Abstract
Rationale: Lymphangioleiomyomatosis (LAM), occurring sporadically (S-LAM) or in patients with tuberous sclerosis complex (TSC), results from abnormal proliferation of LAM cells exhibiting mutations or loss of heterozygosity (LOH) of the TSC genes, TSC1 or TSC2.
Objectives: To identify molecular markers useful for isolating LAM cells from body fluids and determine the frequency of TSC1 or TSC2 LOH.
Methods: Candidate cell surface markers were identified using gene microarray analysis of human TSC2−/− cells. Cells from bronchoalveolar lavage fluid (BALF), urine, chylous effusions, and blood were sorted based on reactivity with antibodies against these proteins (e.g., CD9, CD44v6) and analyzed for LOH using TSC1- and TSC2-related microsatellite markers and single nucleotide polymorphisms in the TSC2 gene.
Measurements and Main Results: CD44v6+CD9+ cells from BALF, urine, and chyle showed TSC2 LOH in 80%, 69%, and 50% of patient samples, respectively. LAM cells with TSC2 LOH were detected in more than 90% of blood samples. LAM cells from different body fluids of the same patients showed, in most cases, identical LOH patterns, that is, loss of alleles at the same microsatellite loci. In a few patients with S-LAM, LAM cells from different body fluids differed in LOH patterns. No patients with S-LAM with TSC1 LOH were identified, suggesting that TSC2 abnormalities are responsible for the vast majority of S-LAM cases and that TSC1-disease may be subclinical.
Conclusions: Our data support a common genetic origin of LAM cells in most patients with S-LAM, consistent with a metastatic model. In some cases, however, there was evidence for genetic heterogeneity between LAM cells in different sites or within a site.
Keywords: lymphangioleiomyomatosis, metastasis, loss of heterozygosity, CD9, CD44v6
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
The multisystem manifestations of lymphangioleiomyomatosis (LAM) are believed to result from a metastatic spread of LAM cells that appear to contain mutations or loss of heterozygosity (LOH) in one of two tuberous sclerosis complex (TSC) tumor suppressor genes, TSC1 or TSC2. LAM cells, however, have not been phenotypically and genetically well characterized, which will be important for their identification in clinical samples.
What This Study Adds to the Field
Here we show that specific cell surface molecules, CD9 and CD44v6, enable identification and isolation of disseminated LAM cells from patients with S-LAM, which appears to be primarily a TSC2-mediated disease. We report also the presence of LAM cells in bronchoalveolar lavage fluid (BALF). Patterns of TSC2 LOH in LAM cells from different sites support a common genetic origin of LAM cells in most patients with S-LAM, but suggest also genetic and phenotypic heterogeneity of LAM cells at different sites or within a site in some cases of S-LAM.
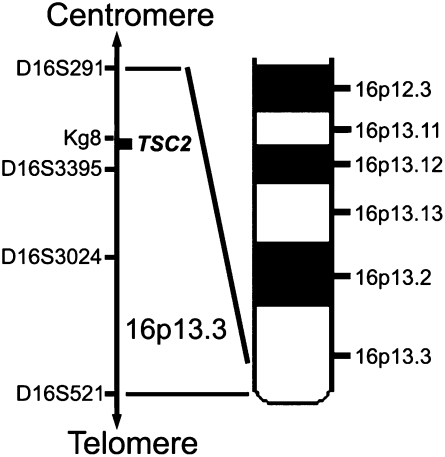
Lymphangioleiomyomatosis (LAM) is a rare multisystem disease affecting primarily women, characterized by abnormal proliferation of smooth muscle–like LAM cells, which leads to cystic destruction of the lungs, formation of fluid-filled cystic structures in the axial lymphatics (e.g., lymphangioleiomyomas), and renal angiomyolipomas (AMLs) (1–5). LAM occurs as a sporadic disease (S-LAM) or in association with tuberous sclerosis complex (TSC) (6–8). TSC is an autosomal dominant syndrome characterized by multiorgan hamartomas, resulting from mutations in one of two tumor suppressor genes, TSC1 on chromosome 9 (9q34) (9) and TSC2 on chromosome 16 (16p13.3) (10, 11). LAM cells in S-LAM were reported to be associated with TSC2 loss of heterozygosity (LOH) (12–15), consistent with Knudson's “two-hit” hypothesis (16).
LAM cells from lung nodules, AMLs, and lymph nodes of the same patient showed identical TSC2 mutations and LOH patterns (13–15), consistent with metastatic spread among organs. Further supporting this model, LAM cells were identified in donor lungs after transplantation (17, 18) and could be isolated from blood, urine, and chyle of patients with LAM (19, 20), consistent with LAM cell dissemination in body fluids. Identification of LAM cells in blood by LOH was aided by fluorescence-activated cell sorting (FACS) removal of non-LAM cells after immunostaining with antibodies against leukocyte common antigen (CD45) and glycophorin A (CD235a) (19), a protein present on LAM cells in lung nodules. In our previous study (19), we were able to isolate LAM cells from only approximately 60% of patients, and thus could not answer questions such as whether sporadic LAM was primarily TSC2 driven, whether LAM cells in different body fluids showed similar LOH patterns, or whether LAM cells could be isolated from bronchoalveolar lavage fluid (BALF).
These questions prompted us to identify cell surface molecules unique to TSC2−/−cells and use these findings to isolate LAM cells with TSC2 LOH from BALF, urine, chyle, and blood. We have shown that CD44v6 is expressed in situ by LAM cells in lung nodules and is present on LAM cells grown from explanted lungs (21). This splice variant of the hyaluronic acid receptor is believed to be involved in tumor metastasis and progression (22–24). In the present study, we showed that the tetraspanin CD9, a highly expressed gene identified by microarray analysis of TSC2−/− cells from TSC skin lesions (25), and CD44v6 identified LAM cells with TSC2 LOH from BALF, urine, and chylous effusions. Similarly, CD45−CD235a− and CD45−CD235a+ cells with TSC2 LOH were detected in blood cell fractions. The majority of TSC2 LOH patterns were identical in LAM cells from blood, urine, and BALF or chyle from the same patients. Different LOH patterns, however, were identified in LAM cells from different body fluids in a minority of patients with S-LAM. Furthermore, we failed to find TSC1 LOH in patients with S-LAM. Some of the results of these studies have been previously reported in the form of an abstract (26).
METHODS
Supplemental description of methods is available in the online supplement.
