Abstract
Rationale: Although inhalation of zinc oxide (ZnO) nanoparticles (NPs) is known to cause systemic disease (i.e., metal fume fever), little is known about mechanisms underlying injury to alveolar epithelium.
Objectives: Investigate ZnO NP–induced injury to alveolar epithelium by exposing primary cultured rat alveolar epithelial cell monolayers (RAECMs) to ZnO NPs.
Methods: RAECMs were exposed apically to ZnO NPs or, in some experiments, to culture fluid containing ZnCl2 or free Zn released from ZnO NPs. Transepithelial electrical resistance (RT) and equivalent short-circuit current (IEQ) were assessed as functions of concentration and time. Morphologic changes, lactate dehydrogenase release, cell membrane integrity, intracellular reactive oxygen species (ROS), and mitochondrial activity were measured.
Measurements and Main Results: Apical exposure to 176 μg/ml ZnO NPs decreased RT and IEQ of RAECMs by 100% over 24 hours, whereas exposure to 11 μg/ml ZnO NPs had little effect. Changes in RT and IEQ caused by 176 μg/ml ZnO NPs were irreversible. ZnO NP effects on RT yielded half-maximal concentrations of approximately 20 μg/ml. Apical exposure for 24 hours to 176 μg/ml ZnO NPs induced decreases in mitochondrial activity and increases in lactate dehydrogenase release, permeability to fluorescein sulfonic acid, increased intracellular ROS, and translocation of ZnO NPs from apical to basolateral fluid (most likely across injured cells and/or damaged paracellular pathways).
Conclusions: ZnO NPs cause severe injury to RAECMs in a dose- and time-dependent manner, mediated, at least in part, by free Zn released from ZnO NPs, mitochondrial dysfunction, and increased intracellular ROS.
Keywords: epithelial monolayers, zinc toxicity, reactive oxygen species, mitochondrial damage, plasma membrane integrity
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
It is well documented that inhaled zinc oxide (ZnO) nanoparticles (NPs) can be associated with systemic adverse effects (e.g., metal fume fever). However, the mechanisms underlying interactions between ZnO NPs and alveolar epithelium, and their adverse effects on alveolar epithelium, are not fully understood.
What This Study Adds to the Field
ZnO NP–induced injury to alveolar epithelium appears to be mediated at least in part by Zn2+ derived from ZnO NPs, increased intracellular reactive oxygen species, and mitochondrial dysfunction.
Nanoparticles (NPs) are used in many commercial products and new applications in biomedicine, yet their fate, potential toxicity, and mechanisms of translocation in biological cells, tissues, and organs (including the lung) have not been well defined. Some, but not all, inhaled NPs (including ambient ultrafine particulates that overlap in size with NPs) have been reported to be associated with adverse health effects (1, 2). There is evidence that measurable amounts of these inhaled NPs are found in end organs (e.g., liver, spleen, and heart) after inhalation (3–6), possibly leading to thrombosis, atherogenesis, and cardiac dysrhythmias (3, 7, 8). NPs found in end organs after inhalation are most likely to enter the systemic circulation across the epithelia of the lung, especially the alveolar epithelium, which constitutes a very thin biological barrier (∼0.5 μm) and affords greater than 95% of the surface area (∼100 m2 in humans) in distal airspaces of the lung.
Of the NPs and ambient ultrafine particulates studied to date, several metal (e.g., Fe, Zn, V, and Ni) and metal oxide (Fe2O3, ZnO, V2O5, and NiO) NPs have the potential to cause inflammation and injury in lungs (9–12). Among metal and metal oxide NPs in anthropogenic air pollution, Zn has a relatively high concentration (0.1–30.0 μg/m3) in inhaled ambient air (13–16). In the atmosphere, Zn exists primarily in oxidized form (ZnO) as aerosolized NPs of 0.01- to 0.10-μm diameter. Although the average concentration of Zn in ambient air in the United States generally is less than 1 μg/m3, several hundred to several thousand-fold higher Zn concentrations may occur in industrial sites for smelting, welding, galvanizing, and brass plating (17–20). Inhalation of very high concentrations of ZnO NPs (77–(600 mg Zn/m3) is commonly associated with acute metal fume fever, characterized by fever, chills, nausea, headache, fatigue, and muscle aches (18). Another Zn compound, ZnCl2, is frequently found in high concentrations at industrial sites for preserving wood and dyeing fabrics, and is reported to constitute an occupational health hazard (18). Inhalation of high levels of ZnCl2, which is corrosive, generally results in more pronounced damage to membranes of the respiratory tract, potentially leading to pulmonary edema (17).
Studies of ZnO NP toxicity show injury to mammalian cells (19–24). Lam and colleagues (19, 20) and Conner and colleagues (22) reported that guinea pigs exposed to ZnO NPs (50-nm diameter) at concentrations greater than 5.9 mg/m3 have pulmonary damage resulting in decreased total lung capacity and vital capacity. Brunner and colleagues (21) reported that exposure of a human mesothelioma and a rodent fibroblast cell line to 15 μg/ml ZnO NPs (19 nm) causes DNA and mitochondrial damage after 3 days. Karlsson and colleagues (23) showed that ZnO NPs (71 nm) decrease cell viability and cause oxidative DNA damage in a human alveolar epithelial cell (AEC) line (A549 cells) at concentrations greater than 40 μg/ml. Lin and colleagues (24) reported that exposure of A549 cells to ZnO NPs (70 and 420 nm) at concentrations greater than 10 μg/ml induces increases in oxidative stress, lipid peroxidation, cell membrane disruption, and DNA damage.
Studies in vivo have provided useful data on pulmonary inflammation and injury in response to NP exposure, although conclusions specific to the alveolar epithelial barrier are often imprecise due to the complex anatomy of the lung. As an alternative, simpler in vitro models of alveolar epithelium have been widely used to study important biological and functional characteristics. Distal airspaces of the lung are lined with a continuous epithelium comprised of alveolar epithelial type I (AT1) and type II (AT2) cells. It is generally accepted that primary cultured rat AT2 cells transdifferentiate into an AT1 cell–like phenotype (25, 26). Primary cultured rat AEC monolayers (RAECMs) derived from freshly isolated AT2 cells exhibit high transepithelial electrical resistance (RT > 2 kΩcm2) with well formed tight junctions and equivalent short-circuit current (IEQ; up to 6 μA/cm2), consistent with the expected properties of alveolar epithelium in vivo.
In this study, we investigated ZnO NP–induced injury to RAECMs assessed by measurements of RT and IEQ, lactate dehydrogenase (LDH) release, intracellular reactive oxygen species (ROS), and mitochondrial activity. We also investigated the effects of Zn2+ (released from ZnO NPs in suspension) on bioelectric properties. Results indicate that ZnO NPs induce severe cell plasma membrane damage, increase cellular ROS, and decrease mitochondrial activity. Injurious effects of ZnO NPs on RAECMs are dose and time dependent, generally irreversible, and, at least in part, mediated by free Zn2+ released from ZnO NPs.
METHODS
ZnO NPs
Most studies were performed with ZnO NPs (spherical-shaped, 20-nm diameter) obtained from Meliorum Technologies (Rochester, NY). To explore the effects of shape, ZnO NPs (rod-shaped, 100- to 200-nm length, with 20- to 70-nm diameter) generated in-house following a procedure described previously (27) were used in some experiments. ZnO NP suspensions (176 μg/ml in water) were sonicated with a probe sonicator (VirSonic 600 Ultrasonic Cell Disrupter; VirTis, Gardiner, NY) at 6 watts for 5 minutes, followed by vortexing for 30 seconds before loading into the Zetasizer (Malvern, Worcestershire, UK) for zeta potential measurement. The measurement cell (disposable cuvette with built-in electrodes) was filled with 1 ml of various ZnO NP suspensions. Zeta potential was estimated by applying an electric field across the two built-in electrodes.
Primary Cultured RAECMs
The detailed procedure for routine generation of RAECMs has been described elsewhere (28, 29). This method involving the usage of rats has been approved by the Institutional Animal Care and Use Committee of the University of Southern California. Briefly, fresh AT2 cells were isolated from adult male, specific pathogen–free, Sprague-Dawley rats (125–150 g) using elastase digestion, and enriched by IgG panning. Enriched AT2 cells were then plated on Day 0 onto tissue culture–treated polycarbonate filters (Transwell, 12-mm diameter, 0.4-μm diameter pores; Corning-Costar, Cambridge, MA) at 1.2 × 106 cells/cm2. Culture media consisted of minimal defined serum-free medium (MDSF) or MDS (MDSF supplemented with 10% newborn bovine serum; Omega, Tarzana, CA). MDSF is a 1:1 mixture of Dulbecco's minimal essential medium and Ham's F-12 (Sigma, St. Louis, MO), supplemented with 0.1 mM nonessential amino acids (Sigma), 0.2% primocin (InvivoGen, San Diego, CA), 10 mM N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) hemisodium salt (Sigma), 1.25 mg/ml bovine serum albumin (BSA; BD Bioscience, San Jose, CA) and 2 mM L-glutamine (Sigma). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 plus 95% air. Confluent monolayers were formed by Day 3. Monolayers were fed every other day starting on Day 3 with MDSF or MDS, as appropriate. Volumes of apical and basolateral fluids were 0.5 and 1.5 ml, respectively. For this study, RAECMs were used on Day 5 or 6.
