Abstract
Rationale: Cerebrovascular regulation is impaired in patients with moderate to severe obstructive sleep apnea; however, it is unknown whether this impairment exists in individuals with less severe sleep-disordered breathing.
Objectives: To test the hypothesis that cerebrovascular responses to hypercapnia are attenuated in a nonclinical population-based cohort.
Methods: A rebreathing test that raised end-tidal CO2 tension by 10 mm Hg was performed during wakefulness in 373 participants of the Wisconsin Sleep Cohort.
Measurements and Main Results: We measured cerebral flow velocity (transcranial Doppler ultrasound); heart rate (electrocardiogram); blood pressure (photoplethysmograph); ventilation (pneumotachograph); and end-tidal CO2 (expired gas analysis). Cerebrovascular CO2 responsiveness was quantified as the slope of the linear relationship between flow velocity and end-tidal CO2 during rebreathing. Linear regression analysis was performed using cerebrovascular CO2 responsiveness as the outcome variable. Main independent variables were the apnea–hypopnea index and the mean level of arterial oxygen saturation during sleep. We observed a positive correlation between cerebrovascular CO2 responsiveness and the mean level of oxygen saturation during sleep that was statistically significant in unadjusted analysis and after adjustment for known confounders and the increase in arterial pressure during rebreathing. Each 5% decrease in SaO2 during sleep predicted a decrease in cerebrovascular reactivity of 0.4 ± 0.2 cm/second/mm Hg PETCO2. In contrast, the negative correlation between cerebrovascular CO2 responsiveness and apnea–hypopnea index was statistically significant only in the unadjusted analysis.
Conclusions: Hypercapnic vasodilation in the cerebral circulation is blunted in individuals with sleep-disordered breathing. This impairment is correlated with hypoxemia during sleep.
Keywords: sleep apnea syndromes, cerebrovascular circulation, blood flow velocity, hypercapnia, endothelial function
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Moderate to severe obstructive sleep apnea is characterized by impaired endothelium-dependent vasodilation in the cerebral circulation.
What This Study Adds to the Field
This study demonstrates the presence of a similar impairment across a wider spectrum of sleep-disordered breathing in the general adult population.
Individuals with obstructive sleep apnea (OSA) syndrome are at increased risk for cardiovascular disease, including hypertension and stroke (1–3); however, the underlying mechanisms remain obscure. Putative links between OSA and stroke include oxidative stress (4), coagulopathies (5–7), thromboembolism associated with atrial fibrillation (8) and patent foramen ovale (9), and carotid atherosclerosis caused by snoring-related vibration (10). Structural abnormalities involving extracranial cerebral arteries (e.g., increased carotid intima–media thickness and augmented carotid–femoral pulse wave velocity) have been documented in patients with moderate to severe OSA (11–13). In addition, functional responses of intracranial cerebral arteries to CO2, a powerful vasodilator in the cerebral circulation, are greatly attenuated (14–16).
The clinical significance of blunted hypercapnic vasodilation is unknown; however, previous investigators have observed an association between cerebral CO2 reactivity and endothelial function in the forearm (17), a biomarker known to be a strong predictor of cardiovascular disease risk (18–20). In addition, impaired vascular responsiveness to CO2 may exacerbate breathing instability during sleep. Because hypercapnic vasodilation minimizes changes in brain Pco2 during fluctuations in arterial Pco2, reductions in vascular reactivity could exaggerate the accumulation and also the washout of CO2 from central chemoreceptors during fluctuations in ventilation. Thus, impaired vascular response to hypercapnia is a potential contributor to both the causes and the consequences of sleep-disordered breathing (SDB).
Current knowledge of the effects of SDB on cerebrovascular function is derived from clinic-based samples of individuals with relatively severe SDB. Similar impairments have not been documented across the spectrum of SDB severity, even though diminished endothelial function in the forearm has been demonstrated in epidemiologic studies (21, 22). Therefore, we sought to test the hypothesis that hypercapnic vasodilation in the cerebral circulation, an endothelium-dependent process, is blunted in individuals with SDB. Accordingly, we examined the relationship between SDB and cerebrovascular responsiveness to hypercapnia in a population-based cohort free of clinical selection biases. Some of the results of this study have been previously reported in abstract form (23).