Patients and Sample Collection
Samples were collected from randomly selected patients with LAM (45 S-LAM and 10 TSC-LAM) and 13 healthy female volunteers who were enrolled between 2007 and 2009 at the National Institutes of Health Clinical Center in clinical protocols (95-H-0186, 96-H-0100) approved by the National Heart, Lung, and Blood Institute Institutional Review Board. The diagnosis of LAM was based on clinical, radiologic, and/or histopathologic findings.
Isolation of Cells from TSC Skin Biopsies
Fibroblasts (TSC2+/−) from postauricular normal-appearing skin and fibroblast-like cells (TSC2−/−) from periungual fibromas of toes from the same female patients with TSC enrolled in protocol 00-H-0051 were isolated and grown as described (25).
Immunofluorescence Analysis of Cultured Cells by Confocal Microscopy
As reported, cells were incubated with a mouse monoclonal antibody against CD9 (1:20; BD Biosciences, San Jose, CA) at 4°C for approximately 12 hours and then with the immunofluorescent fluorescein isothiocyanate (FITC)-labeled goat antibody against mouse IgG (1:100 dilution; Vector Laboratories, Burlingame, CA) for 1 hour at room temperature (21).
Fluorescence-activated Cell Sorting
Anti–CD44v6-FITC (clone VFF-7) and anti–CD9-R-phycoerythrin (PE) (clone MM2/57) antibodies were purchased from Invitrogen (Carlsbad, CA). Anti–CD9-FITC (clone M-L13), anti–CD44-R-PE (clone G44-26), anti–CD45-FITC (clone HI30), and anti–CD235a-PE (clone GA-R2) antibodies were from BD Biosciences. Cells from blood, urine, BALF, and chylous effusions were labeled for flow cytometric analysis and sorting by incubation for 30 minutes at room temperature with the indicated antibodies, followed by two washes with PBS and sorting in a MoFlo Flow Cytometer (Beckman Coulter, Inc., Fullerton, CA). Fluorescence signals were collected using amplifiers that reported on a logarithmic scale. Data acquisition, analysis, and compensation were performed using Summit software (Beckman Coulter).
Polymerase Chain Reaction Analysis of LOH
Genomic DNA was isolated from whole blood and unsorted or sorted cells using the QIAamp DNA Micro Kit (QIAGEN, Valencia, CA) and amplified (19) to determine LOH. Briefly, genomic DNA sequences were amplified at loci D16S291, Kg8, D16S3395, D16S3024, and D16S521 on chromosome 16p13.3, and at loci D9S149, D9S1198, and D9S66 on chromosome 9q34. Primer sequences were obtained from the UniSTS Database (www.ncbi.nlm.nih.gov/unists). Antisense primers were labeled with 6-FAM (Invitrogen). Polymerase chain reaction (PCR) products were analyzed on a 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). QLOH was calculated as described (19). QLOH values of less than 0.5 or more than 0.62 were scored as LOH or retention of heterozygosity (ROH), respectively, whereas no definite decision was made with QLOH values of 0.5 to 0.62 (19, 27).
Single-Nucleotide Polymorphism Analysis
Genomic DNA isolated from whole blood and unsorted or sorted cells was amplified by PCR for the exon 40 polymorphism (T5202C; rs1748) (28, 29) or for a splice site polymorphism (C482–3T; rs1800720) (28, 30), using these primer sequences: TSC40S-Hex, 5′-Hex-ATGGAGGGCCTTGTGGACAC-3′, and TSC40AS, 5′-CGGAGCCGCTTGATGTG-3′; TSCspliceS, 5′-GGAGATGTAGATTCGGCGTC-3′, and TSCspliceAS-Hex, 5′-Hex-CTGCGGAGCTGAACTTAGG-3′. PCR products were digested with the appropriate restriction enzyme (EcoRV for T5151C; PvuII for C482–3T) (New England Biolabs, Beverly, MA), and then analyzed with a 3100 Genetic Analyzer (Applied Biosystems).
Statistical Analysis
Fisher exact test was performed with the SPSS 15.0 (SPSS, Inc., Chicago, IL). Statistical significance was accepted for P < 0.05.
RESULTS
We had first detected LAM cells with TSC2 LOH in blood by OncoQuick density-gradient fractionation, and from urine and chyle specimens based on centrifugation. To improve yield and purity of LAM cells we focused on identification of potential LAM cell surface markers by comparing gene expression in TSC fibroblasts (TSC2+/−) grown from normal-appearing skin and in fibroblastic cells (TSC2−/−) grown from TSC-associated skin tumors of the same patient. By microarray analysis, levels of CD9 were higher in TSC2−/− cells than in their TSC2+/− counterparts (25). We reported previously that LAM cells grown from lungs contained CD44v6, a splice variant of the hyaluronic acid receptor CD44 (21), prompting us to use antibodies to these proteins to isolate circulating LAM cells.
CD9 Expression on TSC2−/− Cells
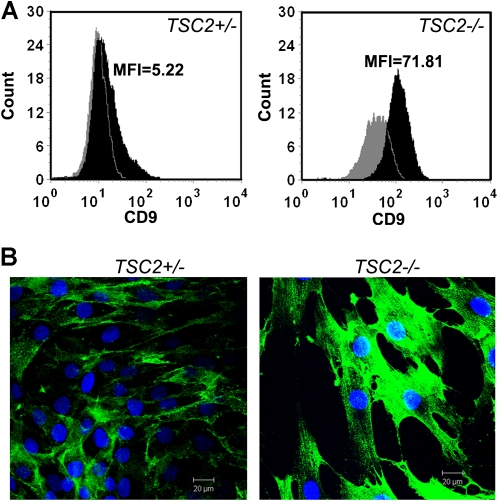
Amounts of CD9 assessed by flow cytometric analysis and immunostaining were greater in TSC2−/− skin tumor cells than in TSC2+/− skin fibroblasts (Figure 1). As determined by mean fluorescence intensity (MFI), the levels of CD9 were much higher in TSC2−/− (MFI = 71.81) than in TSC2+/− cells (MFI = 5.22) (Figure 1A). Most of the CD9 appeared to be concentrated at the plasma membrane, with a small amount located within the cells (Figure 1B). There appeared to be more intracellular CD9 in the null than in the heterozygous cells. Altogether, these data prompted us to isolate cells from body fluids based in part on the presence of this cell surface antigen.
Figure 1.
CD9 protein in TSC2−/− fibroblastic cells grown from human tuberous sclerosis complex (TSC)-associated skin tumors. (A) Flow cytometric analysis showed significantly higher levels of CD9 (clone M-L13, black histogram) as assessed by mean fluorescence intensity (MFI) in TSC2−/− cells (MFI = 71.81) than in TSC2+/− cells (MFI = 5.22). Gray histogram, negative control. (B) Immunostaining analysis of TSC2+/− and TSC2−/− skin cells with anti-CD9 antibody. Reactivity was greater in TSC2−/− than in TSC2+/− cells. Blue, nuclear staining (DAPI). Bar, 20 μm. Experiments were replicated three times.
