leg FM. Only current rhGH use contributed significantly to leg FM Z-score: children treated with rhGH at the time of the study visit had a mean leg FM Z-score that was 0.78 [0.01, 1.56] SD lower than participants not treated with rhGH (P = 0.048).
DISCUSSION
This study demonstrated that greater CKD severity was associated with progressively greater leg LM deficits in children and adolescents, independent of poor growth, delayed maturation, or potentially abnormal body proportions.27,28 To our knowledge, this is the first study to assess whole body and regional LM and FM across the spectrum of CKD severity, and the first to include concurrent, robust individual-level reference data to facilitate adjustment of body composition measures for growth and maturation. An important limitation of some prior studies of body composition in children with CKD11,13 was a failure to account for the substantial differences in height and pubertal status between healthy children and those with CKD. Short children with delayed sexual maturation have lower absolute LM than their taller, more developed, age-matched peers; however, this does not necessarily represent muscle wasting.
Among studies that accounted for differences in height between children with CKD and healthy children, LM deficits were observed in some12,29, but not in others.14 Rashid et al. evaluated DXA whole body LM and FM in children with CKD and reported LM deficits.12 However, the study had two important limitations. First, LM and FM were expressed relative to height-age, rather than relative to height. Although this approach is appealing in its simplicity, its validity is unconfirmed. Expressing measures of body composition relative to height-age assumes that children of the same height-age will have the same pubertal status. This may not be true. Second, CKD patients were compared with published reference data30 collected over a decade earlier in a different country on a different generation scanner. Large and systematic differences have been reported in whole body DXA measurements obtained from different scanners.31 Furthermore, reliance on published reference data precluded multivariable analyses comparing CKD participants with controls, adjusting for age, body size, maturation, or study site. Our approach of expressing LM and FM relative to height within sex and race categories and adjusting for age, pubertal status, and study site permitted a more accurate comparison of CKD participants with controls.
As observed in this study, muscle wasting may not be evident when evaluating DXA whole body LM. Organs, which make up a substantial proportion of whole body LM, may be relatively preserved in CKD—even in the presence of skeletal muscle wasting. We detected deficits in whole body LM only among dialysis patients. Muscle wasting was masked on whole body scans in other CKD participants, likely because of the higher than expected trunk LM in CKD participants compared with controls. The reasons for the higher trunk LM in CKD participants is not clear, but may include preserved organ mass and preferential distribution of excess fluid to the trunk. DXA does not distinguish between normally hydrated and overhydrated lean tissue;32 volume overload will result in overestimation of LM by DXA. The higher trunk LM among CKD participants could not be explained by differences in body proportions; trunk LM was expressed relative to sitting height, controlling for any differences in body proportions.
Unlike some studies, which reported elevated adiposity in CKD,33 we observed significantly elevated whole body FM Z-scores only among CKD 4 to 5 participants. However, our findings regarding fat distribution were consistent with prior reports, indicating central distribution of adiposity in both children12 and adults20 with CKD.
Significant deficits in leg LM, proportional to the severity of CKD, were observed. Whereas CKD 2 to 3 participants showed no leg LM deficits, CKD 4 to 5 participants showed moderate deficits and dialysis participants showed substantial deficits. DXA-derived limb LM was previously demonstrated to accurately reflect skeletal muscle mass measured by whole body magnetic resonance imaging in children.19 In this study leg LM represented limb LM, and therefore the skeletal muscle compartment. In adults with CKD, limb LM deficits accounted for observed deficits in whole body LM, as measured by DXA.20 Deficits in calf muscle CSA were seen only in dialysis participants; the discrepancy between DXA and pQCT findings likely reflects lower sensitivity of the pQCT measure, which captures only a small slice of the leg LM, compared with the DXA measure, which captures the entire leg.
Recent literature suggests that muscle wasting in the context of CKD may be due to an inflammatory state.34 CRP levels were moderately elevated, on average, among CKD participants in this study, similar to levels observed in other studies of CKD.13,35,36 Interestingly, CRP levels were not significantly associated with leg LM Z-scores. This should not be interpreted as evidence that inflammation is not an important mediator of muscle wasting. Rather, this may reflect the limited ability of a cross-sectional study to detect these associations, or may indicate that the degree of inflammation present in the study was modest, such that other factors played a greater role in the muscle deficits observed here.