Effects of ZnO NPs on Bioelectric Properties of RAECMs
RT and potential difference (PD [mV]) in the presence or absence of varying concentrations of ZnO NPs in apical fluid as functions of exposure time (up to 24 h) were measured using a Millicell-ERS device (Millipore, Bedford, MA). All RT and PD values were corrected for background measured across blank filters. We used RAECMs the RT and PD values of which on Day 5 or 6 were greater than 2.0 kΩcm2 and greater than 4.0 mV. Equivalent active ion transport rate (i.e., IEQ, μA/cm2) was estimated as PD/RT. ZnO NPs (up to 176 μg/ml) were added at time 0 to apical fluid and effects of ZnO NP concentration on bioelectric properties (i.e., RT and IEQ) assessed at times 0.5, 1, 2, 4, 6, and 24 hours. To investigate effects of ZnO NP washout on bioelectric properties, RAECMs exposed apically to ZnO NPs for 1, 3, or 7 hours were washed three times with fresh MDSF or MDS, as appropriate, and RT and IEQ were assessed for up to 24 hours after replacement of apical fluid.
Effects of Free Zn2+ on Bioelectric Properties of RAECMs
To determine if observed ZnO NP effects on alveolar epithelial barrier properties are in part related to free Zn2+ (that may have dissociated from ZnO NPs [30]), MDSF- or MDS-grown RAECMs were exposed to 18 and 295 μg/ml apical ZnCl2 (Acros Organics, Morris Plains, NJ). These concentrations of ZnCl2 are used to match the equivalent Zn2+ content in 11 and 176 μg/ml ZnO NPs, respectively. In addition, ZnO NPs (11 and 176 μg/ml) were incubated in fresh MDSF or MDS for 24 hours, followed by centrifugation (10,000 × g for 10 min) to obtain ZnO NP–free supernatants. MDSF- or MDS-grown RAECMs were exposed apically to the corresponding supernatant obtained from ZnO NP suspensions, and changes in RT and IEQ assessed for up to 24 hours. Zn2+ concentrations in supernatants and bathing fluids were measured using a colorimetric method (31) by monitoring absorbance at 490 nm (SpectraMax M2; Molecular Devices, Sunnyvale, CA) of the solution containing Zn2+ to which 4-(2-pyridylazo) resorcinol sodium salt was added, and pH adjusted to 9.5. Standard curves were generated using zinc standard solution (Fluka, Milwaukee, WI) for determination of unknown [Zn2+]. In addition, we used a cell membrane–impermeable Zn chelator, diethylenetriaminepentacetic acid (DTPA; Sigma), to investigate further if observed ZnO NP effects on alveolar epithelial barrier properties are in part related to free Zn2+ released from dissolved ZnO NPs in apical fluid. In general, DTPA binds Zn2+ with a 1:1 stoichiometry in apical fluid after exposure. Briefly, MDSF- or MDS-grown RAECMs were apically exposed to ZnO NPs (22 and 176 μg/ml) in the presence or absence of 100 μM DTPA, and bioelectric properties (i.e., RT and IEQ) were assessed for up to 24 hours.
Measurements of ZnO NP Fluxes
To study rates of ZnO NP trafficking across RAECMs grown in MDSF or MDS, apical fluid was replaced with fresh MDSF or MDS, respectively, containing ZnO NPs (apical [ZnO NP] = 176 μg/ml) at time 0. Apical-to-basolateral flux was estimated by monitoring ZnO NP accumulation over time in basolateral fluid. At 6 and 24 hours, 1 ml of basolateral fluid was taken for measurement of [ZnO NP] and replaced immediately with the same volume of appropriate fresh culture fluid. At 24 hours, 0.1 ml of apical fluid was sampled for determination of [ZnO NP]. Concentrations of ZnO NPs in apical and basolateral fluids were estimated using an inductively coupled plasma–mass spectrometer (Agilent 7500; Agilent Technologies, Santa Clara, CA). The ZnO NP flux, J, is given by J = (V/S)(C/Δt), where V is basolateral fluid volume, S is nominal monolayer surface area (1.13 cm2), Δt is measurement time period, and C is basolateral concentration of ZnO NPs at Δt.
Confocal Laser Scanning Microscopy
In some experiments, MDSF- or MDS-grown RAECMs exposed apically to 176 μg/ml ZnO NPs for 24 hours were processed for confocal laser scanning microscopy. ZnO NP–exposed RAECMs were washed with ice-cold phosphate-buffered saline (PBS, pH 7.2) and fixed in 3.7% formaldehyde (J.T. Baker, Phillipsburg, NJ) for 15 minutes at room temperature (25°C). Fixed RAECMs were permeabilized with 0.5% Triton X-100 (TX-100, Bio-Rad, Hercules, CA) for 15 minutes at room temperature. After rinsing 3 times with ice-cold PBS, these permeabilized RAECMs were blocked with PBS containing 5% BSA (Sigma) and 0.2% TX-100 for 1 hour at room temperature. Rabbit antibody against midregion human zonula occludens–1 (Zymed Laboratories, San Francisco, CA) diluted 1:100 in 1% BSA in PBS was incubated with these monolayers overnight at 4°C, followed by washing three times with PBS for 10 minutes each at room temperature. Monolayers were then incubated with secondary antibody (goat anti-rabbit antibody conjugated with Alexa 488; Invitrogen, Carlsbad, CA) in 1% BSA in PBS for 1 hour at room temperature followed by rinsing four times with ice-cold PBS, and mounted on microscope slides (Curtin Matheson Scientific, Baltimore, MD) with Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (Vector, Burlingame, CA). Monolayers not exposed to ZnO NPs, and those incubated with primary or secondary antibody alone, were similarly processed, serving as negative controls. Images were acquired with a Zeiss laser scanning microscope (510 Meta NLO Confocal Laser Scanning Microscope; Zeiss, Jena, Germany) equipped with argon, red/green HeNe and Chameleon lasers mounted on a vibration-free table and attached to an incubation chamber.
LDH Release
LDH is a cytoplasmic enzyme that exists in all cells. When the cell membrane is damaged, LDH is released into extracellular medium. We assessed LDH activity in extracellular medium of ZnO NP–exposed RAECMs grown in MDS or MDSF using an LDH Assay Kit (Roche Applied Science, Indianapolis, IN). Briefly, after 24-hour exposure to apically instilled ZnO NPs (up to 176 μg/ml), 100 μl of apical and basolateral fluids of RAECMs were reacted with 100 μl of the reaction mixture of the LDH Assay Kit for 30 minutes at room temperature. RAECMs exposed to 0.1% TX-100 for 15 minutes and those not exposed to ZnO NPs served as positive and negative controls, respectively. The absorbance of reacted LDH at 492 nm in the apical and basolateral fluids was assessed using a UV-Vis spectrophotometer (SpectraMax M2).
Flux of Paracellular Transport Marker Fluorescein Sulfonic Acid across RAECMs
Fluorescein-5 (and 6)-sulfonic acid, trisodium salt (FS; Molecular Probes, Eugene, OR) is hydrophilic and cell membrane impermeant. To investigate the effects of ZnO NPs on integrity of RAECMs grown with MDSF or MDS as a biological barrier, FS apical-to-basolateral flux (200 μg/ml FS in apical fluid) and apparent permeability coefficient (Papp) across RAECMs, after 24-hour apical exposure to ZnO NPs (up to 176 μg/ml), were measured. Basolateral samples (1 ml) were taken at 2.5 hours after FS instillation into apical fluid. At the end of 2.5-hour flux experiments, apical fluid (0.1 ml) was sampled for determination of [FS]. RAECMs exposed apically to 0.1% TX-100 for 15 minutes and those exposed to FS in the absence of ZnO NPs served as positive and negative controls, respectively. Concentrations of FS in apical and basolateral fluids were estimated fluorometrically at excitation/emission wavelengths of 495/519 nm. Unknown [FS] in the samples was assessed using standard curves generated using appropriate solutions with known concentrations of FS. Papp was estimated as J/Co, where J is flux of FS and Co the FS concentration in apical fluid at time 0.
Measurements of Intracellular ROS
Changes in intracellular ROS produced by apical exposure of RAECMs to ZnO NPs were measured using a fluorometric assay (32, 33). Nonfluorescent 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) is lipophilic and freely taken up by cells. DCFH-DA is then cleaved by intracellular esterases to yield DCFH, which is trapped within the cell due to charge. DCFH is oxidized to fluorescent dichlorofluorescein (DCF) by cellular oxidants (e.g., •OH, H2O2, and ONOO•−) (33). The fluorescence intensity of DCF is proportional to the concentration of intracellular ROS. Briefly, RAECMs in MDSF or MDS were exposed apically to 176 μg/ml ZnO NPs for 1–24 hours, followed by washing twice with PBS at room temperature. Washed monolayers were then incubated with 100 μM DCFH-DA (Sigma) for 30 minutes at 37°C in a humidified atmosphere of 5% CO2 plus 95% air. DCFH-DA–exposed RAECMs were washed with PBS to remove DCFH-DA that did not enter the cells. Washed RAECMs were lysed with 0.5% TX-100 for 30 minutes at room temperature. Fluorescence of oxidized DCF in cell lysates was measured using a spectrofluorometer with excitation/emission wavelengths of 485/530 nm, and used as an index of intracellular ROS.