Detection of TSC2 LOH in CD44v6+/CD9+ Cells from BALF and Urine
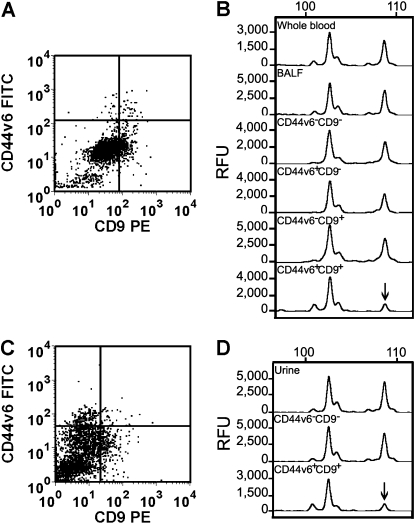
Cells from BALF, urine, and chylous effusion were incubated with anti-CD9 and anti-CD44v6 antibodies and separated by cell sorting. We found that the percentage of cells reactive with anti-CD44v6 and anti-CD9 antibodies differed considerably among body fluids and among patients, and ranged from 0.51 to 6.13% in BALF samples (n = 12), 0.12 to 8.25% in urine samples (n = 55), and 0.10 to 0.55% in chylous effusions (n = 5). Genomic DNA from these cells was isolated to determine TSC2 LOH using microsatellites near the TSC2 locus (i.e., D16S291 [∼ 331 kb centromeric to TSC2], Kg8 [∼ 100 bp centromeric to TSC2], D16S3395 [∼ 96 kb telomeric to TSC2], D16S3024 [∼ 443 kb telomeric to TSC2], and D16S521 [∼ 981 kb telomeric to TSC2]). We specified that a cell population had TSC2 LOH when at least one of the informative markers (heterozygosity of the alleles) had a QLOH of less than 0.5 (see Methods). Figures 2A and 2C show representative FACS plots of BALF and urine cell samples from a single patient, respectively, which were incubated with anti-CD44v6 and anti-CD9 antibodies. The CD44v6+CD9+ cell population from BALF showed TSC2 LOH at the Kg8 microsatellite (Figure 2B, bottom panel), as did a CD44v6+CD9+ cell population from urine (Figure 2D, bottom panel). Furthermore, we were able to determine that CD44v6+CD9+ cells from chylous effusions had TSC2 LOH (Table 1). These data suggest that LAM cells could be identified in BALF, urine, and chyle based on the expression of the tetraspanin CD9 and the splice variant of the hyaluronic receptor, CD44v6.
Figure 2.
Fluorescence-activated cell sorting (FACS) of cells in bronchoalveolar lavage fluid (BALF) and urine samples from a patient with lymphangioleiomyomatosis (LAM). Cells were reacted with anti–CD44v6-FITC and anti–CD9-PE antibodies and sorted cells were analyzed for TSC2 loss of heterozygosity (LOH) of chromosome 16p13.3 microsatellite marker Kg8. Four populations of BALF cells were separated (A), and LOH was detected only in the CD44v6+CD9+ population (B, lowest histogram). Two populations of urine cells were separated: CD44v6−CD9− and CD44v6+CD9+ (C). LOH was observed only in the CD44v6+CD9+ population (D, lowest histogram). Arrows indicate positions of allelic loss compared with that seen in whole blood from the same patient. Numbers at the top of histograms indicate the number of DNA bases and the Y-axis indicates relative fluorescence units (RFU).
TABLE 1.
DETECTION OF TSC2-RELATED LOSS OF HETEROZYGOSITY IN DIFFERENT CELL POPULATIONS FROM DIFFERENT BODY FLUIDS OF PATIENTS WITH LYMPHANGIOLEIOMYOMATOSIS
| Type of LAM | Fluid | Cell Population | Number of Informative Samples* | % Samples with LOH† |
|---|---|---|---|---|
| S-LAM | Blood fraction | Unsorted | 43 | 35 |
| CD45−/CD235a− | 39 | 85 | ||
| CD45−/CD235a+ | 36 | 86 | ||
| Urine‡ | Unsorted | 43 | 14 | |
| CD44v6−/CD9− | 42 | 0 | ||
| CD44v6+/CD9+ | 42 | 67 | ||
| BALF | Unsorted | 8 | 0 | |
| CD44v6−/CD9− | 8 | 0 | ||
| CD44v6+/CD9− | 8 | 0 | ||
| CD44v6−/CD9+ | 8 | 0 | ||
| CD44v6+/CD9+ | 8 | 63 | ||
| Chyle | Unsorted | 4 | 0 | |
| CD44v6−/CD9− | 4 | 0 | ||
| CD44v6+/CD9+ | 4 | 50 | ||
| TSC-LAM | Blood fraction | Unsorted | 10 | 30 |
| CD45−/CD235a− | 9 | 89 | ||
| CD45−/CD235a+ | 9 | 100 | ||
| Urine‡ | Unsorted | 10 | 10 | |
| CD44v6−/CD9− | 10 | 0 | ||
| CD44v6+/CD9+ | 10 | 80 | ||
| BALF | Unsorted | 2 | 0 | |
| CD44v6−/CD9− | 2 | 0 | ||
| CD44v6+/CD9− | 2 | 0 | ||
| CD44v6−/CD9+ | 2 | 0 | ||
| CD44v6+/CD9+ | 2 | 100 |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; LAM = pulmonary lymphangioleiomyomatosis; LOH = loss of heterozygosity; PCR = polymerase chain reaction; S-LAM = sporadic LAM; TSC = tuberous sclerosis complex.
Samples were collected from 45 patients with S-LAM and 10 patients with TSC-LAM, but only samples that were heterozygous for the markers tested and that were amplified well by PCR were included.
Results of PCR assays are based on total of five microsatellite markers on chromosome 16p13.3: D16S291, Kg8, D16S3395, D16S3024, and D16S521.
Due to limited cell numbers in urine specimens, simultaneous sorting of four cell populations led to too few cells collected for each population to be analyzed for TSC2 LOH. We randomly selected five cases that showed TSC2 LOH in CD44v6+CD9+ cells from urine to separate subsequently CD44v6+CD9− and CD44v6−CD9+ cells, but did not identify TSC2 LOH in these populations.