Concurrent rhGH therapy was associated with significantly higher leg LM Z-scores, compared with participants not currently treated with rhGH. Prior studies have also suggested a relationship between rhGH use and higher muscle mass.11,37,12 Few participants used rhGH at the time of the study visit in this study; these results need to be confirmed in larger, prospective studies. However, there is a sound biologic plausibility for this relation. In addition to its key role in growth, growth hormone is important to maintaining normal LM and FM.38 Randomized trials of rhGH in wasted adult dialysis patients showed benefits including weight gain,39 increased LM,40 and improved muscle performance.41
Significantly higher leg LM was observed in participants with glomerulonephritis and FSGS compared with participants with CAKUT. Fluid overload in participants with glomerulonephritis or FSGS, and/or volume depletion in participants with CAKUT, is a possible explanation for this finding.
Muscle deficits were larger among girls than boys, and among non–African-American than African-American participants, adjusted for the other covariates in Table 4. The reasons for this are not clear and need further investigation.
CKD participants from CHOP had greater muscle deficits than those from CCHMC. Although we cannot exclude the possibility that calibration of the DXA scanners at the two sites may have been imperfect, leading to systematic differences by site, no site differences in body composition were evident among controls, and the term for study site was not statistically significant in the model comparing CKD and controls. Therefore, it is likely that differences between CHOP and CCHMC CKD participants represent real differences, due to unmeasured differences in subject characteristics or treatment strategies.
The positive association between leg FM Z-score and leg LM Z-score was expected; numerous prior studies have demonstrated greater relative LM in association with greater adiposity.24,25 Consistent with prior studies demonstrating lower adiposity after rhGH therapy,11,12 we found that current rhGH use was significantly and independently associated with lower leg FM Z-scores.
This is the first study to consider the influence of physical activity on body composition in children with CKD. Average daily duration of physical activity had a very small impact on whole body and regional LM and FM; physical activity was not a significant determinant of leg LM Z-score. However, our measure of physical activity estimated duration but not intensity of activity. Longitudinal data and a measure of physical activity capturing quantity and intensity of activity may demonstrate greater associations with body composition.
Although a number of potential determinants of muscle wasting were identified, some caution is advised in interpreting the results presented in Table 4. The relatively small number of CKD participants in each of the sex, race, disease, and treatment categories, and the cross-sectional design, may have limited our ability to accurately identify all important determinants.
Possible overestimation of GFR in CKD participants with muscle deficits is another limitation. However, overestimation of GFR in CKD participants with muscle deficits would result in underestimation of the association between CKD stage and muscle deficits. In addition, dialysis participants would not be affected by this limitation.
The greatest limitations of this study were the cross-sectional design and the lack of nutritional intake data. Protein-energy malnutrition may be a mechanism for growth restriction and LM wasting among children with CKD.23 However, outside infancy, energy intake among children with CKD is generally appropriate for body size,15 and protein intake has been reported to be 1.5 to 2 times that recommended.15,23,42 Accumulating evidence suggests that LM wasting in CKD is unrelated to protein-calorie intake; rather, systemic inflammation, acidosis, growth hormone resistance, and disturbances in neuropeptide signaling have been proposed as important mediators of muscle wasting.34,43–48 Furthermore, other nutritional indicators such as FM Z-scores indicated that our CKD participants likely had adequate energy intake.
In conclusion, these data demonstrate significant muscle deficits in children and adolescents, which are proportional to CKD severity. In addition, these observational data suggest that rhGH may protect against muscle deficits. Future studies may demonstrate muscle deficits to be a better predictor of poor outcomes than low BMI. Longitudinal observational studies are needed to examine the associations between body composition, growth, maturation, dietary intake, medications, inflammation, physical activity, and morbidity and mortality.
CONCISE METHODS
Study Participants
Children and adolescents with CKD were recruited from the nephrology clinics at CHOP and CCHMC as part of a larger study of bone health and body composition in CKD. A total of 205 participants with estimated GFR (eGFR) <90 ml/min per 1.73 m2 49 were enrolled. Participants were excluded if they had significant cognitive or developmental disorders precluding cooperation with study procedures. This report is limited to the 143 participants with no prior history of renal transplantation, and no other diseases known to affect body composition such as neuromuscular disease, inflammatory bowel disease, sickle cell anemia, malignancy, or prior liver or cardiac transplantation. CKD participants were categorized into three groups according to CKD stage:21,22 CKD 2 to 3 (eGFR 30 to 89 ml/min per 1.73 m2; n = 64), CKD 4 to 5 (eGFR <30 ml/min per 1.73 m2; n = 45), and maintenance dialysis (n = 34). eGFR was calculated using the updated Schwartz formula.21
Healthy controls were recruited from general pediatric clinics in Philadelphia and Cincinnati and the surrounding communities and through advertisements. Children with a history of illnesses or medication use that may affect growth, nutritional status, or pubertal development were ineligible. Obesity was not an exclusion criterion.