Antioxidant Effects on Bioelectric Properties of RAECMs Exposed to ZnO NPs
To determine if ZnO NP effects on alveolar epithelial barrier properties are related to intracellular ROS generated by apical exposure to ZnO NPs, RAECMs were apically treated with an antioxidant, N-acethyl-L-cysteine (NAC; Sigma), before apical exposure to ZnO NPs. NAC acts as a precursor in the formation of glutathione in cells, and helps protect cells from intracellular ROS. Briefly, MDSF- or MDS-grown RAECMs were apically pretreated with 10 mM NAC for 2 hours. RAECMs were then apically exposed to 22 μg/ml ZnO NPs and bioelectric properties (i.e., RT and IEQ) were assessed for up to 24 hours.
Mitochondrial Activity Assay
Mitochondrial activity of RAECMs was assessed using the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI), which uses 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine ethosulfate. Briefly, MTS is taken up by live cells and converted into water-soluble formazan. This reaction is governed by mitochondrial dehydrogenase activity. After apical exposure of RAECMs grown in MDSF or MDS to 176 μg/ml ZnO NPs for 24 hours, RAECMs were washed three times with the respective fresh culture medium. A mixture of MTS and phenazine ethosulfate was then added to apical fluid of washed RAECMs. After 1-hour incubation at 37°C in a humidified atmosphere of 5% CO2 plus 95% air, the absorbance at 490 nm of water-soluble formazan formed in apical fluid was assessed using a UV-Vis spectrophotometer. Formazan absorbance was used as an index of mitochondrial activity. RAECMs exposed to 0.1% TX-100 for 15 minutes at 37°C served as positive control, whereas those not exposed to ZnO NPs served as negative control.
Data Analysis
Data are presented as mean (±SD). Comparisons of multiple (≥3) group means were performed using one-way analyses of variance and post hoc procedures based on Newman-Keuls tests. Student's t tests were used for comparisons of two group means. A P value less than 0.05 is considered statistically significant.
RESULTS
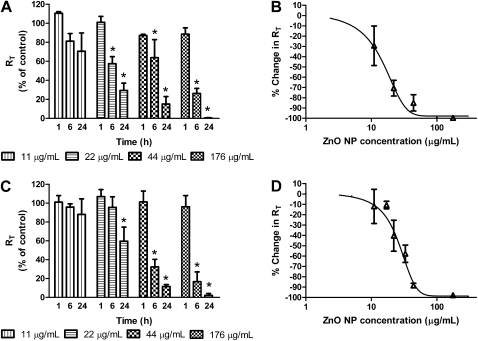
We investigated the effects of apically exposing MDSF-grown RAECMs to ZnO NPs (spherical shaped, zeta potential of −5.10 [±1.68] mV [n = 5]). Changes in RT observed after 1, 6, and 24 hours of MDSF-grown RAECMs exposed to 11, 22, 44, and 176 μg/ml are shown in Figure 1A. Average RT and IEQ of these RAECMs before ZnO NPs exposure were 2.33 (±0.37) kΩcm2 and 2.28 (±0.54) μA/cm2, respectively (n = 27). ZnO NP exposure led to decreased RT in a dose- and time-dependent manner, with higher doses and longer exposure times exhibiting greater decreases in RT. At the highest ZnO NP concentration (176 μg/ml) studied, RT decreased by approximately 100% after 24-hour exposure. However, at a lower concentration (11 μg/ml), RT did not decrease even after 24-hour exposure. Similarly, apical exposure to 176 μg/ml ZnO NPs decreased IEQ of monolayers by approximately 100% at 24-hour exposure, whereas, at 11 μg/ml, no effect on IEQ was observed for up to 24 hours (data not shown). Figure 1B shows the dose–response curve for RT versus ZnO NP concentration of MDSF-grown RAECMs determined after 24-hour exposure (n = 3–9 for each concentration). Dose-dependent effects of ZnO NPs on RT and IEQ, respectively, assessed after 24-hour exposure yielded half-maximal concentrations (IC50) of approximately 17 μg/ml and approximately 32 μg/ml (data not shown).
Figure 1.
Effects of apical exposure to spherical zinc oxide (ZnO) nanoparticles (NPs) on RT of minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (C)-grown rat alveolar epithelial cell monolayers (RAECMs) after 1, 6, and 24 hours. RAECMs were exposed apically to ZnO NPs at 11, 22, 44, and 176 μg/ml. RT of MDSF- and MDS-grown RAECMs before ZnO NP exposure was 2.33 (±0.37) kΩcm2 (n = 27) and 2.63 (±0.47) kΩcm2 (n = 33), respectively. Dose–response curves after 24-hour exposure are shown for RT of MDSF (B)- or MDS (D)-grown RAECMs versus ZnO NPs. *Significantly different (P < 0.05) from control (RAECMs not exposed to ZnO NPs).
Figure 1C shows changes in RT observed after 1, 6, and 24 hours of MDS-grown RAECMs exposed apically to 11, 22, 44, and 176 μg/ml. Average RT and IEQ of these RAECMs before ZnO NP exposure were 2.63 (±0.47) kΩcm2 and 5.24 (±0.65) μA/cm2, respectively (n = 33). Similar to the changes in RT of MDSF-grown RAECMs, 176 μg/ml ZnO NPs decreased RT by approximately 100% after 24-hour exposure, whereas 11 μg/ml ZnO NPs had little effect on RT. At 176 μg/ml ZnO NPs, IEQ decreased by approximately 100% after 24-hour exposure (data not shown). No changes in IEQ were observed for up to 24 hours of exposure to 11 μg/ml apical [ZnO NP] (data not shown). Figure 1D shows ZnO NP effects on RT and IEQ, respectively, of MDS-grown RAECMs after 24-hour exposure, yielding an IC50 of approximately 28 μg/ml and approximately 52 μg/ml (data not shown). These results indicate that some factor(s) in serum ameliorate(s) the ZnO NP–induced decrements in bioelectric properties of RAECMs.
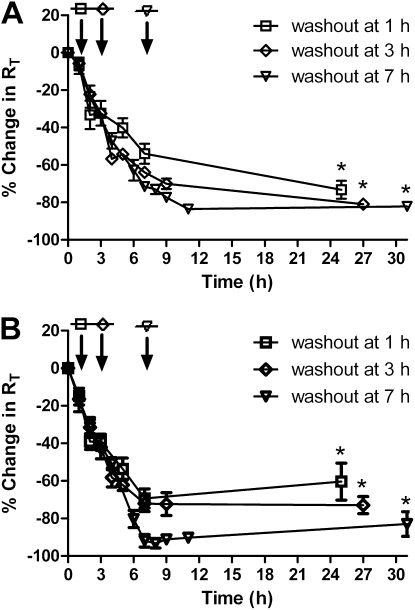
Figure 2A shows changes in RT of MDSF-grown RAECMs exposed to 176 μg/ml apical [ZnO NP] for 1, 3, or 7 hours, followed by replacement of apical fluid with fresh MDSF, and monitoring RT for 24 hours thereafter. Average RT and IEQ of these RAECMs before ZnO NP exposure were 2.12 (±0.21) kΩcm2 and 3.29 (±0.29) μA/cm2, respectively (n = 9). None of the washed monolayers exhibited recovery in RT. After washout at 1, 3, and 7 hours, on the other hand, IEQ continued to decrease, and reached 30–50% of control at 24 hours after washout (data not shown). Figure 2B shows changes in RT of MDS-grown RAECMs after washout of apical ZnO NPs. Average RT and IEQ of these RAECMs before ZnO NP exposure were 2.46 (±0.73) kΩcm2 and 5.87 (±0.32) μA/cm2, respectively (n = 18). Similar to the changes in RT of MDSF-grown RAECMs, RT of monolayers washed at 1, 3, and 7 hours did not recover toward control. IEQ recovered to approximately 70% of control at 24 hours after washout at 1 and 3 hours, whereas no appreciable recovery in IEQ was seen with monolayers washed after 7 hours (data not shown).
Figure 2.
Effects of washout of zinc oxide (ZnO) nanoparticles (NPs) (176 μg/ml) at 1, 3, and 7 hours after NPs instillation by replacing apical fluid with fresh minimal defined serum-free medium (MDSF) (A) or MDSF supplemented with 10% newborn bovine serum (MDS) (B). RT of MDSF- and MDS-grown rat alveolar epithelial cell monolayers (RAECMs) before ZnO NP exposure was 2.12 (±0.21) kΩcm2 (n = 9) and 2.46 (±0.71) kΩcm2 (n = 18), respectively. Arrow indicates the time when apical fluid was replaced with fresh culture medium. *Significantly different (P < 0.05) from control (RAECMs not exposed to ZnO NPs).