We had reported TSC2 LOH in cells grown from LAM lung nodules and sorted with anti-CD44 and anti-CD44v6 antibodies (21), but LOH was not consistently seen in cells similarly separated from BALF (1 out of 6) and urine (2 out of 15) (see Figure E1 in the online supplement). Populations reactive with anti-CD45/CD235a antibodies were not seen in BALF and urine cell samples (Figure E2), nor were those reactive with anti-CD44v6/CD44 and anti-CD44v6/CD9 antibodies seen in blood cell fractions (Figure E3). Thus, LAM cells in different locations appear to show differences in surface protein expression, which is consistent with their phenotypic heterogeneity in different tissues. LAM cells in lung nodules appear both spindle-shaped and epithelioid, whereas TSC2−/− cells in renal AMLs may resemble adipocytes, vasculature, or smooth muscle cells.
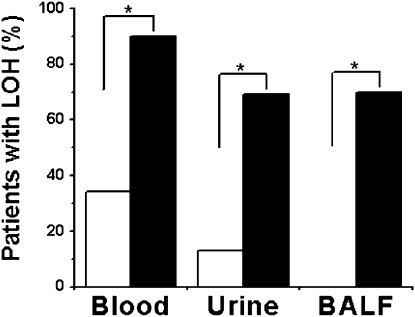
In our series of 55 patients (Table E1), detection of TSC2 LOH in blood fractions, urine, and BALF was markedly enhanced after cell sorting with the specified cell surface markers (Table 1 and Figure 3). Despite the ability to show allelic imbalance and LOH in unsorted cells from urine and blood fractions, we failed to find TSC2 LOH in cell pellets isolated from BALF (Figure 3 and Table 1). Percentage of detection of TSC2 LOH, however, was increased from 34% (18 of 53 patients) in unsorted blood samples separated using the OncoQuick density-gradient system to 90% (47 of 52 patients) after sorting with anti-CD45 and anti-CD235a antibodies (P < 0.001). Cells from urine with TSC2 LOH were more readily detected after sorting with anti-CD44v6 and anti-CD9 antibodies (from 13% [7 of 53 patients] to 69% [36 of 52 patients]; P < 0.001). Furthermore, the success rate of detection of TSC2 LOH was 70% (7 of 10 patients) in the CD44v6+CD9+ cell population separated from BALF samples (P = 0.003).
Figure 3.
Effect of sorting on detection of TSC2 loss of heterozygosity (LOH) in cell samples. Cells separated from blood by OncoQuick density-gradient centrifugation were reacted with anti–CD45-FITC and anti–CD235a-PE antibodies and cells from urine and bronchoalveolar lavage fluid (BALF) with anti–CD44v6-FITC and anti–CD9-PE antibodies. Percentage TSC2 LOH detected in cell samples of sorted cells (solid bars) was significantly higher than that in unsorted cells (open bars) from low-density cell fractions of blood, urine, and BALF from patients with lymphangioleiomyomatosis (LAM). For all comparisons, *P < 0.001 for blood (n = 52) and urine (n = 52); *P = 0.003 for BALF (n = 10).
To assess the reproducibility of detection of cells with TSC2 LOH, blood and urine samples from 10 patients with S-LAM at two visits, separated by 6 to 18 months, were analyzed and found to show reproducible detection of TSC2 LOH using the same microsatellites in 100% of blood samples and 80% of urine samples (Table 2 and Figure E4).
TABLE 2.
TSC2-RELATED LOSS OF HETEROZYGOSITY IN BLOOD AND URINE FROM PATIENTS ON TWO VISITS
| Allelic Status of TSC2-Related Microsatellites |
||||||||
|---|---|---|---|---|---|---|---|---|
| Case no. | Visit | Interval (mo) | Fluid | D16S291 | Kg8 | D16S3395 | D16S3024 | D16S521 |
| S-LAM1 | 1 | Blood | NA | ROH | LOH | NI | LOH | |
| 17 | Urine | NA | ROH | ROH | ROH | |||
| 2 | Blood | NA | ROH | LOH | LOH | |||
| Urine | NA | ROH | ROH | ROH | ||||
| S-LAM2 | 1 | Blood | NA | NI | LOH | NA | NI | |
| 18 | Urine | NA | ROH | NA | ||||
| 2 | Blood | NA | LOH | NA | ||||
| Urine | NA | ROH | LOH | |||||
| S-LAM8 | 1 | Blood | NI | LOH | LOH | NI | NI | |
| 12 | Urine | LOH | NA | |||||
| 2 | Blood | LOH | NA | |||||
| Urine | LOH | ROH | ||||||
| S-LAM9 | 1 | Blood | NI | NA | NI | NA | LOH | |
| 18 | Urine | ROH | ROH | LOH | ||||
| 2 | Blood | NA | NA | LOH | ||||
| Urine | ROH | ROH | LOH | |||||
| S-LAM27 | 1 | Blood | NI | LOH | LOH | NI | NI | |
| 6 | Urine | LOH | ROH | |||||
| 2 | Blood | LOH | LOH | |||||
| Urine | ROH | ROH | ||||||
| S-LAM28 | 1 | Blood | NI | LOH | NA | NI | NI | |
| 12 | Urine | LOH | ROH | |||||
| 2 | Blood | LOH | NA | |||||
| Urine | LOH | ROH | ||||||
| S-LAM30 | 1 | Blood | NI | LOH | LOH | NA | NI | |
| 12 | Urine | LOH | ROH | NA | ||||
| 2 | Blood | LOH | LOH | NA | ||||
| Urine | LOH | ROH | NA | |||||
| S-LAM31 | 1 | Blood | NI | NI | LOH | LOH | LOH | |
| 12 | Urine | LOH | LOH | LOH | ||||
| 2 | Blood | LOH | NA | NA | ||||
| Urine | LOH | NA | LOH | |||||
| S-LAM40 | 1 | Blood | NI | LOH | LOH | NI | NA | |
| 14 | Urine | ROH | ROH | LOH | ||||
| 2 | Blood | LOH | NA | NA | ||||
| Urine | ROH | NA | NA | |||||
| S-LAM43 | 1 | Blood | NI | LOH | NI | NI | NI | |
| 13 | Urine | LOH | ||||||
| 2 | Blood | LOH | ||||||
| Urine | ROH | |||||||
Definition of abbreviations: LOH = loss of heterozygosity; NA = not amplified; NI = noninformative: homozygosity of the markers tested; ROH = retention of heterozygosity; S-LAM = sporadic lymphangioleiomyomatosis.