The study was approved by the Institutional Review Boards of both sites. Written informed consent was obtained from study participants older than 18 years and assent along with parental consent from participants under 18 years of age.
Disease and Treatment Characteristics
Medical charts were reviewed to obtain information regarding participant, disease, and treatment characteristics. Primary renal disease was categorized as CAKUT, FSGS, glomerulonephritis, and other (cystinosis, renal ischemia, hemolytic uremic syndrome, tubulointerstitial nephritis, and Alport syndrome). Participants and parents were interviewed at the study visit to confirm current medications and review prior therapies.
Anthropometry and Tanner Staging
Height (0.1 cm), sitting height (0.1 cm), and weight (0.1 kg) were assessed by standard techniques.50 Leg length was calculated as height minus sitting height. Pubertal status was determined by self-assessment51 and classified according to the method of Tanner.52 Study participants and their parents were asked to categorize the participant's race according to the National Institute of Health categories.
Age- and sex-specific Z-scores (SD scores) for height and BMI were calculated using National Center for Health Statistics 2000 Center for Disease Control growth data.53 In addition, BMI-for-height-age Z-scores were also calculated, as recommended in the 2008 K/DOQI nutrition guidelines;23 height-age is the age at which the child's height is at the 50th percentile.
DXA Scans
All CKD and control participants underwent whole body DXA scans using a Delphi Discovery densitometer (Hologic, Inc., Bedford, MA) with a fan beam in the array mode, with standard positioning. All scans were analyzed at CHOP with software version 12.4. The LM and FM (kg) measures were divided into trunk, leg, and arm components using standard reference lines, excluding the head.54,26 LM was calculated as fat-free mass minus bone mineral content. It should be noted that fat-free mass measured by DXA assumes normal hydration of lean tissue; fluid overload will result in overestimation of fat-free mass and volume depletion in underestimation. DXA is a precise (coefficient of variation [CV] 1% to 4%)55 method used extensively to describe age-, sex-, race-, and pubertal maturation–related variability in body composition.56–59 DXA-derived appendicular lean soft tissue mass has been previously demonstrated to reflect skeletal muscle.19 The scanners at each site were calibrated daily using an hydroxyapatite spine phantom and weekly with a whole body phantom. The intraindividual CVs of DXA for whole body lean and fat mass are 1.0% and 2.1%, respectively.60 Precision data for regional body composition are available only for fat masses; the CV for trunk fat was reported at 2.1% and for leg fat at 3.1%.61
pQCT Scans
Muscle CSA in the left calf were obtained by pQCT using a Stratec XCT2000 device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s. All scans were analyzed at CHOP with Stratec software version 5.50. A scout view was obtained to place the reference line at the proximal border of the distal tibia growth plate, and measurements were obtained at 66% of tibia length proximal to the reference line. The manufacturer's hydroxyapatite phantom was scanned daily for quality assurance. In our laboratory, the CVs for short-term precision ranged from 0.5% to 1.6% for pQCT outcomes in children and adolescents. A single European Forearm Phantom was scanned on the pQCT devices at CHOP and CCHMC. Calibration scans were obtained twice over a 3-year interval during the conduct of this study. Measures were highly stable over time.
Physical Activity
All CKD participants and/or parents responded to a validated questionnaire62 that determined the number of minutes per week and weeks per month that the participant engaged in each of a comprehensive list of 55 physical activities during each month over the prior year. From these responses, the total number of hours of physical activity per year was calculated and expressed as the average number of hours of activity per day. The healthy controls at the CCHMC site and a subset of controls at CHOP completed the questionnaire, providing data in 401 controls.