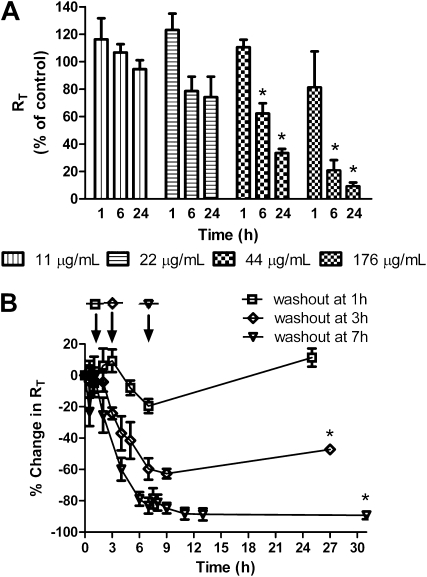
Effects of ZnO NPs shape on bioelectric properties of MDS-grown RAECMs are shown in Figure 3A, in which decreases in RT over 24 hours in response to apical rod-shaped ZnO NP exposure (11–176 μg/ml) are shown. Zeta potential of ZnO NPs was −0.77 (±2.49) mV (n = 3). Average RT and IEQ before ZnO NP exposure of these RAECMs were 3.31 (±0.77) kΩcm2 and 5.50 (±0.77) μA/cm2, respectively (n = 26). As can be seen, rod-shaped ZnO NPs decreased RT in a dose- and time-dependent manner. At 176 μg/ml ZnO NPs, RT decreased by approximately 91% after 24-hour exposure. Similarly, IEQ decreased by approximately 100% after 24 hours (data not shown). IC50 of approximately 31 μg/ml and approximately 45 μg/ml for RT and IEQ, respectively, were obtained (data not shown). Figure 3B shows RT after washout of ZnO NPs by replacement of apical fluid with fresh MDS at 1, 3, or 7 hours after the onset of ZnO NP exposure. Average RT and IEQ of MDS-grown RAECMs before ZnO NP exposure were 2.43 (±0.41) kΩcm2 and 5.14 (±0.50) μA/cm2, respectively (n = 12). After apical ZnO NPs were washed out at 3 hours, RT recovered to approximately 44% of control after 24 hours, whereas washing monolayers at 7 hours led to only approximately 2% recovery toward control after 24 hours. IEQ of monolayers that were washed at 3 hours recovered to approximately 80% of control after 24 hours, whereas no appreciable recovery in IEQ was seen for RAECMs washed at 7 hours (data not shown). These results indicate generally similar effects of rod- and spherical-shaped ZnO NPs.
Figure 3.
Effects of apical exposure to rod-shaped zinc oxide (ZnO) nanoparticles (NPs) on RT of minimal defined serum-free medium supplemented with 10% newborn bovine serum (MDS)–grown rat alveolar epithelial cell monolayers (RAECMs) after 1, 6, and 24 hours (A). RT before ZnO NP exposure was 3.31 (±0.77) kΩcm2 (n = 26). RAECMs were exposed apically to rod-shaped ZnO NPs at 11, 22, 44, and 176 μg/ml. Effects of washout of rod-shaped ZnO NPs (176 μg/ml) at 1, 3, and 7 hours are shown in B. RT before ZnO NP exposure was 2.43 (±0.41) kΩcm2 (n = 12). Arrow indicates the time when apical fluid was replaced with fresh culture medium. *Significantly different (P < 0.05) from control (RAECMs not exposed to ZnO NPs).
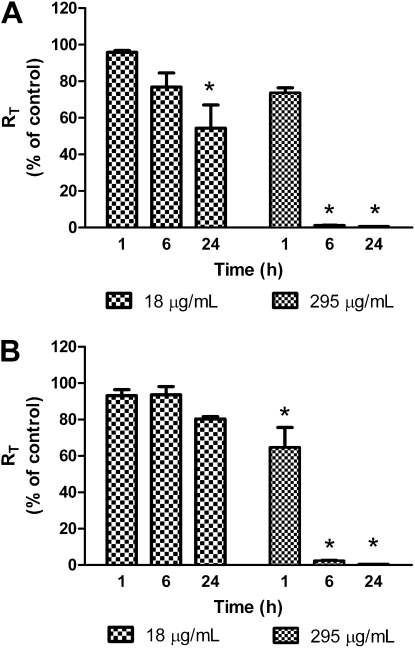
Changes in RT of MDSF-grown RAECMs exposed apically to ZnCl2 (18 and 295 μg/ml) for 24 hours are shown in Figure 4A. Average RT and IEQ of these RAECMs before ZnCl2 exposure were 2.13 (±0.16) kΩcm2 and 1.91 (±0.18) μA/cm2, respectively (n = 6). ZnCl2 (295 μg/ml, corresponding to 141 μg/ml Zn2+) decreased RT by approximately 100% after 6-hour exposure, and 18 μg/ml ZnCl2 (corresponding to 8.8 μg/ml Zn2+) decreased RT by approximately 40% after 24 hours. IEQ of monolayers exposed apically to 295 μg/ml ZnCl2 decreased by approximately 100% after 6 hours, whereas no changes in IEQ at 18 μg/ml ZnCl2 were observed for up to 24 hours (data not shown).
Figure 4.
Effects of apical exposure to zinc chloride (ZnCl2) on RT of minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs) after 1, 6, and 24 hours ( n = 3). RAECMs were exposed apically to ZnCl2 at 18 and 295 μg/ml. RT of MDSF- and MDS-grown RAECMs before ZnCl2 exposure was 2.13 (±0.16) kΩcm2 (n = 6) and 2.95 (±0.37) kΩcm2 (n = 6), respectively. *Significantly different (P < 0.05) from control (RAECMs not exposed to ZnCl2).
Figure 4B shows changes in RT of MDS-grown RAECMs exposed apically to ZnCl2. Average RT and IEQ of these RAECMs before ZnCl2 exposure were 2.95 (±0.37) kΩcm2 and 5.26 (±0.65) μA/cm2, respectively (n = 6). Similar to the changes in RT of MDSF-grown RAECMs, 295 μg/ml ZnCl2 decreased RT of MDS-grown RAECMs by approximately 100% after 6 hours, whereas 18 μg/ml ZnCl2 did not decrease RT after 24 hours. ZnCl2 (295 μg/ml) decreased IEQ by approximately 100% after 6-hour exposure, whereas 18 μg/ml ZnCl2 did not appreciably affect IEQ for up to 24 hours (data not shown).
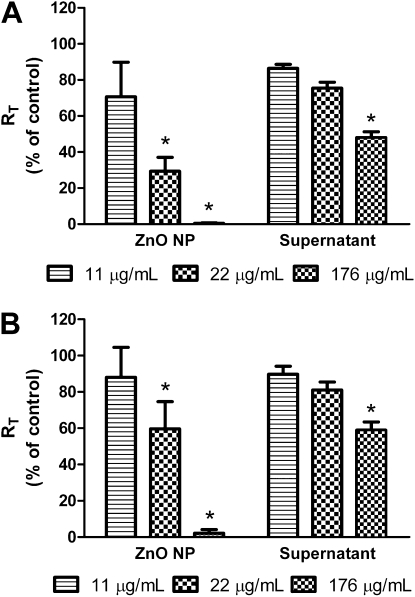
Figure 5A shows the effects of apical exposure to ZnO NP–free supernatants (obtained from fresh MDSF incubated with 11, 22, or 176 μg/ml ZnO NPs for 24 h) on RT of MDSF-grown RAECMs compared with those seen with apical exposure to corresponding concentrations of ZnO NPs. Average RT and IEQ of these RAECMs before supernatant exposure were 2.88 (±0.29) kΩcm2 and 2.22 (±0.36) μA/cm2, respectively (n = 9). Apical exposure to ZnO NP–free supernatants taken from 176 μg/ml ZnO NP suspension led to decreases in RT by approximately 50% after 24 hours, compared with approximately 100% decrease in RT with 176 μg/ml ZnO NP exposure. Supernatants taken from 22 μg/ml ZnO NP suspension decreased RT by approximately 25% after 24 hours, whereas 22 μg/ml ZnO NPs decreased RT by approximately 70% after 24 hours. IEQ of monolayers exposed apically to ZnO NP–free supernatants (taken from 176 μg/ml ZnO NP suspension) decreased by approximately 20% after 24 hours, whereas supernatants (taken from 22 or 11 μg/ml ZnO NP suspension) did not decrease IEQ for up to 24 hours (data not shown).
Figure 5.
Effects of apical exposure to supernatants separated from zinc oxide (ZnO) nanoparticles (NPs) after incubation for 24 hours in fresh minimal defined serum-free medium (MDSF) or MDSF supplemented with 10% newborn bovine serum (MDS) on RT of MDSF (A)- or MDS (B)-grown rat alveolar epithelial cell monolayers (RAECMs) after 24-hour exposure (n = 3). The supernatants were obtained by centrifugation of ZnO NPs (11, 22 and 176 μg/ml) suspensions in MDSF or MDS. RT of MDSF- and MDS-grown RAECMs before supernatant exposure was 2.88 (±0.29) kΩcm2 (n = 9) and 2.16 (±0.16) kΩcm2 (n = 9), respectively. *Significantly different (P < 0.05) from control (RAECMs not exposed to ZnO NPs or supernatants).
Figure 5B shows the effects of ZnO NP–free supernatants on RT of MDS-grown RAECMs. ZnO NP–free supernatants used in MDS-grown RAECMs were taken from fresh MDS incubated with ZnO NPs for 24 hours. Average RT and IEQ of these RAECMs before supernatant exposure were 2.16 (±0.16) kΩcm2 and 5.93 (±0.26) μA/cm2, respectively (n = 9). Apical exposure to supernatants (taken from 176 μg/ml ZnO NP suspension) led to decreases in RT of approximately 40% after 24 hours, whereas 176 μg/ml ZnO NPs decreased RT by approximately 100% after 24 hours. Exposure to supernatants (taken from 22 or 11 μg/ml ZnO NP suspension) did not decrease RT after 24 hours. Changes in IEQ of MDS-grown RAECMs exposed apically to various supernatants for 24 hours were similar (data not shown) to the corresponding changes in IEQ of MDSF-grown RAECMs described previously here.