TSC2 LOH Patterns in LAM Cells from Different Body Fluids
Because the microsatellite markers on chromosome 16p13.3 near the TSC2 gene cover a large region, we compared the patterns of TSC2 LOH in samples from different sources in the same patients. Twenty-seven of 47 patients showed LOH at one informative microsatellite, and 20 of 47 patients at two informative microsatellites, which involved different regions of chromosome 16 (Table E1). We found that CD44v6+CD9+ cells from BALF, urine, and chylous effusions and CD45−CD235a− or CD45−CD235a+ cells from blood fractions showed, in general, identical TSC2 LOH patterns, that is, loss or retention of the alleles at the same microsatellite loci (Table E1). Exceptions were observed, however, in eight cases of S-LAM and two of TSC-LAM, in which LAM cells from different body fluids did not show consistent LOH or ROH for each informative marker (Table E1, see S-LAM2, 11, 23, 27, 30, 40, 41, 45, and TSC-LAM5, 8). In addition, we observed that allelic loss in LAM cells from different body fluids consistently occurred at the same alleles for the same microsatellite markers (Figures 2B and 2D).
We analyzed also the frequency of detection of LOH with different chromosome 16p13.3 microsatellite markers (Table 3). The TSC2 gene is closest to the Kg8 microsatellite; other markers are more distant (D16S3395 < D16S291 < D16S3024 < D16S521) and span a large region from approximately 981 kb telomeric through approximately 331 kb centromeric to the TSC2 gene. Percentage of LOH detection by PCR using Kg8 in informative patients approached 97% in blood (n = 34), 71% in urine (n = 35), and 100% in BALF (n = 5), whereas percentage for other markers were significantly lower (P < 0.05). Isolated LOH was observed both centromeric and telomeric to the TSC2 gene.
TABLE 3.
FREQUENCY OF DETECTION OF TSC2-RELATED LOSS OF HETEROZYGOSITY BY POLYMERASE CHAIN REACTION AMPLIFICATION OF DIFFERENT MICROSATELLITES ON CHROMOSOME 16P13.3
FACS of Samples from Patients with LAM and Healthy Volunteers with Anti-CD44v6 and Anti-CD9 Antibodies
Investigating whether identification of CD44v6+CD9+ cells with TSC2 LOH would distinguish patients with LAM (S-LAM or TSC-LAM) from healthy volunteers, we found that using similar cell fractionation and sorting, cells reactive with anti-CD44v6 and anti-CD9 antibodies were also seen in BALF and urine samples from healthy volunteers. We did not observe significant differences in reactivity to anti-CD44v6 and anti-CD9 antibodies among cells from S-LAM, TSC-LAM, and healthy volunteers. Table 4 presents data for patients with S-LAM (45 blood and urine, 10 BALF, and 5 chylous effusions), patients with TSC-LAM (10 blood and urine, and 2 BALF), and healthy volunteers (13 blood, urine, and BALF). Of importance regarding the specificity of TSC2 LOH, LOH was not detected in blood, urine, and BALF specimens from healthy volunteers (data not shown). We observed TSC2 LOH in 38 of 43 (88%) blood specimens, 28 of 42 (67%) urine specimens, 5 of 8 (63%) BALF specimens, and 2 of 4 (50%) chylous effusions from informative patients with S-LAM. In patients with TSC-LAM, LOH was detected in 9 of 9 (100%) blood specimens, 8 of 10 (80%) urine specimens, and 2 of 2 (100%) BALF specimens. The frequency of detection of TSC2 LOH was greater in patients with TSC-LAM than in patients with S-LAM, but it was not statistically significant. The overall detection rate in patients with LAM was 90% in blood, 69% in urine, and 70% in BALF. TSC2 LOH was not found in five informative patients with S-LAM; two patients with S-LAM were noninformative due to homozygosity of all five tested markers. We further analyzed these patients using two single-nucleotide polymorphisms (SNPs) within the TSC2 gene. Three patients with S-LAM (S-LAM15, 35, 39) were informative for one of two SNPs. LOH at the exon 40 polymorphism was detected in CD44v6+CD9+ cells from BALF of S-LAM39 (data not shown), increasing the overall detection rate to 80% in BALF samples.
TABLE 4.
DETECTION OF TSC2-RELATED LOSS OF HETEROGEITY IN CELL SAMPLES SORTED FROM BODY FLUIDS FROM PATIENTS WITH SPORADIC-LYMPHANGIOLEIOMYOMATOSIS AND TUBEROUS SCLEROSIS COMPLEX–LYMPHANGIOLEIOMYOMATOSIS, AND FROM HEALTHY VOLUNTEERS
| Informative‡ |
% Patients with LOH |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | Fluid* | Number of Cases | Noninformative† | Not Amplified | ROH | LOH | All Cases | Informative Cases |
| S-LAM | Blood | 45 | 2 | 0 | 5 | 38 | 84 | 88 |
| Urine | 45 | 2 | 1 | 14 | 28 | 62 | 67 | |
| BALF | 10 | 2 | 0 | 3 | 5 | 50 | 63 | |
| Chyle | 5 | 0 | 1 | 2 | 2 | 40 | 50 | |
| TSC-LAM | Blood | 10 | 0 | 1 | 0 | 9 | 90 | 100 |
| Urine | 10 | 0 | 0 | 2 | 8 | 80 | 80 | |
| BALF | 2 | 0 | 0 | 0 | 2 | 100 | 100 | |
| Healthy volunteers | Blood | 13 | 0 | 2 | 11 | 0 | 0 | 0 |
| Urine | 13 | 0 | 1 | 12 | 0 | 0 | 0 | |
| BALF | 13 | 0 | 0 | 13 | 0 | 0 | 0 | |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; LAM = pulmonary lymphangioleiomyomatosis; LOH = loss of heterozygosity; PCR = polymerase chain reaction; ROH = retention of heterozygosity; S-LAM = sporadic LAM; TSC = tuberous sclerosis complex.
Cells separated from blood by OncoQuick density-gradient centrifugation were reacted with anti–CD45-FITC and anti–CD235a-PE antibodies and cells from urine, BALF, and chylous effusions with anti–CD44v6-FITC and anti–CD9-PE antibodies.
Noninformative: homozygosity of the markers tested.
Results of PCR assays are based on a total of five microsatellite markers on chromosome 16p13.3: D16S291, Kg8, D16S3395, D16S3024, and D16S521.
Because LAM is believed to result from mutations in the TSC1 or TSC2 gene, we looked to see if those lacking TSC2 LOH had TSC1 LOH. We therefore assessed microsatellite markers at the TSC1 locus, but none of these samples showed TSC1 LOH (Table E2). In addition, patients with TSC2 ROH in blood consistently showed ROH in urine and/or BALF.