Laboratory Studies
Nonfasting blood samples were collected during the study visit in all CKD participants. Serum creatinine (mg/dl) was measured by spectrophotometric enzymatic assay (Vitros; Johnson & Johnson Co., Rochester, NY) with a CV of 1% to 5%. eGFR (ml/min per 1.73 m2) was calculated using the recently updated pediatric estimating equations.21 Serum bicarbonate (mmol/L) was measured in 136 CKD participants by reflectance spectrophotometry with a CV of 3% to 8%. C reactive protein (CRP) and IL-6 were measured in 134 CKD participants. CRP (low sensitivity) was measured using a fixed-point immuno-rate method (Vitros) with a CV of 3% to 8%. IL-6 was measured by high-sensitivity solid-phase ELISA (R&D Systems, Inc., Minneapolis) with CV of 8%. Serum albumin was measured in 143 CKD participants by spectrophotometric enzymatic assay (Vitros) with a CV of 1% to 2%.
Statistical Analysis
Analyses were performed using Stata 10.0 (College Station, TX) and LMS Chartmaker Pro (Institute for Child Health, London, UK63). Two-sided tests were used throughout, and a P value <0.05 was considered significant. Group differences were assessed using the χ2 test for categorical variables, and t test or the Wilcoxon rank sum test for continuous variables, as appropriate for the distribution of the data.
Body composition outcomes were expressed as sex- and race- specific Z-scores, as described previously.64,65 In this study, Z-scores were used to normalize measures of body composition to height or body segment length; this was critical to distinguish LM deficits due to muscle wasting from apparent deficits due simply to growth failure or abnormal body proportions. DXA whole body LM and FM were expressed relative to height, trunk LM and FM relative to sitting height (i.e., trunk length), leg LM and FM relative to leg length, and pQCT calf muscle and fat CSA relative to tibia length. Data from the CHOP control sample were used to construct sex- and race-specific smoothed reference centile curves for each outcome using the LMS method,66 including whole body LM-for-height, whole body FM-for-height, trunk LM-for-sitting height, trunk FM-for-sitting height, leg LM-for-leg length, and leg FM-for-leg length. The LMS method accounts for the nonlinearity, heteroscedasticity, and skew of body composition data in growing children.66 Z-scores are interpreted as the difference, in SD units, between the observed value and the median value for healthy controls of the same sex, race, and height (sitting height or leg length). As such, Z-scores provide a sense of the relative position of an individual, or the relative distribution of a group, compared with the distribution observed in healthy individuals of the same sex, race, and height. Z-scores are interchangeable with percentiles. An example of one of the centile curves generated from healthy control data, with individual data points representing CKD participants, is provided in Figure 3. A limitation of Z-score–based comparisons is that the absolute difference in LM or FM between groups is not determined. However, an average absolute LM or FM difference may be difficult to interpret when the body sizes in the groups vary widely, as in this study.
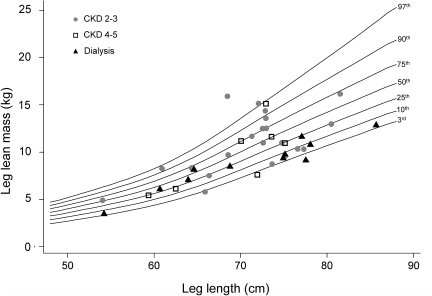
Figure 3.
An example of one of the body composition centile curves constructed to allow generation of z-scores in controls and CKD participants (DXA leg LM for leg length centile curves for non–African-American girls). The leg LM-for-leg length centile curves constructed using data from healthy non-African-American girls are shown, with 3rd, 10th, 25th, 50th, 75th, 90th, and 97th percentile lines. In addition, leg LM is plotted relative to leg length for each individual non-African-American female CKD participant. CKD 2 to 3 participants are represented with circles, CKD 4 to 5 participants with squares, and dialysis participants with triangles. Similar centile curves were created for each sex/race category for each of the considered body composition measures. It should be noted that this graph represents an unadjusted comparison between CKD participants and controls, and that the severity of deficits is likely to be underestimated from the appearance of the graph. On average, CKD participants will be older than controls with the same leg length.