The concentrations of free Zn2+ released from spherical ZnO NP suspensions were measured, and are summarized in Table 1. ZnO NP–free supernatants taken from ZnO NPs resuspended in MDS contained relatively more Zn2+ than those taken from ZnO NPs resuspended in MDSF. At a similar Zn2+ concentration (e.g., ∼9 μg/ml) in ZnO NP–free supernatant, similar changes in RT of MDSF- or MDS-grown RAECMs after 24-hour exposure were seen (Figures 4 and 5), suggesting that the effects of ZnO NP–free supernatants on RT of RAECMs are likely due to Zn2+ released from ZnO NP suspensions.
TABLE 1.
ZN2+ CONCENTRATIONS IN SUPERNATANTS FROM ZINC OXIDE NANOPARTICLE SUSPENSIONS
| Zn2+(μg/ml) |
||
|---|---|---|
| Supernatant Source | MDSF | MDS |
| 11 μg/ml ZnO NP suspension | 4.5 ± 0.2 | 7.9 ± 0.2 |
| 22 μg/ml ZnO NP suspension | 5.3 ± 0.2 | 9.2 ± 0.1 |
| 176 μg/ml ZnO NP suspension | 6.8 ± 0.2 | 10.8 ± 0.3 |
Definition of abbreviations: MDS = minimal defined serum-free medium supplemented with 10% newborn bovine serum; MDSF = minimal defined serum-free medium; NP = nanoparticle; ZnO = zinc oxide.
Zn2+ concentrations were measured from ZnO NP–free supernatants obtained by centrifugation after incubating ZnO NP in fresh MDSF or MDS for 24 hours (n = 3).
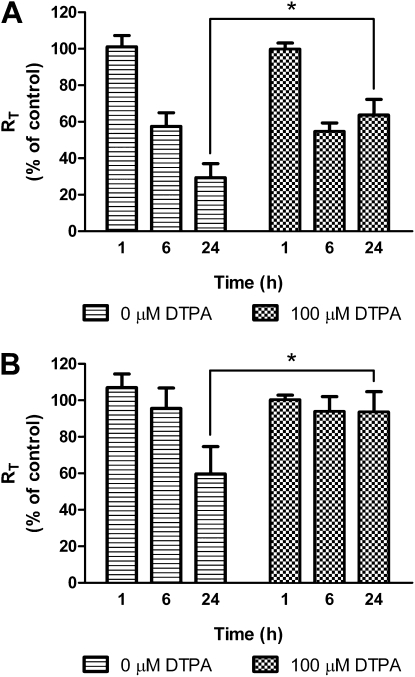
Figure 6A shows the effects of the zinc chelator, DTPA, on RT of MDSF-grown RAECMs exposed apically to 22 μg/ml ZnO NPs for 24 hours. Average RT and IEQ of these RAECMs before ZnO exposure were 2.23 (±0.73) kΩcm2 and 2.69 (±0.17) μA/cm2, respectively (n = 6). When DTPA (100 μM) and ZnO NPs (22 μg/ml) were added simultaneously to apical fluid, decreases in RT of RAECMs were significantly less than those of RAECMs not exposed to DTPA for 24 hours, whereas 100 μM DTPA did not inhibit the reduction of RT of monolayers exposed to 176 μg/ml ZnO NPs after 24 hours (data not shown). Exposure to 22 μg/ml ZnO NPs in the presence or absence of 100 μM DTPA did not decrease IEQ for up to 24 hours (data not shown). Decreases in RT of MDS-grown RAECMs exposed apically to 22 μg/ml ZnO NPs in the presence or absence of 100 μM DTPA for 24 hours are shown in Figure 6B. Average RT and IEQ of these RAECMs before ZnO NP exposure were 2.72 (±0.14) kΩcm2 and 5.41 (±0.38) μA/cm2, respectively (n = 9). Similar to the changes in RT of MDSF-grown RAECMs, 100 μM DTPA inhibited the reduction of RT of monolayers exposed to 22 μg/ml ZnO NPs for 24 hours, but not that of monolayers exposed to 176 μg/ml ZnO NPs. IEQ of monolayers exposed to 22 μg/ml ZnO NPs in the presence or absence of 100 μM DTPA did not decrease for up to 24 hours (data not shown).
Figure 6.
Effects of apical exposure to 22 μg/ml zinc oxide (ZnO) nanoparticles (NPs) on RT of minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs) in the presence or absence of 100 μM diethylenetriaminepentacetic acid (DTPA) after 24 hours (n = 3). RT of MDSF- and MDS-grown RAECMs before 100 μM DTPA exposure was 2.23 (±0.73) kΩcm2 (n = 6) and 2.72 (±0.14) kΩcm2 (n = 9), respectively. *Significantly different (P < 0.05) from control (RAECMs not exposed to DTPA).
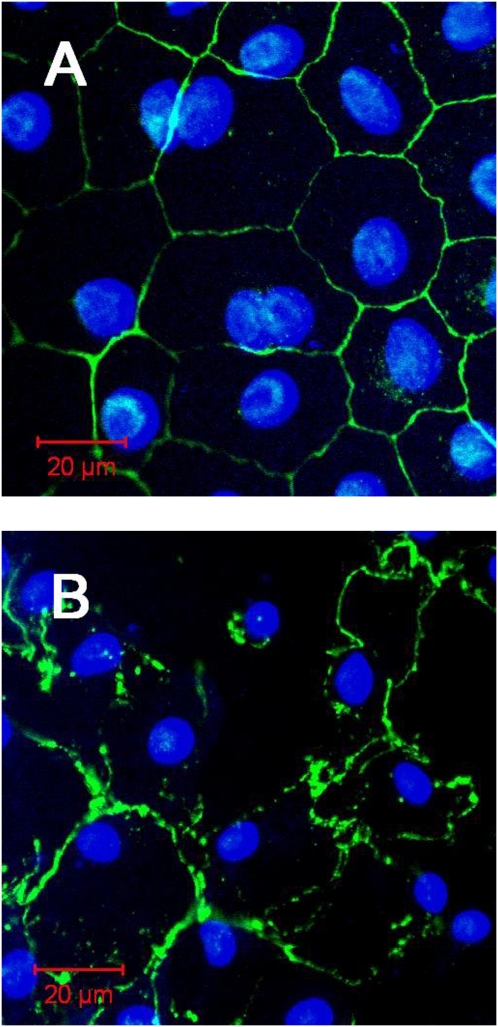
Confocal photomicrographs of MDSF- or MDS-grown RAECMs exposed apically to 176 μg/ml ZnO NPs for 24 hours are shown in Figure 7. Compared with control (i.e., MDSF-grown RAECMs not exposed to ZnO NPs), disruption of continuity of tight junctions and plasma membranes were observed. A similar result was observed in MDS-grown RAECMs exposed apically to 176 μg/ml ZnO NPs (data not shown). Flux of ZnO NPs across MDSF- and MDS-grown RAECMs over 24 hours at apical fluid [ZnO NP] of 176 μg/ml was estimated to be 72.4 (±7.2) and 81.7 (±20.5) pg/cm2/s, respectively (not significantly different).
Figure 7.
Confocal photomicrographs of minimal defined serum-free medium (MDSF)–grown rat alveolar epithelial cell monolayers not exposed to zinc oxide (ZnO) nanoparticles (NPs) (A) versus exposed apically to 176 μg/ml ZnO NPs for 24 hours (B). Cell–cell borders (zonula occludens [ZO]–1 staining) are seen as green and nuclei (4′,6-diamidino-2-phenylindole [DAPI] staining) are seen as blue.
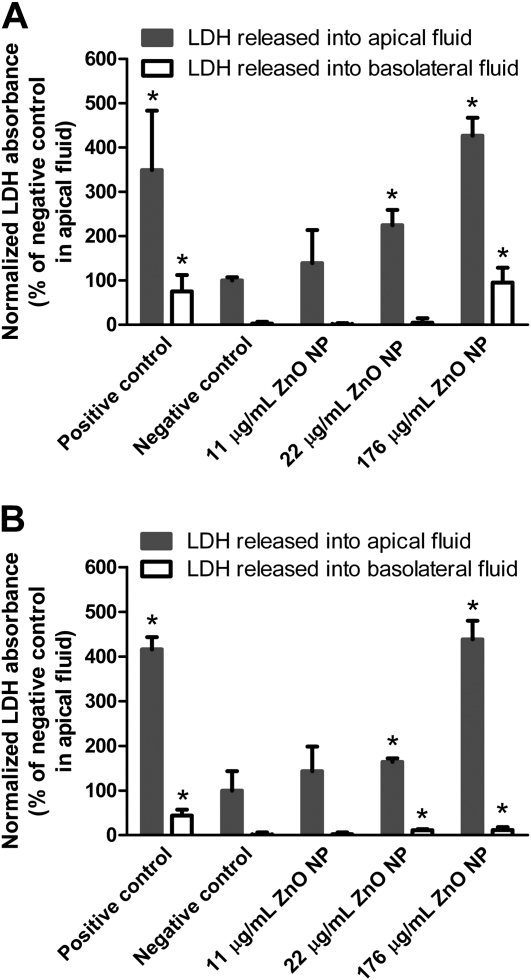
Figure 8A shows the profile of LDH release from MDSF-grown RAECMs after exposure to apical ZnO NPs (up to 176 μg/ml) for 24 hours. Exposure of these monolayers to 176 μg/ml ZnO NPs for 24 hours led to increases in the levels of LDH in apical and basolateral fluids, compared with those observed in negative control (i.e., RAECMs not exposed to ZnO NPs). Similarly, increases in release of LDH from MDS-grown RAECMs exposed apically to 176 μg/ml ZnO NPs were observed (Figure 8B). No increases in release of LDH from either MDSF- or MDS-grown RAECMs were found after apical exposure to 11 μg/ml ZnO NPs for 24 hours.