TSC2 LOH and Clinical Phenotypes of Patients with LAM
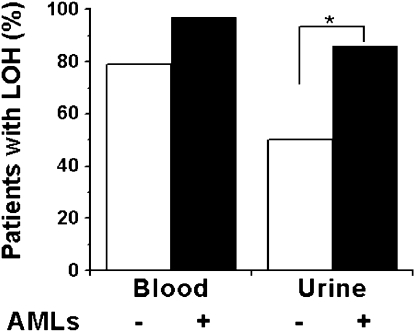
LAM is characterized by renal AMLs, lymphatic abnormalities, and pulmonary cystic lesions. We therefore assessed the association between the presence of AMLs and lymphatic involvement, which may represent the presence of more metastatic cells, and detection of LOH in cells from blood and urine. LOH was detected in 86% (n = 28) of urine samples from patients with AMLs, but in only 50% (n = 24) of urine samples from those without AMLs (P = 0.007), although the frequency of TSC2 LOH was not significantly higher in blood cell fractions from patients with LAM with AMLs (n = 29) than in those without AMLs (n = 24; P = 0.08) (Figure 4). These data suggest that LAM cells in blood might be shed into the urine in patients with LAM, or that necrosis in the AMLs might result in TSC2−/− cells in the urine. There was no significant association between the presence of lymphangioleiomyomas, adenopathy, or lymphangioleiomyomas/adenopathy in patients with LAM with or without AMLs and detection of TSC2 LOH in blood and urine cell fractions (Figure E5).
Figure 4.
Association of TSC2 loss of heterozygosity (LOH) with angiomyolipomas (AMLs). Presence of AMLs is indicated with solid bars, and their absence with open bars. LOH was detected more frequently in blood and urine from patients with lymphangioleiomyomatosis (LAM) with AMLs (blood, n = 29; urine, n = 28) than those without AMLs (blood, n = 24; urine, n = 24) but was statistically significant only for urine samples (*P = 0.007).
DISCUSSION
We have identified two cell surface proteins, CD44v6 and CD9, that are useful for isolation of disseminated LAM cells from BALF, urine, and chylous effusions. LAM cells from patients with S-LAM exhibit mutations and LOH most frequently in the TSC2 locus. For the majority of patients with S-LAM, LAM cells isolated from blood, urine, BALF, or chyle of the same patients show identical TSC2 LOH patterns for specific microsatellites, although in some, LAM cells from different body fluids appeared to differ in the extent of TSC2 LOH regions, consistent with genetic heterogeneity.
In general, these findings support the hypothesis that multisystem manifestations of LAM appear to result from a metastatic process (31). Previously in five patients with S-LAM, identical TSC2 mutations or LOH were identified in pulmonary and renal LAM lesions (13–15). LAM cells from the recipient were identified in a transplanted donor lung (17, 18). As LAM cells were also detected in blood (19) or chylous fluids (19, 20), it was hypothesized that LAM cells could migrate or metastasize via blood and/or lymphatic circulations (19, 20). The LAM cells found in BALF could result from release from the LAM lung nodules or perhaps shedding from the lymphatic circulation within the nodules. Patients with LAM may experience chyloptysis. LAM cells were identified in chyle and BALF may contain components of chyle.
Identification of proteins on the surface of circulating or disseminated cells has been of interest in human cancers for use potentially as therapeutic targets (32–34). Gene expression microarray analysis revealed that TSC2−/− cells grown from TSC-associated skin tumors contained highly increased mRNA levels of the tetraspanin CD9 (25). We demonstrated here using flow cytometric analysis and immunostaining that CD9 protein was abundant on TSC2−/− skin tumor cells (Figure 1). A correlation between greater CD9 content and potential for metastasis is evident in some tumor types (e.g., bone, cervix, head and neck, stomach) (35), although CD9 is considered to suppress metastasis by decreasing cell motility (35–38). Tetraspanins are present widely among mammals and play important roles in cell morphology, motility, invasion, adhesion, and signaling (39–42). Tetraspanins form complexes with other tetraspanins and a variety of transmembrane proteins at tetraspanin-enriched membrane microdomains (42, 43). The diverse actions of CD9 are probably due to its association with other molecules in the tetraspanin-enriched membrane microdomains. In our studies, high levels of CD9 protein correlated with cells having TSC2 LOH (Figure 1).
Our group had reported earlier an association between the presence of CD44v6 protein and TSC2 LOH in LAM cells grown from explanted lungs (21). In the present study, LAM cells from BALF, urine, and chylous effusions reactive with anti-CD44v6 and anti-CD9 antibodies showed TSC2 LOH. Thus, disseminated LAM cells contained prometastatic molecules that could enable their mobilization and subsequent anchorage to sites of metastasis. Phenotypic changes in metastatic cells may occur as they migrate to sites of metastasis (44). We observed that LAM cells grown from explanted lungs (as identified with the markers CD44v6/CD44), in blood (CD235a), and in BALF, urine, and chyle (CD44v6/CD9) differed in the expression of surface proteins, suggesting that LAM cells within different microenvironments have different phenotypic characteristics, which is consistent with their phenotypic heterogeneity in different tissues. In fact, human TSC2−/− cells are known to exhibit different morphologies in different locations. In renal AMLs, TSC2−/− cells appear as smooth muscle, fat, and vascular cells. LAM cells in the lungs may be spindle-shaped or epithelioid.
S-LAM is considered to be associated most frequently with mutations in TSC2. In our studies, TSC2 LOH was detected in 38 of 43 (88%) patients with S-LAM, consistent with the hypothesis that more patients with S-LAM have dysfunctional TSC2 than TSC1. We did not find TSC2 LOH in five informative patients with S-LAM; two patients with S-LAM were noninformative because of their homozygosity for all five tested markers (Table 4). To increase detection of TSC2 LOH, we did SNP-based LOH analysis on these patients using two SNPs within the TSC2 gene. We identified LOH at the exon 40 polymorphism in BALF cell samples from one of three patients informative for two SNPs; blood and urine cell samples from this patient were not amplified well, probably due to low DNA amounts (data not shown). To determine whether or not these seven patients with S-LAM have TSC1 abnormalities, we further assessed TSC1 LOH but did not identify any patients with TSC1 LOH. Failure to detect TSC1 LOH could result from the fact that the second hit for TSC1 may be subtle sequence changes (e.g., point mutations, small deletions), which are not detectable by LOH analysis. It is also possible that methylation may be responsible for dysregulation of the TSC2 gene (45). The absence of TSC1 mutations in LAM cells from patients with S-LAM suggests that pulmonary disease due to this mutation may be subclinical.