The difference in Z-scores between healthy controls and each of the three CKD groups was determined by multivariable linear regression, adjusting for sex, age, pubertal status, study site (CHOP versus CCHMC), and height (sitting height or leg length). The LM models were also adjusted for FM Z-scores to account for the known positive association between FM-for-height and LM-for-height.24–26 The LMS method does not allow for simultaneous adjustment for age and height (sitting height or leg length). Therefore, the Z-scores that were generated relative to height (sitting height or leg length) were subsequently adjusted for age and height (sitting height or leg length) using linear regression analyses to capture the differences in the joint distributions of age and height (sitting height or leg length) in children with CKD compared with controls. Additional multivariable linear regression models limited to CKD participants were used to identify potential determinants of LM deficits; the following potential determinants were considered: CKD severity, medications, inflammatory markers, and demographic and disease characteristics.
DISCLOSURES
None.
Acknowledgments
This study was funded by grants from the NIH (R01-DK060030, R01-HD040714, K24-DK076808, F32-DK062637) and by the General Clinical Research Centers at CHOP and CCHMC. Dr. Foster is currently a member of the McGill University Health Centre Research Institute (supported in part by the Fonds de la recherche en santé du Québec [FRSQ]). During data analysis, Dr. Foster was support by the Fonds de la recherche en santé du Québec and by a KRESCENT New Investigator award, jointly funded by the Kidney Foundation of Canada, the Canadian Institutes of Health Research, and the Canadian Society of Nephrology. Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; October 27 through November 1, 2009; San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB: Survival advantages of obesity in dialysis patients. Am J Clin Nutr 81: 543–554, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Kalantar-Zadeh K, Kuwae N, Wu DY, Shantouf RS, Fouque D, Anker SD, Block G, Kopple JD: Associations of body fat and its changes over time with quality of life and prospective mortality in hemodialysis patients. Am J Clin Nutr 83: 202–210, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ: Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis 31: 997–1006, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Wong CS, Gipson DS, Gillen DL, Emerson S, Koepsell T, Sherrard DJ, Watkins SL, Stehman-Breen C: Anthropometric measures and risk of death in children with end-stage renal disease. Am J Kidney Dis 36: 811–819, 2000 [DOI] [PubMed] [Google Scholar]
- 5. O'Sullivan AJ, Lawson JA, Chan M, Kelly JJ: Body composition and energy metabolism in chronic renal insufficiency. Am J Kidney Dis 39: 369–375, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevinho-Becerra A, Wanner C: A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 73: 391–398, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Mehrotra R, Kopple JD: Nutritional management of maintenance dialysis patients: Why aren't we doing better? Annu Rev Nutr 21: 343–379, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Mitch WE: Mechanisms causing loss of lean body mass in kidney disease. Am J Clin Nutr 67: 359–366, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD: Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr 80: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients [comment]. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Johnson VL, Wang J, Kaskel FJ, Pierson RN: Changes in body composition of children with chronic renal failure on growth hormone. Pediatr Nephrol 14: 695–700, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Rashid R, Neill E, Smith W, King D, Beattie TJ, Murphy A, Ramage IJ, Maxwell H, Ahmed SF: Body composition and nutritional intake in children with chronic kidney disease. Pediatr Nephrol 21: 1730–1738, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Sylvestre LC, Fonseca KP, Stinghen AE, Pereira AM, Meneses RP, Pecoits-Filho R: The malnutrition and inflammation axis in pediatric patients with chronic kidney disease. Pediatr Nephrol 22: 864–873, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Baur LA, Knight JF, Crawford BA, Reed E, Roy LP, Allen BJ, Gaskin KJ: Total body nitrogen in children with chronic renal failure and short stature. Eur J Clin Nutr 48: 433–441, 1994 [PubMed] [Google Scholar]
- 15. Foreman JW, Abitbol CL, Trachtman H, Garin EH, Feld LG, Strife CF, Massie MD, Boyle RM, Chan JC: Nutritional intake in children with renal insufficiency: A report of the growth failure in children with renal diseases study. J Am Coll Nutr 15: 579–585, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Jones RW, Rigden SP, Barratt TM, Chantler C: The effects of chronic renal failure in infancy on growth, nutritional status and body composition. Pediatr Res 16: 784–791, 1982 [DOI] [PubMed] [Google Scholar]