Figure 8.
Effects of zinc oxide (ZnO) nanoparticles (NPs) on lactate dehydrogenase (LDH) release from minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers. Monolayers were exposed apically to ZnO NPs (up to 176 μg/ml) for 24 hours before assessing LDH release. Positive control was monolayers exposed to 0.1% TX-100 for 15 minutes. Negative control was monolayers not exposed to ZnO NPs. *Significantly different (P < 0.05) from negative control.
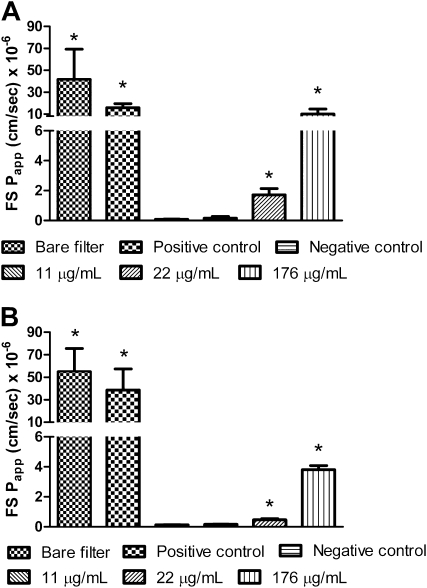
Barrier integrity of MDSF-grown RAECMs after exposure to apical ZnO NPs (up to 176 μg/ml) for 24 hours was further assessed using the fluorescent hydrophilic probe, FS (Figure 9A). Papp of FS in MDSF-grown RAECMs exposed apically to 22 and 176 μg/ml ZnO NPs increased compared with that observed for negative control (i.e., RAECMs not exposed to ZnO NPs). No differences in Papp of FS were seen for MDSF-grown RAECMs exposed to 11 μg/ml ZnO NPs versus negative control. Similarly, Papp of FS in MDS-grown RAECMs increased with increasing apical ZnO NP concentration (Figure 9B). These data indicate that loss of barrier integrity of RAECMs is induced by apical exposure for 24 hours to 22 and 176, but not 11, μg/ml ZnO NPs. In addition, MDS-grown RAECMs are slightly more resistant to disruption of barrier integrity by ZnO NP exposure than MDSF-grown RAECMs, consistent with changes in bioelectric properties (especially RT) described previously here.
Figure 9.
Effects of zinc oxide (ZnO) nanoparticles (NPs) on apparent permeability of fluorescein sulfonic acid (FS) for minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs) after 24 hours. Positive control was monolayers exposed to 0.1% TX-100 for 15 minutes. Negative control was monolayers not exposed to ZnO NPs. *Significantly different (P < 0.05) from negative control.
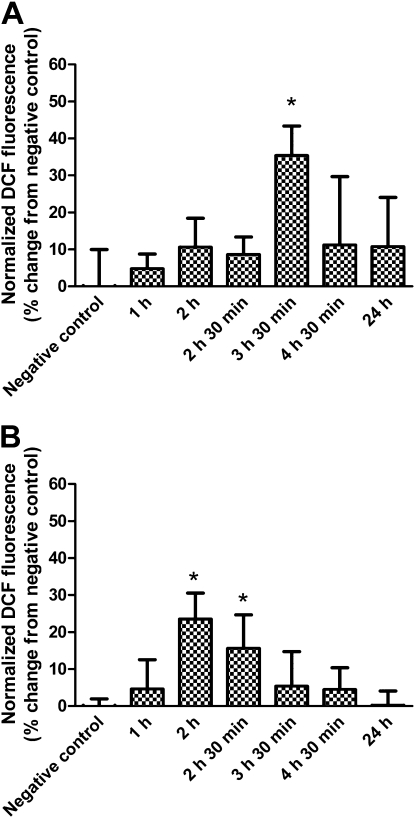
Changes in intracellular ROS levels induced by apical exposure of MDSF-grown RAECMs to 176 μg/ml ZnO NPs are shown in Figure 10A. Intracellular ROS in MDSF-grown RAECMs peaked at 3.5-hour exposure to ZnO NPs. Similarly, apical exposure of MDS-grown RAECMs to 176 μg/ml ZnO NPs revealed increased intracellular ROS generation at 2.0–2.5 hours of exposure (Figure 10B). These increases in ROS were no longer seen after 4-hour exposure of either MDSF- or MDS-grown RAECMs.
Figure 10.
Changes in intracellular reactive oxygen species levels in minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs) exposed apically to 176 μg/ml zinc oxide (ZnO) nanoparticles (NPs). Monolayers were assayed at 1, 2, 2.5, 3.5, 4.5, and 24 hours after ZnO NP exposure. *Significantly different (P < 0.05) from negative control.
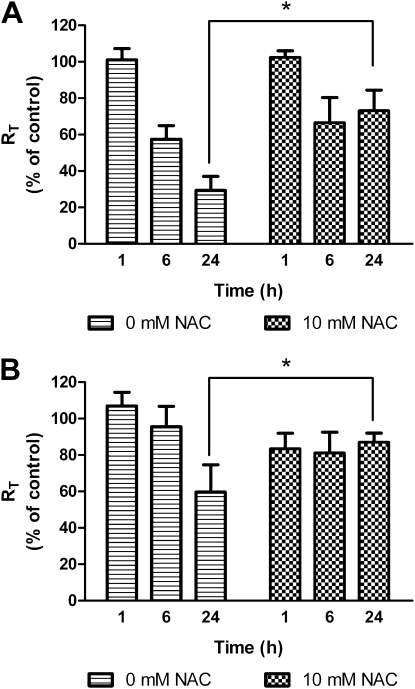
Figure 11A shows the effects of pretreatment with 10 mM NAC on RT of MDSF-grown RAECMs exposed apically to 22 μg/ml ZnO NPs for 24 hours. Average RT and IEQ of these RAECMs before treatment with NAC were 2.47 (±0.03) kΩcm2 and 2.88 (±0.23) μA/cm2, respectively (n = 3). When RAECMs were apically pretreated with NAC (10 mM) for 2 hours, decreases in RT of RAECMs after apical exposure to ZnO NPs (22 μg/ml) were significantly less than those of RAECMs not treated with NAC for 24 hours, whereas no effect on IEQ was observed for up to 24 hours in the case of pretreatment or without pretreatment (data not shown). Decreases in RT of MDS-grown RAECMs pretreated with 10 mM NAC after apical exposure to 22 μg/ml ZnO NPs for 24 hours are shown in Figure 11B. Average RT and IEQ of these RAECMs before treatment with NAC were 2.67 (±0.21) kΩcm2 and 4.80 (±0.33) μA/cm2, respectively (n = 3). Similarly, pretreatment with 10 mM NAC led to significant inhibition of RT reduction after apical exposure to 22 μg/ml ZnO NPs for 24 hours. IEQ of monolayers exposed to 22 μg/ml ZnO NPs with and without pretreatment with 10 mM NAC did not decrease for up to 24 hours (data not shown).
Figure 11.
Effects of apical exposure to 22 μg/ml zinc oxide (ZnO) nanoparticles (NPs) on RT of minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs) pretreated with 10 mM N-acethyl-L-cysteine (NAC) after 24 hours (n = 3). RT of MDSF- and MDS-grown RAECMs before 10 mM NAC pretreatment was 2.47 (±0.03) kΩcm2 (n = 3) and 2.67 (±0.21) kΩcm2 (n = 3), respectively. *Significantly different (P < 0.05) from control (RAECMs not pretreated with NAC).
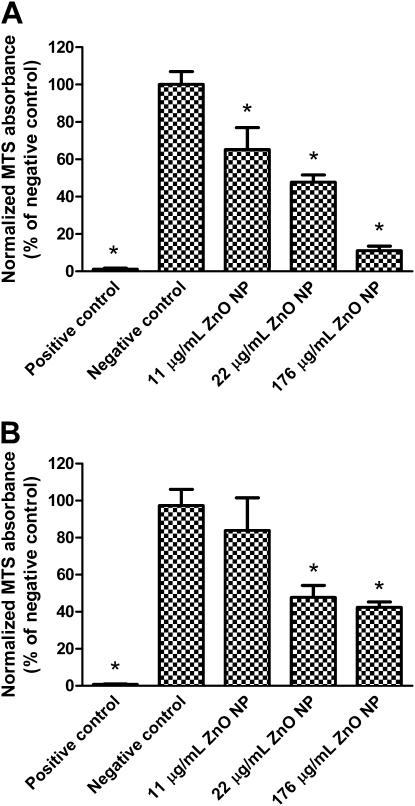
Figure 12A shows the changes in mitochondrial activity of MDSF-grown RAECMs after apical exposure to ZnO NPs (up to 176 μg/ml) for 24 hours. Exposure to 11 and 176 μg/ml ZnO NPs decreased mitochondrial activity of these monolayers by approximately 35% and approximately 90%, respectively, whereas exposure of MDS-grown RAECMs to 11 and 176 μg/ml ZnO NPs decreased mitochondrial activity by approximately 16% and approximately 58%, respectively. Changes in mitochondrial activity of MDS-grown RAECMs after exposure to ZnO NPs (up to 176 μg/ml) for 24 hours are shown in Figure 12B. Similar to the results obtained from measurements of bioelectric properties (e.g., RT and IEQ) described previously here, mictochondrial activity of MDS-grown RAECMs decreased somewhat less than that of MDSF-grown RAECMs after exposure to ZnO NPs.