METHODS
Subjects
Detailed sampling methods and characteristics of the Wisconsin Sleep Cohort Study have been described previously (24). Briefly, over the past 20 years, a random sample of 1,550 males and females was recruited from a sampling frame of payroll records of several Wisconsin state agencies to undergo polysomnographic examinations at 4-year intervals. Four hundred and twenty male and female participants of the Wisconsin Sleep Cohort Study who were scheduled to undergo follow-up evaluations during the years 2004–2008 were recruited to also participate in the present ancillary investigation of cerebrovascular function. Potential subjects were screened for current, serious medical problems via a self-reported history questionnaire that was followed-up by a physical examination, if necessary. In this manner, physician clearance was obtained for all subjects before participation. Twenty-one potential participants were deemed not healthy enough to undergo the rebreathing test, two declined participation after receiving an explanation of procedures, in seven instances there were equipment failures, and in 17 subjects we were unable to obtain an adequate Doppler signal. Characteristics of the remaining 373 subjects are shown in Table 1. The parent study and this subprotocol were approved by the University of Wisconsin-Madison's Health Sciences Institutional Review Board.
TABLE 1.
DESCRIPTION OF SAMPLE (N = 373) BY SLEEP-DISORDERED BREATHING CATEGORY
| Apnea–Hypopnea Index Category |
|||||||
|---|---|---|---|---|---|---|---|
| Total (n = 373) | <1 (n = 112) | 1–4.9 (n = 100) | 5–14.9 (n = 94) | 15–29.9 (n = 25) | ≥30 (n = 13) | CPAP Users (n = 29) | |
| Continuous variables, mean (SD) | |||||||
| Age, yr | 60 (8) | 58 (8) | 61 (7) | 61 (8) | 63 (8) | 58 (7) | 59 (8) |
| Body mass index, kg/m2 | 31 (6.9) | 28 (4.7) | 30 (6.4) | 33 (6) | 34 (7) | 36 (8.7) | 40 (6.9) |
| Waist:hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 1 (0.1) | 0.9 (0.1) | 1 (0.1) | 1 (0.1) |
| Alcoholic drinks, number per week | 3.7 (4.7) | 3.9 (4.6) | 3.7 (4.3) | 3.8 (5.3) | 4.2 (5.6) | 2.6 (4.2) | 2.6 (4.4) |
| Sleep SaO2, % | 94.9 (2) | 95.8 (1.3) | 95.2 (1.5) | 94.7 (1.6) | 93.8 (1.5) | 92.2 (3.3) | 93.5 (3.2) |
| Insulin sensitivity, HOMA model | 3.6 (4.5) | 2.3 (1.6) | 3.1 (2.6) | 4.1 (4.1) | 4.7 (5.6) | 7.6 (11.6) | 6.6 (7.8) |
| Binary variables, n (%) | |||||||
| Current smoking | 35 (9) | 12 (11) | 13 (13) | 4 (4) | 1 (4) | 3 (23) | 2 (7) |
| Sex, male | 216 (58) | 56 (50) | 51 (51) | 67 (71) | 14 (56) | 9 (69) | 20 (70) |
| Sleepiness, Epworth >10* | 103 (31) | 27 (25) | 31 (32) | 35 (38) | 4 (16) | 6 (50) | 15 (52) |
| Cardiovascular disease† | 40 (11) | 10 (29) | 9 (9) | 10 (11) | 4 (16) | 3 (23) | 4 (14) |
| Hypertension | 187 (50) | 46 (41) | 42 (42) | 55 (59) | 16 (64) | 7 (54) | 22 (76) |
| Diabetes† | 38 (10) | 6 (5) | 7 (7) | 14 (15) | 3 (12) | 0 (0) | 8 (28) |
Definition of abbreviations: CPAP = continuous positive airway pressure; HOMA = homeostatic model assessment.
Epworth data not available in 10 participants.
Obtained by participant self-report.
Measurements
Study participants completed overnight studies that included nocturnal polysomnography and other clinical tests. Information on medical history, current medication use, smoking, alcohol use, age, and other sociodemographic factors was obtained by interview and questionnaire. Body habitus measurements were made using standard procedures (25). Body mass index (BMI) was calculated from measured weight and height (kilogram per square meter). Insulin sensitivity was estimated using homeostatic model assessment (26). Daytime sleepiness was measured by the Epworth Scale (27). Blood pressure was measured with arm cuff sphygmomanometry in the seated position according to established guidelines (28). The average of two consecutive blood pressure measurements was computed for each subject.