- 17. Salusky IB, Fine RN, Nelson P, Blumenkrantz MJ, Kopple JD: Nutritional status of children undergoing continuous ambulatory peritoneal dialysis. Am J Clin Nutr 38: 599–611, 1983 [DOI] [PubMed] [Google Scholar]
- 18. Norman LJ, Coleman JE, Macdonald IA, Tomsett AM, Watson AR: Nutrition and growth in relation to severity of renal disease in children. Pediatr Nephrol 15: 259–265, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Shen W, Gallagher D, Jones A, Jr., Wang Z, Wang J, Heshka S, Heymsfield SB: Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr 84: 1014–1020, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woodrow G, Oldroyd B, Turney JH, Tompkins L, Brownjohn AM, Smith MA: Whole body and regional body composition in patients with chronic renal failure. Nephrol Dial Transplant 11: 1613–1618, 1996 [PubMed] [Google Scholar]
- 21. Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4: 1832–1843, 2009 [DOI] [PubMed] [Google Scholar]
- 22. National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis 39: S128–S142, 2002 [PubMed] [Google Scholar]
- 23. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53: S11–S104, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Forbes GB: Lean body mass-body fat interrelationships in humans. Nutr Rev 45: 225–231, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Forbes GB, Welle SL: Lean body mass in obesity. Int J Obes 7: 99–107, 1983 [PubMed] [Google Scholar]
- 26. Foster BJ, Shults J, Zemel BS, Leonard MB: Interactions between growth and body composition in children treated with high-dose chronic glucocorticoids. Am J Clin Nutr 80: 1334–1341, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Zivicnjak M, Franke D, Filler G, Haffner D, Froede K, Nissel R, Haase S, Offner G, Ehrich JH, Querfeld U: Growth impairment shows an age-dependent pattern in boys with chronic kidney disease. Pediatr Nephrol 22: 420–429, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lucke T, Franke D, Clewing JM, Boerkoel CF, Ehrich JH, Das AM, Zivicnjak M: Schimke versus non-Schimke chronic kidney disease: an anthropometric approach. Pediatrics 118: e400–e407, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Weber HP, Michalk D, Rauh W, Romahn A, Scharer K: Total body potassium in children with chronic renal failure. Int J Pediatr Nephrol 1: 42–47, 1980 [PubMed] [Google Scholar]
- 30. van der Sluis IM, de Ridder MA, Boot AM, Krenning EP, de Muinck Keizer-Schrama SM: Reference data for bone density and body composition measured with dual energy x ray absorptiometry in white children and young adults. Arch Intern Med 87: discussion 341–347, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, Mahboubi S, Fan B, Frederick MM, Winer K, Shepherd JA: The bone mineral density in childhood study: Bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab 92: 2087–2099, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Foster BJ, Leonard MB: Measuring nutritional status in children with chronic kidney disease. Am J Clin Nutr 80: 801–814, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Rashid R, Neill E, Maxwell H, Ahmed SF: Growth and body composition in children with chronic kidney disease. Br J Nutr 97: 232–238, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Mak RH, Cheung W: Cachexia in chronic kidney disease: Role of inflammation and neuropeptide signaling. Curr Opin Nephrol Hypertens 16: 27–31, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Coll B, Betriu A, Martinez-Alonso M, Borras M, Craver L, Amoedo ML, Marco MP, Sarro F, Junyent M, Valdivielso JM, Fernandez E: Cardiovascular risk factors underestimate atherosclerotic burden in chronic kidney disease: Usefulness of non-invasive tests in cardiovascular assessment. Nephrol Dial Transplant 25: 3017–3025, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Navarro-Gonzalez JF, Mora-Fernandez C, Muros M, Herrera H, Garcia J: Mineral metabolism and inflammation in chronic kidney disease patients: A cross-sectional study. Clin J Am Soc Nephrol 4: 1646–1654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Sluis IM, Boot AM, Nauta J, Hop WC, de Jong MC, Lilien MR, Groothoff JW, van Wijk AE, Pols HA, Hokken-Koelega AC, de Muinck Keizer-Schrama SM: Bone density and body composition in chronic renal failure: Effects of growth hormone treatment. Pediatr Nephrol 15: 221–228, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Iglesias P, Diez JJ: Recombinant human growth hormone therapy in adult dialysis patients. Int J Artif Organs 23: 802–804, 2000 [PubMed] [Google Scholar]
- 39. Iglesias P, Diez JJ, Fernandez-Reyes MJ, Aguilera A, Burgues S, Martinez-Ara J, Miguel JL, Gomez-Pan A, Selgas R: Recombinant human growth hormone therapy in malnourished dialysis patients: A randomized controlled study. Am J Kidney Dis 32: 454–463, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, Pedersen FB: Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clin Nephrol 53: 99–107, 2000 [PubMed] [Google Scholar]