Based on earlier reports that AMLs and pulmonary LAM cells from the same patients with S-LAM have the same TSC2 mutations and identical TSC2 LOH patterns (13, 14), it was hypothesized that pulmonary LAM cells and AML cells could have a common genetic origin, and LAM cells could metastasize in vivo. Here, we described identical LOH patterns at the chromosome 16p13.3 region in LAM cells isolated from blood, urine, BALF, or chyle from the same patient in 27 of 37 (73%) cases. In 8 of 29 (23%) patients with S-LAM and 2 of 8 (25%) patients with TSC-LAM, however, LAM cells from different body fluids appeared to differ in the extent of LOH regions based on the informative microsatellites. Our data from two patients with TSC-LAM are consistent with the report that two AMLs from the same patient with TSC with multiple AMLs showed different regions of LOH on 16p13 (46). These findings from eight patients with S-LAM are discordant with a prior report (14) and suggest that in some patients with S-LAM, LAM cells may show genetic heterogeneity, which (a) could result from a different second mutation in cells containing the same first mutation, or (b) result from the introduction of new independent genetic changes in the existing LAM cells during the metastatic process, or (c) could represent a second novel LAM cell with two different mutations. In support of the first model, skin lesions in patients with TSC appear to arise from cells with independent second mutations in the TSC2 genes (47). The second model of chromosomal instability appears to occur frequently in cancer cells (48). The third model is least likely. Further studies would be required to define the extent of the deletion and the identification of specific genes involved as well as the mechanism(s) of LOH (e.g., mitotic nondisjunction with reduplication of the mutant chromosome).
Among five microsatellites on chromosome 16p13.3, Kg8, closer in proximity to the TSC2 gene than the other microsatellite markers (i.e., D16S291, D16S3395, D16S3024, and D16S521), was more frequently affected in patients with LAM (Table 3). In one case, ROH was observed at the Kg8 locus, but LOH was observed in microsatellite markers D16S3395 and D16521, which span a region telomeric to the TSC2 locus (Table E1, see S-LAM1). Nearly half of patients with S-LAM showed LOH of two informative microsatellites; the patterns include LOH at Kg8 and D16S3395, two adjacent microsatellites that span the TSC2 locus, and LOH at two distant microsatellites mapped centromerically (from D16S291 through Kg8) or telomerically (from D16S3395 through D16S521) to the TSC2 locus or spanning the TSC2 locus (from Kg8 through D16S521). Our data indicate that LAM cells from these patients might have loss of a larger part and a different region of chromosome 16.
An association between the presence of AMLs and lymphatic involvement and detection of TSC2 LOH in blood and urine was also assessed, but no significant differences were found between detection of TSC2 LOH in blood cell fractions and urine and the presence of lymphangioleiomyomas, adenopathy, or lymphangioleiomyomas/adenopathy in patients with LAM with or without AMLs (Figure E5). A statistically significant association between TSC2 LOH in cells from urine and the presence of AMLs in patients with LAM (Figure 4) suggests that circulating LAM cells are more likely to be shed from blood into the urine in patients with LAM with AMLs than those without AMLs. Alternatively, cells from AMLs might be shed directly into urine, perhaps due to necrosis within the tumors.
Overall, we found that the presence of specific surface proteins (e.g., CD44v6, CD9, CD235a) was associated with LAM cells exhibiting TSC2 LOH in blood, BALF, urine, and chyle, which supports, in most cases, a metastatic dissemination of LAM cells via blood and/or lymphatic circulatory systems. Contrary to previous observations, however, our data suggest that LAM cells may exhibit genetic as well as phenotypic heterogeneity in some of patients with S-LAM.
Supplementary Material
Acknowledgments
The authors thank Dr. Martha Vaughan (NHLBI, NIH) for helpful discussions and critical review of the manuscript, and Drs. Christian Combs and Daniela Malide of the microscopy core facility for technical assistance. We thank the LAM Foundation and the Tuberous Sclerosis Alliance for their assistance in recruiting patients for our studies.
Supported in part by the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute (J.M.); and by RO1 CA100907 (T.N.D.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201003-0489OC on July 16, 2010
Author Disclosure: X.C. is employed by the National Institutes of Health (NIH) as a postdoctoral visiting fellow. G.P-R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Q-Y.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. is an employee of the NIH. L.S. is a full-time employee of the NIH. S.E-C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H-P.W. is an employee of the NIH. J.P.M. and J.P.M.'s spouse/life partner are employees of the NIH and owned $5,001–$10,000 in stock ownership or options in Altria within the last 12 months or at present. W.K.S. is an employee of the NIH. J-P.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.N.D. received more than $100,001 from the NIH in sponsored grants (R01, R21) and more than $100,001 from the Department of Defense in sponsored grants (CDMRP, DMRDP grants). J.M. has received $1,001–$5000 in patent royalties from the NIH for an invention licensed by Emiliem. J.M. is employed by the NIH.