Sleep-disordered breathing.
An 18-channel polysomnography recording system (Polygraph model 78, Grass Instruments, Quincy, MA) was used to record sleep stage, and respiratory and cardiovascular variables. Electroencephalography, electrooculography, and chin electromyography were used to score sleep stage for each 30-second epoch using standard criteria (29). Arterial oxyhemoglobin saturation (SaO2) was measured by pulse oximetry (Ohmeda 3740, Englewood, CO). Oral and nasal airflow were measured using thermocouples (ProTec, Hendersonville, TN). Nasal air pressure was measured with a pressure transducer (Validyne, Northridge, CA). Thoracic cage and abdominal respiratory motion was measured with inductance plethysmography (Respitrace, Ambulatory Monitoring, Ardsley, NY). These signals were used to identify SDB events. Apnea was defined as cessation of airflow lasting greater than or equal to 10 seconds. Hypopnea was defined as a decrease in tidal volume (plethysmograph signal) accompanied by a greater than or equal to 4% reduction in SaO2. The apnea-hypopnea index (AHI) was defined as the average number of apneas plus hypopneas per hour of objectively measured sleep. To compute mean SaO2 during sleep, the pulse oximeter signal was sampled at 100 Hz and an average SaO2 derived for each 30-second epoch. The average SaO2 during all epochs of non-REM and REM sleep was recorded as the mean SaO2 during sleep.
Medications.
Two hundred and four participants were chronically treated with medications that could affect cerebrovascular CO2 sensitivity, either by interfering with mechanisms of hypercapnic vasodilation or by affecting baseline cerebrovascular reactivity. The categories and number of participants who took these medications were as follows: antihypertensives (n = 123); statins (n = 76); estrogen (n = 9); allopurinol (n = 10); and nonsteroidal antiinflammatory agents (n = 131).
Cerebrovascular reactivity protocol.
All subjects were studied during wakefulness in a semirecumbent position in the same room (ambient temperature, 24 ± 1°C.) at the same time of day (between 13:00 and 14:30 hours). A 2-MHz pulsed Doppler ultrasound system (Neurovision 500 M, Multigon Industries, Younkers, NY) was used to measure blood flow velocity in the proximal (M1) segment of the middle cerebral artery. We used previously published search techniques (30) to insonate the artery through the right temporal window in most subjects. In a small fraction of subjects, we used the left temporal window when an adequate signal could not be obtained on the right side. After the quality of the signal was maximized, the Doppler probe was secured using a headband device to provide a fixed angle of insonation. Heart rate was measured from the electrocardiogram. Beat-by-beat arterial pressure was measured by photoelectric plethysmography (Finapres, Ohmeda, Louisville, CO). Ventilation was measured via a mouthpiece and pneumotachograph (Model 5719, Hans Rudolph, Kansas City, MO). End-tidal O2 tension and end-tidal CO2 tension (PETCO2) were sampled from the mouthpiece and measured with infrared gas analyzers (Models CD3A and S-3A/I, Ametek, Pittsburgh, PA). Each of the physiologic signals was routed to a signal conditioner/amplifier module and a physiologic chart recorder (TA-4000, Gould, Cleveland, OH), and was digitized and stored on a personal computer (sampling rate, 120 Hz) for off-line analysis using custom-written software.
We used a modified Read rebreathing test (31) to assess cerebrovascular responses to hyercapnia. A 6-L anesthesia bag was attached by means of a two-way valve to a mouthpiece. Before initiation of the rebreathing test, this bag was filled with a volume of air equal to the subject's predicted vital capacity plus 1 L that had a gas composition of 3% CO2 and 40% O2. Predicted vital capacity was calculated using the following equations (32):
Males: 0.052 · height (cm) − 0.022 · age −3.6
Females: 0.047 · height (cm) − 0.029 · age −2.9.
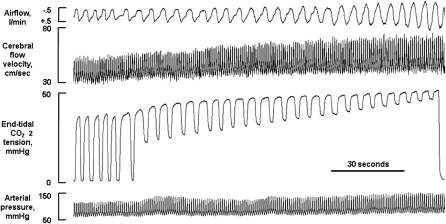
The test began with a baseline period of room air breathing through the mouthpiece with the nose occluded in which at least 4 minutes of stable normoxic, normocapnic breathing were recorded. After the baseline recordings were complete, the valve was turned to allow rebreathing from the bag. The PETCO2 level of the first breath from the bag was noted, and rebreathing continued until PETCO2 reached +10 mm Hg above this level. In most subjects, this level was reached within 2.5 minutes. Then, the valve was opened to room air, and after the first room air breath, the mouthpiece and noseclip were removed. Typical responses to this rebreathing test are shown in Figure 1.