Figure 12.
Effects of zinc oxide (ZnO) nanoparticles (NPs) on changes in mitochondrial activity of minimal defined serum-free medium (MDSF) (A)- or MDSF supplemented with 10% newborn bovine serum (MDS) (B)-grown rat alveolar epithelial cell monolayers (RAECMs). Monolayers were exposed apically to ZnO NPs (up to 176 μg/ml) for 24 hours before 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Positive control was monolayers exposed to 0.1% Triton X-100 for 15 minutes. Negative control was monolayers not exposed to ZnO NPs. *Significantly different (P < 0.05) from negative control.
DISCUSSION
In this study, we report that apical exposure of RAECMs to ZnO NPs causes severe injury to the alveolar epithelial barrier, involving loss of cell plasma membrane integrity, mitochondrial dysfunction, increased intracellular ROS, and tight junction disruption. Growth of RAECMs in MDSF or MDS, and exposure of RAECMs to spherical- or rod-shaped ZnO NPs, each results in generally similar effects on RAECMs. Apical exposure to free Zn2+ released from ZnO NPs or to ZnCl2 similarly alters monolayer barrier properties (i.e., RT and IEQ) in a dose- and time-dependent manner, suggesting that ZnO NP–induced injury to AEC is mediated, at least in part, by Zn2+ released from ZnO NPs.
Based on published human exposure data, we decided to use concentrations of ZnO NP exposure from 11 to 176 μg/ml. Hammond (34) reported that workers exposed to ZnO NP concentrations of approximately 725 mg/m3 (i.e., ∼580 mg Zn/m3) for 3 hours have experienced metal fume fever. ZnO deposited in the lung in this particular case (assuming a respiratory minute volume of 20 L/min, ∼70 m2 surface area of airspaces of the lung, and 30% of inhaled ZnO deposition in the lung [35, 36]) would be 1.1 μg/cm2. In other words, if the workers are exposed to ZnO NPs in the same environment for 24 hours, the ZnO deposited in their lungs could be 8.8 μg/cm2. In our in vitro rodent model, 11, 22, 44, and 176 μg/ml ZnO NPs were used to expose RAECMs apically for 24 hours. These concentrations are equivalent to 4.9, 9.7, 19.4, and 77.8 μg ZnO NPs/cm2, respectively, and are relevant to ZnO concentrations that can cause inflammation in human lungs.
ZnO NP–induced injury to RAECMs appears to be generally irreversible, because, when apical fluid was replaced with fresh culture medium after 3- or 7-hour exposure to 176 μg/ml ZnO NPs, RT continued to decrease by approximately 60–80% compared with control 24 hours after washout. Similarly, Gojova and colleagues (27) reported that viability of human aortic endothelial cells (HAECs) decreased at 24 hours from washout after exposure for 4 hours to 50 μg/ml ZnO NPs. These findings suggest that early cellular damage due to ZnO NP exposure results in a sequence of events that can lead to cell death. Cellular damage appeared to be slightly reduced in RAECMs grown with 10% serum (i.e., MDS-grown RAECMs) compared with that with serum-free media. Specific factor(s) in serum that provide protection against ZnO NP–induced injury are unknown. However, MDS-grown RAECMs appear to exhibit higher RT and IEQ than MDSF-grown RAECMs, which may have been a contributing factor for lesser decrements in bioelectric properties after ZnO NP exposure. In line with this observation, Okeson and colleagues (37) reported that, when serum is present in culture medium, significantly smaller toxicities of zinc on rat AECs are apparent, perhaps because zinc binds strongly/readily to serum component(s) before zinc can affect the cells to manifest cytotoxic effects.
Solubility of ZnO in water is very low at pH 8, but much higher at pH 6 (38, 39). ZnO dissolved in water dissociates into Zn2+ and Zn(OH)n+, depending on pH. At pH less than 8, Zn2+ is the dominant ionic species, whereas Zn(OH)n+ is dominant at pH greater than 8 (38). Excess Zn2+ in mammalian cells is toxic, although a trace amount of Zn2+ serves as an effective antioxidant and a critical structural and functional component of zinc-binding proteins (40, 41). Impaired mitochondrial function due to increased intracellular Zn2+ causes elevated intracellular ROS, leading to apoptotic or necrotic cell death (42–44). Recently, Nel and colleagues (30) reported that cytotoxicity of ZnO NPs may be directly related to dissolution of ZnO NPs suspended in bathing medium of RAW 264.7 and BEAS-2B cells, and Lin and colleagues (24) showed that viability of A549 cells is reduced in a dose- and time-dependent manner after apical exposure to ZnO NPs. Gojova and colleagues (27) previously reported that a pronounced inflammatory response is found in HAECs exposed for 4 hours to 50 μg/ml ZnO NPs, leading to 50% cell death. Our present findings indicate that RAECMs may be more resistant to ZnO NPs (i.e., greater IC50) compared with HAECs, suggesting that ZnO NP–induced injury is, in part, cell type–dependent.
In our study, apical exposure to ZnCl2 induced severe injury to RAECMs (Figure 4). Evans (45) first reported cases of deaths caused by ZnCl2 fume inhalations. Milliken and colleagues (46) reported that a worker who accidentally inhaled ZnCl2 fumes (from a smoke bomb) developed advanced pulmonary fibrosis that ended in death. In a related situation, where soluble Zn in air pollution particulates may lead to injury, Adamson and colleagues (13) reported that pulmonary inflammation after atmospheric dust exposure might be activated by the high level of soluble Zn in atmospheric dust. With respect to a soluble Zn effect, we demonstrated that free Zn2+ (from 22 μg/ml ZnO NPs) apical exposure induced injury to RAECMs that was significantly attenuated by the Zn chelator, DTPA. Inhaled NPs may traffic across the lung air–blood barrier into the systemic circulation via endocytotic or nonendocytotic mechanisms of normal uninjured cells (47). However, because ZnO NP exposure leads to disruption of both paracellular pathways and cell plasma membranes of RAECMs, translocation of ZnO NPs across RAECMs from apical to basolateral fluid likely occurs via damaged cell membranes and/or tight junctions.
Cell plasma membrane damage can generally be correlated with LDH release into extracellular fluid (24, 48, 49). As described previously here, disruption of cell–cell borders and cell plasma membranes caused by ZnO NP exposure leads to increased LDH (molecular weight, ∼140 kD) release into extracellular fluid. Apparent permeability of FS (molecular weight, 478 Da) across RAECMs increased with increasing apical ZnO NP concentration, indicating severe cell plasma membrane damage at high ZnO NP concentrations. With respect to NP-induced effects, cell plasma membrane damage or cell injury may be related to elevated levels of intracellular ROS. The level of intracellular ROS in RAECMs is increased by apical exposure to ZnO NPs in a time-dependent manner (Figure 10). We demonstrated that pretreatment of RAECMs with the antioxidant, NAC, attenuated injury related to intracellular ROS, suggesting that ZnO NP–induced injury to RAECMs is associated with intracellular ROS. Disruption of mitochondrial respiration can augment intracellular ROS levels (50, 51). We demonstrated that apical exposure to ZnO NPs for 24 hours reduced mitochondrial activity (Figure 12). Mitochondrial dysfunction is partly associated with depletion of sulfhydryl groups in metallothionein-1 proteins caused by excess Zn2+ arising from dissolved ZnO NPs (43, 44). Our data suggest that excess free Zn2+ (from dissolved ZnO NPs) disrupts zinc–protein interactions in mitochondria, leading to disruption of mitochondrial redox state and an increase in intracellular ROS levels, resulting in cell plasma membrane damage and cell injury.
In contrast to inhalation of ZnCl2, which can cause severe acute and chronic effects on lung (e.g., advanced pulmonary fibrosis and fatal respiratory distress syndrome), inhalation of high concentrations of ZnO NPs generally cause short-term effects (e.g., metal fume fever), which resolve without treatment within 48 hours (17). Brown (52), however, reported that a worker who accidentally inhaled ultrafine ZnO particles developed a predominantly interstitial edema with inflammatory infiltrates and tissue damage in the lung periphery. Large numbers of in vitro studies show that exposure to ZnO NPs induces severe injury to various cells (e.g., AECs, epidermal, microphage, endothelial, and stem cells). To better correlate the results observed in ZnO NP exposure studies using in vitro cell culture studies with those of in vivo pulmonary diseases caused by ZnO NP exposure, additional long-term human studies and well validated in vitro studies are needed.
In summary, we have shown that apical exposure to ZnO NPs induces severe injury to RAECMs in a dose- and time-dependent manner. ZnO NP–induced injury appears to reflect an increased level of intracellular ROS and resultant loss of cell membrane integrity (seen as elevated LDH release), likely mediated by Zn2+ released from ZnO NPs suspended in apical bathing medium of RAECMs. Although additional studies are required to further delineate specific mechanisms underlying cytotoxicity due to ZnO NP inhalation, it is clear that Zn2+ derived from ZnO NPs, mitochondrial dysfunction, and increased intracellular ROS play important roles in ZnO NP–induced injury to RAECMs.
Acknowledgments
The authors appreciate Dr. Shane Que Hee (University of California, Los Angeles) for help with inductively coupled plasma–mass spectrometer analysis, and the expert technical assistance of Mr. Juan Ramon Alvarez in the generation of primary cultured cell monolayers. E.D.C. is Hastings Professor and Kenneth T. Norris Jr. Chair of Medicine, and Z.B. is Ralph Edgington Chair in Medicine.