References
- 1.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Contr 2006;13:276–285. [DOI] [PubMed] [Google Scholar]
- 2.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, Cordier JF. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'etudes et de recherche sur les maladies “Orphelines” Pulmonaires (germ"O"P). Medicine (Baltimore) 1999;78:321–337. [DOI] [PubMed] [Google Scholar]
- 3.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008;133:507–516. [DOI] [PubMed] [Google Scholar]
- 4.Johnson SR, Tattersfield AE. Lymphangioleiomyomatosis. Semin Respir Crit Care Med 2002;23:85–92. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow CG, Taveira-Dasilva AM, Darling TN, Moss J. Lymphatic involvement in lymphangioleiomyomatosis. Ann N Y Acad Sci 2008;1131:206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormack FX. Lymphangioleiomyomatosis. MedGenMed 2006;8:15. [PMC free article] [PubMed] [Google Scholar]
- 7.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, Hedin CJ, Hunsberger S, Kristof AS. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med 2001;164:669–671. [DOI] [PubMed] [Google Scholar]
- 8.Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000;75:591–594. [DOI] [PubMed] [Google Scholar]
- 9.van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997;277:805–808. [DOI] [PubMed] [Google Scholar]
- 10.Consortium TECTS. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305–1315. [DOI] [PubMed] [Google Scholar]
- 11.Povey S, Burley MW, Attwood J, Benham F, Hunt D, Jeremiah SJ, Franklin D, Gillett G, Malas S, Robson EB, et al. Two loci for tuberous sclerosis: One on 9q34 and one on 16p13. Ann Hum Genet 1994;58:107–127. [DOI] [PubMed] [Google Scholar]
- 12.Smolarek TA, Wessner LL, McCormack FX, Mylet JC, Menon AG, Henske EP. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: Chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet 1998;62:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2000;97:6085–6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Astrinidis A, Henske EP. Chromosome 16 loss of heterozygosity in tuberous sclerosis and sporadic lymphangiomyomatosis. Am J Respir Crit Care Med 2001;164:1537–1540. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Seyama K, Fujii H, Maruyama H, Setoguchi Y, Iwakami S, Fukuchi Y, Hino O. Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis. J Hum Genet 2002;47:20–28. [DOI] [PubMed] [Google Scholar]
- 16.Knudson AG. Hereditary cancer: two hits revisited. J Cancer Res Clin Oncol 1996;122:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbowniczek M, Astrinidis A, Balsara BR, Testa JR, Lium JH, Colby TV, McCormack FX, Henske EP. Recurrent lymphangiomyomatosis after transplantation: genetic analyses reveal a metastatic mechanism. Am J Respir Crit Care Med 2003;167:976–982. [DOI] [PubMed] [Google Scholar]
- 18.Bittmann I, Rolf B, Amann G, Lohrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol 2003;34:95–98. [DOI] [PubMed] [Google Scholar]
- 19.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP Jr, Wang JA, Kumaki F, Darling T, Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci USA 2004;101:17462–17467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, Sato T, Kubo H, Gomi K, Shibuya K, et al. Lymphangiogenesis-mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol 2005;29:1356–1366. [DOI] [PubMed] [Google Scholar]
- 21.Pacheco-Rodriguez G, Steagall WK, Crooks DM, Stevens LA, Hashimoto H, Li S, Wang JA, Darling TN, Moss J. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res 2007;67:10573–10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33–45. [DOI] [PubMed] [Google Scholar]
- 23.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol 2004;35:211–231. [DOI] [PubMed] [Google Scholar]
- 24.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991;65:13–24. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Takeuchi F, Wang JA, Fan Q, Komurasaki T, Billings EM, Pacheco-Rodriguez G, Moss J, Darling TN. Mesenchymal-epithelial interactions involving epiregulin in tuberous sclerosis complex hamartomas. Proc Natl Acad Sci USA 2008;105:3539–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Pacheco-Rodriguez G, Fan Q, Haughey M, El-Chemaly S, Gochuico B, Samsel L, McCoy JP, Darling TN, Moss J. Identification of disseminated CD44v6+/CD9+ cells with TSC2 loss of heterozygosity in patients with lymphangioleiomyomatosis [abstract]. Am J Respir Crit Care Med 2009;179:A4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomlinson IP, Lambros MB, Roylance RR. Loss of heterozygosity analysis: practically and conceptually flawed? Genes Chromosomes Cancer 2002;34:349–353. [DOI] [PubMed] [Google Scholar]
- 28.Roberts PS, Chung J, Jozwiak S, Dabora SL, Franz DN, Thiele EA, Kwiatkowski DJ. SNP identification, haplotype analysis, and parental origin of mutations in TSC2. Hum Genet 2002;111:96–101. [DOI] [PubMed] [Google Scholar]
- 29.van Bakel I, Sepp T, Yates JR, Green AJ. An EcoRV polymorphism in exon 40 of the tuberous sclerosis 2 (TSC2) gene. Mol Cell Probes 1997;11:75–76. [DOI] [PubMed] [Google Scholar]
- 30.Platten M, Meyer-Puttlitz B, Blumcke I, Waha A, Wolf HK, Nothen MM, Louis DN, Sampson JR, von Deimling A. A novel splice site associated polymorphism in the tuberous sclerosis 2 (TSC2) gene may predispose to the development of sporadic gangliogliomas. J Neuropathol Exp Neurol 1997;56:806–810. [PubMed] [Google Scholar]
- 31.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer 2003;38:376–381. [DOI] [PubMed] [Google Scholar]
- 32.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008;8:329–340. [DOI] [PubMed] [Google Scholar]
- 33.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micrometastases. Nat Rev Clin Oncol 2009;6:339–351. [DOI] [PubMed] [Google Scholar]
- 34.Mostert B, Sleijfer S, Foekens JA, Gratama JW. Circulating tumor cells (CTCs): detection methods and their clinical relevance in breast cancer. Cancer Treat Rev 2009;35:463–474. [DOI] [PubMed] [Google Scholar]
- 35.Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 2009;9:40–55. [DOI] [PubMed] [Google Scholar]
- 36.Boucheix C, Duc GH, Jasmin C, Rubinstein E. Tetraspanins and malignancy. Expert Rev Mol Med 2001;2001:1–17. [DOI] [PubMed] [Google Scholar]
- 37.Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens 2004;64:533–542. [DOI] [PubMed] [Google Scholar]
- 38.Lazo PA. Functional implications of tetraspanin proteins in cancer biology. Cancer Sci 2007;98:1666–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J 1997;11:428–442. [PubMed] [Google Scholar]
- 40.Stipp CS, Kolesnikova TV, Hemler ME. Functional domains in tetraspanin proteins. Trends Biochem Sci 2003;28:106–112. [DOI] [PubMed] [Google Scholar]
- 41.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 2003;19:397–422. [DOI] [PubMed] [Google Scholar]
- 42.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005;6:801–811. [DOI] [PubMed] [Google Scholar]
- 43.Levy S, Shoham T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda) 2005;20:218–224. [DOI] [PubMed] [Google Scholar]
- 44.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006;127:679–695. [DOI] [PubMed] [Google Scholar]
- 45.Lesma E, Sirchia SM, Ancona S, Carelli S, Bosari S, Ghelma F, Montanari E, Di Giulio AM, Gorio A. The methylation of the TSC2 promoter underlies the abnormal growth of TSC2 angiomyolipoma-derived smooth muscle cells. Am J Pathol 2009;174:2150–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henske EP, Neumann HP, Scheithauer BW, Herbst EW, Short MP, Kwiatkowski DJ. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer 1995;13:295–298. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Li S, Fan Q, Pacheco-Rodriguez G, Moss J, Darling TN. Independent origins for multifocal skin tumors in tuberous sclerosis complex [abstract]. J Invest Dermatol 2007;127:S23. [Google Scholar]
- 48.Loeb LA, Bielas JH, Beckman RA. Cancers exhibit a mutator phenotype: Clinical implications. Cancer Res 2008;68:3551–3557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.