Figure 1.
Physiologic record showing typical cardiovascular and respiratory responses to the rebreathing test.
Calculation of Outcome Variables
All signals were analyzed using custom-written software. Cerebral flow velocity for each cardiac cycle was determined by integrating the Doppler signal and dividing by the length of the cycle (i.e., velocity-time integral). Mean arterial pressure (MAP) was calculated as one third pulse pressure plus diastolic pressure. Within-breath averages were computed for velocity-time integral, heart rate, and MAP. To quantify vascular responsiveness to CO2, we performed breath-by-breath linear regression analysis of velocity-time integral versus PETCO2. The slope of this relationship was used to make between-subject comparisons of cerebrovascular CO2 responsiveness (CCR) (centimeters per seconds per millimeters of mercury PETCO2). The day-to-day reliability of these measurements, assessed in nine healthy subjects, was good (intraclass correlation coefficient = 0.80). Linear regression analysis was also used to determine the change, per millimeter of mercury PETCO2, in secondary outcome measures of MAP, heart rate, and ventilation during rebreathing. Quantification of physiologic variables associated with the rebreathing test was performed by one of two study personnel. To assess the reliability of our analysis procedures, we performed duplicate quantifications for every 15th subject tested. This examination revealed excellent repeatability (intraclass correlation coefficients >0.99).
Statistical Analysis
The distributions of all variables assessed in the Cerebrovascular Reactivity Protocol were approximately normal. We performed linear regression analyses with CCR as the primary outcome variable. The MAP, heart rate, and ventilatory responses during rebreathing were secondary outcome variables. We examined SDB severity as the independent variable, using two different representations. One was the AHI, categorized into severity levels (level 1, AHI <1; level 2, AHI 1–4.9; level 3, AHI 5–14.9; level 4, 15–29.9; and level 5, AHI ≥30). We tested this categorical variable using a linear trend test. We also examined log-transformed AHI (log[AHI+1]) as a continuous variable. The other measure of SDB severity was mean level of SaO2 during sleep, a continuous variable. In this SaO2 analysis, we excluded individuals with lung disease (those reporting a diagnosis of emphysema or use of β agonist, adrenal glucocorticoid, methylxanthine, leukotriene inhibitor, or mast cell stabilizer medications). In all linear regression analyses, we excluded subjects who were current users of nasal continuous positive airway pressure (CPAP) (n = 29) because their polysomnographic data are not accurate reflections of the amount of SDB experienced on a nightly basis.
Initially, we examined unadjusted relationships. Secondarily, we adjusted for age; sex; BMI; waist-to-hip ratio; current smoking; alcohol use (drinks per week); and the presence of diabetes. We adjusted for these potential confounders because of their known influence on SDB and endothelial function (33–38). In addition, for CCR, we also examined models that included an adjustment for the increase in MAP during rebreathing. It was necessary to adjust for MAP because it is an indicator of cerebral perfusion pressure, an important determinant of cerebral blood flow (39). Finally, we also examined two-way interactions between SDB:CCR relationships and age, sex, and excessive daytime sleepiness.
We used β coefficients from the model and population means for the confounding variables to estimate least square means of our outcomes for the AHI severity level categories. We used the β coefficient to estimate the effect of log AHI and mean level of oxygen saturation. Chi-square t tests were used to assess statistical significance and P values less than 0.05 were considered statistically significant for main effects. For interaction terms, we used a more conservative α level of P less than 0.01. SAS software (version 9.1.3, SAS Institute, Carey, NC) was used for all analysis.
RESULTS
Seventeen of 390 participants (5 males and 12 females) were excluded from analysis because of inability to obtain an adequate Doppler signal. These participants tended to have higher BMI, but did not differ from the remaining subjects in terms of AHI, presence of diabetes, or history of cardiovascular disease (data not shown).
Baseline Cardiovascular and Respiratory Variables
Summary data for cardiovascular and respiratory variables measured before the rebreathing test are shown in Table 2. Minute ventilation was somewhat elevated in patients in the highest AHI category and in CPAP users relative to other categories; however, PETCO2 was comparable in all groups.