Supported in part by the Hastings Foundation, Whittier Foundation, and research grants EY011386, EY017923, ES017034, ES018782, HL038578, HL038621, HL062569, HL064365, and HL089445 from the National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.201002-0185OC on July 16, 2010
Author Disclosure: Y.H.K. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.F. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.M.K. has received industry-sponsored grants from Synthia LLC ($1,001–$5,000), and sponsored grants from National Institute of Environmental Health Sciences (more than $100,000). N.R.Y. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.F.H.-A. has received industry-sponsored grants from Alcon (more than $100,000), has a patent pending from University of Southern California related to tear biomarkers for Sjogren's Syndrome, and has received a sponsored grant from National Institutes of Health (NIH) (more than $100,000). Z.B. has received sponsored grants from NIH (more than $100,000); K.-J.K. received $1,000 from Springer for book editing activities. E.D.C. has received sponsored grants from NIH (more than $100,000).
References
- 1.Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr, Speizer FE. An association between air pollution and mortality in six US Cities. N Engl J Med 1993;329:1753–1759. [DOI] [PubMed] [Google Scholar]
- 2.Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health 2009;30:137–150. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004;109:2655–2671. [DOI] [PubMed] [Google Scholar]
- 4.Kreyling WG, Semmler-Behnke M, Seitz J, Scymczak W, Wenk A, Mayer P, Takenaka S, Oberdorster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol 2009;21:55–60. [DOI] [PubMed] [Google Scholar]
- 5.Oberdorster G, Oberdorster E, Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 2005;113:823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semmler-Behnke M, Kreyling WG, Lipka J, Fertsch S, Wenk A, Takenaka S, Schmid G, Brandau W. Biodistribution of 1.4- and 18-nm gold particles in rats. Small 2008;4:2108–2111. [DOI] [PubMed] [Google Scholar]
- 7.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, Radomski MW. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol 2005;146:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol 2002;39:935–942. [DOI] [PubMed] [Google Scholar]
- 9.Fond AM, Meyer GJ. Biotoxicity of metal oxide nanoparticles. In: Kumar CSSR, editor. Nanomaterials—toxicity, health and environmental issues. Weinheim: WILEY-VCH Verlag GmbH & Co. KGaA; 2006. pp. 3–34.
- 10.Li N, Xia T, Nel AE. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 2008;44:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemery B. Metal toxicity and the respiratory tract. Eur Respir J 1990;3:202–219. [PubMed] [Google Scholar]
- 12.Schaumann F, Borm PJ, Herbrich A, Knoch J, Pitz M, Schins RP, Luettig B, Hohlfeld JM, Heinrich J, Krug N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am J Respir Crit Care Med 2004;170:898–903. [DOI] [PubMed] [Google Scholar]
- 13.Adamson IY, Prieditis H, Hedgecock C, Vincent R. Zinc is the toxic factor in the lung response to an atmospheric particulate sample. Toxicol Appl Pharmacol 2000;166:111–119. [DOI] [PubMed] [Google Scholar]
- 14.Beckett WS, Chalupa DF, Pauly-Brown A, Speers DM, Stewart JC, Frampton MW, Utell MJ, Huang LS, Cox C, Zareba W, et al. Comparing inhaled ultrafine versus fine zinc oxide particles in healthy adults: a human inhalation study. Am J Respir Crit Care Med 2005;171:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison RM, Yin J. Particulate matter in the atmosphere: which particle properties are important for its effects on health? Sci Total Environ 2000;249:85–101. [DOI] [PubMed] [Google Scholar]
- 16.Kodavanti UP, Schladweiler MC, Ledbetter AD, Hauser R, Christiani DC, Samet JM, McGee J, Richards JH, Costa DL. Pulmonary and systemic effects of zinc-containing emission particles in three rat strains: multiple exposure scenarios. Toxicol Sci 2002;70:73–85. [DOI] [PubMed] [Google Scholar]
- 17.Barceloux DG. Zinc. J Toxicol Clin Toxicol 1999;37:279–292. [DOI] [PubMed] [Google Scholar]
- 18.Gerberding JL. Toxicological profile for zinc. Atlanta: U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; 2005. pp. 11–118. [PubMed]
- 19.Lam HF, Chen LC, Ainsworth D, Peoples S, Amdur MO. Pulmonary function of guinea pigs exposed to freshly generated ultrafine zinc oxide with and without spike concentrations. Am Ind Hyg Assoc J 1988;49:333–341. [DOI] [PubMed] [Google Scholar]
- 20.Lam HF, Conner MW, Rogers AE, Fitzgerald S, Amdur MO. Functional and morphologic changes in the lungs of guinea pigs exposed to freshly generated ultrafine zinc oxide. Toxicol Appl Pharmacol 1985;78:29–38. [DOI] [PubMed] [Google Scholar]
- 21.Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 2006;40:4374–4381. [DOI] [PubMed] [Google Scholar]
- 22.Conner MW, Flood WH, Rogers AE, Amdur MO. Lung injury in guinea pigs caused by multiple exposures to ultrafine zinc oxide: changes in pulmonary lavage fluid. J Toxicol Environ Health 1988;25:57–69. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson HL, Cronholm P, Gustafsson J, Moller L. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 2008;21:1726–1732. [DOI] [PubMed] [Google Scholar]
- 24.Lin W, Xu Y, Huang C-C, Ma Y, Shannon KB, Chen D-R, Huang Y-W. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J Nanopart Res 2009;11:25–39. [Google Scholar]
- 25.Cheek JM, Evans MJ, Crandall ED. Type I cell–like morphology in tight alveolar epithelial monolayers. Exp Cell Res 1989;184:375–387. [DOI] [PubMed] [Google Scholar]
- 26.Elbert KJ, Schafer UF, Schafers HJ, Kim KJ, Lee VH, Lehr CM. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm Res 1999;16:601–608. [DOI] [PubMed] [Google Scholar]
- 27.Gojova A, Guo B, Kota RS, Rutledge JC, Kennedy IM, Barakat AI. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ Health Perspect 2007;115:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borok Z, Danto SI, Zabski SM, Crandall ED. Defined medium for primary culture de novo of adult rat alveolar epithelial cells. In Vitro Cell Dev Biol Anim 1994;30A:99–104. [DOI] [PubMed] [Google Scholar]
- 29.Borok Z, Hami A, Danto SI, Zabski SM, Crandall ED. Rat serum inhibits progression of alveolar epithelial cells toward the type I cell phenotype in vitro. Am J Respir Cell Mol Biol 1995;12:50–55. [DOI] [PubMed] [Google Scholar]
- 30.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater 2009;8:543–557. [DOI] [PubMed] [Google Scholar]
- 31.Lampugnani L, Maccheroni M, Rotunno T, Zamboni R. A simple colorimetric method for the zinc assay in blood. Anal Lett 1990;23:1665–1683. [Google Scholar]
- 32.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 1992;5:227–231. [DOI] [PubMed] [Google Scholar]
- 33.Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 2003;65:1575–1582. [DOI] [PubMed] [Google Scholar]
- 34.Hammond JW. Metal fume fever in the crushed stone industry. J Ind Hyg Toxicol 1944;26:117–119. [Google Scholar]
- 35.Bide RW, Armour SJ, Yee E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J Appl Toxicol 2000;20:273–290. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health 1985;15:197–214. [DOI] [PubMed] [Google Scholar]
- 37.Okeson CD, Riley MR, Riley-Saxton E. In vitro alveolar cytotoxicity of soluble components of airborne particulate matter: Effects of serum on toxicity of transition metals. Toxicol In Vitro 2004;18:673–680. [DOI] [PubMed] [Google Scholar]
- 38.Degen A, Kosec M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J Eur Ceram Soc 2000;20:667–673. [Google Scholar]
- 39.Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 2007;41:8484–8490. [DOI] [PubMed] [Google Scholar]
- 40.Borovansky J, Riley PA. Cytotoxicity of zinc in vitro. Chem Biol Interact 1989;69:279–291. [DOI] [PubMed] [Google Scholar]
- 41.Palmiter RD. Protection against zinc toxicity by metallothionein and zinc transporter 1. Proc Natl Acad Sci USA 2004;101:4918–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpkins C, Lloyd T, Li S, Balderman S. Metallothionein-induced increase in mitochondrial inner membrane permeability. J Surg Res 1998;75:30–34. [DOI] [PubMed] [Google Scholar]
- 43.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 2007;292:L165–L177. [DOI] [PubMed] [Google Scholar]
- 44.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol 2006;291:C555–C568. [DOI] [PubMed] [Google Scholar]
- 45.Evans EH. Casualties following exposure to zinc chloride smoke. Lancet 1945;246:368–370. [Google Scholar]
- 46.Milliken JA, Waugh D, Kadish ME. Acute interstitial pulmonary fibrosis caused by a smoke bomb. Can Med Assoc J 1963;88:36–39. [PMC free article] [PubMed] [Google Scholar]
- 47.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003;422:37–44. [DOI] [PubMed] [Google Scholar]
- 48.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 2005;88:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Liu C, Yang D, Zhang H, Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 2009;29:69–78. [DOI] [PubMed] [Google Scholar]
- 50.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009;3:279–290. [DOI] [PubMed] [Google Scholar]
- 51.Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol 2004;286:L1220–L1227. [DOI] [PubMed] [Google Scholar]
- 52.Brown JJ. Zinc fume fever. Br J Radiol 1988;61:327–329. [DOI] [PubMed] [Google Scholar]