- 41. Johannsson G, Bengtsson BA, Ahlmen J: Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: Anabolic effect and functional improvement. Am J Kidney Dis 33: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Wingen AM, Fabian-Bach C, Schaefer F, Mehls O: Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet 349: 1117–1123, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Mitch WE: Insights into the abnormalities of chronic renal disease attributed to malnutrition. J Am Soc Nephrol 13[Suppl 1]: S22–S27, 2002 [PubMed] [Google Scholar]
- 44. Mitch WE: Malnutrition is an unusual cause of decreased muscle mass in chronic kidney disease. J Ren Nutr 17: 66–69, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Cheung WW, Mak RH: Ghrelin and its analogues as therapeutic agents for anorexia and cachexia in end-stage renal disease. Kidney Int 76: 135–137, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Mak RH, Cheung W, Cone RD, Marks DL: Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int 69: 794–797, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Mak RH, Cheung WW, Roberts CT, Jr.: The growth hormone-insulin-like growth factor-I axis in chronic kidney disease. Growth Horm IGF Res 18: 17–25, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mitch WE: Proteolytic mechanisms, not malnutrition, cause loss of muscle mass in kidney failure. J Ren Nutr 16: 208–211, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Schwartz GJ, Feld LG, Langford DJ: A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr 104: 849–854, 1984 [DOI] [PubMed] [Google Scholar]
- 50. Lohman TG, Roche AF, Martorell R: Anthropometric Standardization Reference Manual, Champaign, IL, Human Kinetics Publishers, 1988 [Google Scholar]
- 51. Morris NM, Udry JR: Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9: 271–280, 1980 [DOI] [PubMed] [Google Scholar]
- 52. Tanner JM: Growth at Adolescence, Oxford, Blackwell Scientific Publication, 1962 [Google Scholar]
- 53. Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL: Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 109: 45–60, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Burnham JM, Shults J, Semeao E, Foster BJ, Zemel BS, Stallings VA, Leonard MB: Body-composition alterations consistent with cachexia in children and young adults with Crohn disease. Am J Clin Nutr 82: 413–420, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Ellis KJ, Shypailo RJ, Abrams SA, Wong WW: The reference child and adolescent models of body composition. A contemporary comparison. Ann N Y Acad Sci 904: 374–382, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Ellis KJ: Body composition of a young, multiethnic, male population. Am J Clin Nutr 66: 1323–1331, 1997 [DOI] [PubMed] [Google Scholar]
- 57. Goulding A, Taylor RW, Gold E, Lewis-Barned NJ: Regional body fat distribution in relation to pubertal stage: A dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr 64: 546–551, 1996 [DOI] [PubMed] [Google Scholar]
- 58. Ogle GD, Allen JR, Humphries IR, Lu PW, Briody JN, Morley K, Howman-Giles R, Cowell CT: Body-composition assessment by dual-energy x-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr 61: 746–753, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Lloyd T, Chinchilli VM, Eggli DF, Rollings N, Kulin HE: Body composition development of adolescent white females: The Penn State Young Women's Health Study. Arch Pediatr Adolesc Med 152: 998–1002, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Fuerst T, Genant HK: Evaluation of body composition and total bone mass with the Hologic QDR 4500. Osteoporosis Int 6: 203, 1996 [Google Scholar]
- 61. Cavalcanti RB, Cheung AM, Raboud J, Walmsley S: Reproducibility of DXA estimations of body fat in HIV lipodystrophy: implications for clinical research. J Clin Densitom 8: 293–297, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE: Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol 142: 191–201, 1995 [DOI] [PubMed] [Google Scholar]
- 63. Cole TJ, Green PJ: LMS Chartmaker Pro 2.3 ed., Child Growth Foundation, London, UK, 2006 [Google Scholar]
- 64. Thayu M, Denson LA, Shults J, Zemel BS, Burnham JM, Baldassano RN, Howard KM, Ryan A, Leonard MB: Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology 139: 430–438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thayu M, Shults J, Burnham JM, Zemel BS, Baldassano RN, Leonard MB: Gender differences in body composition deficits at diagnosis in children and adolescents with Crohn's disease. Inflamm Bowel Dis 13: 1121–1128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cole TJ: The LMS method for constructing normalized growth standards. Eur J Clin Nutr 44: 45–60, 1990 [PubMed] [Google Scholar]