TABLE 2.
BASELINE VALUES FOR PHYSIOLOGIC VARIABLES BY SLEEP-DISORDERED BREATHING CATEGORY*
| Apnea–Hypopnea Index Category |
|||||||
|---|---|---|---|---|---|---|---|
| Total (n = 373) | <1 (n = 112) | 1–4.9 (n = 100) | 5–14.9 (n = 94) | 15–29.9 (n = 25) | ≥30 (n = 13) | CPAP Users (n = 29) | |
| PETCO2, mm Hg | 40 (4) | 40 (4) | 40 (3) | 40 (3) | 40 (3) | 40 (7) | 39 (3) |
| Ventilation, L/min | 8.3 (2.1) | 7.7 (1.9) | 7.8 (1.6) | 8.7 (2) | 8.4 (2) | 10.6 (3.2) | 9.8 (2.5) |
| Heart rate, beats/min | 68 (11) | 68 (11) | 68 (11) | 67 (10) | 72 (9) | 74 (12) | 69 (13) |
| Systolic pressure, mm Hg | 122 (14) | 119 (14) | 122 (13) | 125 (14) | 127 (10) | 125 (11) | 127 (15) |
| Diastolic pressure, mm Hg | 78 (9) | 76 (9) | 78 (7) | 78 (10) | 80 (11) | 80 (8) | 78 (8) |
Definition of abbreviations: CPAP = continuous positive airway pressure; PETCO2, end-tidal CO2 tension.
Values shown are means (SD).
Cardiovascular and Respiratory Variables During Rebreathing
The rebreathing protocol was generally well-tolerated. We did not observe signs of hemodynamic instability in any participant. Transient breathlessness was the most commonly reported symptom. The rebreathing test elicited, on average, increases in heart rate of one beat per minute, MAP of 12 mm Hg, and minute ventilation of 17 liters per minute. Summary data for these variables and CCR are shown in Table 3.
TABLE 3.
CARDIOVASCULAR AND RESPIRATORY VARIABLES MEASURED DURING REBREATHING BY SLEEP-DISORDERED BREATHING CATEGORY*
| Apnea–Hypopnea Index Category |
|||||||
|---|---|---|---|---|---|---|---|
| Total (n = 373) | <1 (n = 112) | 1–4.9 (n = 100) | 5–14.9 (n = 94) | 15–29.9 (n = 25) | ≥30 (n = 13) | CPAP users (n = 29) | |
| CCR, cm/s/mm Hg | 2.3 (1) | 2.4 (0.9) | 2.4 (1.1) | 2.1 (0.9) | 2.3 (0.8) | 2 (0.8) | 2.3 (1) |
| Change in MAP, mm Hg/mm Hg | 1.2 (1) | 1.3 (1) | 1.2 (1) | 1.1 (1) | 1.2 (0.7) | 0.8 (0.8) | 1 (1) |
| Change in HR, beats/min/mm Hg | 0.1 (0.6) | 0.2 (0.6) | 0.2 (0.5) | 0.1 (0.6) | 0.1 (0.4) | −0.2 (0.5) | −0.0 (0.5) |
| HCVR, L/min/mm Hg | 1.7 (1.1) | 1.7 (1) | 1.6 (1) | 1.8 (1) | 1.3 (0.9) | 1.2 (1) | 2 (1.9) |
Definition of abbreviations: CCR = cerebrovascular CO2 responsiveness; CPAP = continuous positive airway pressure; HCVR = hypercapnic ventilatory response; HR = heart rate; MAP = mean arterial pressure.
Values shown are means (SD).
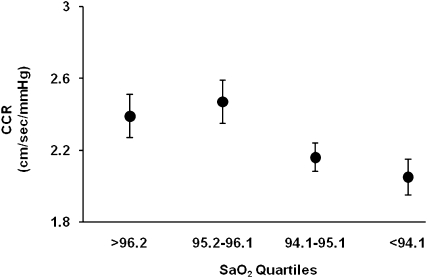
We observed a significant positive correlation between CCR and mean SaO2 during sleep (Table 4 and Figure 2). This relationship was evident in the unadjusted analysis (P = 0.013) and it persisted after adjustments for age; sex; BMI; alcohol consumption; smoking; diabetes; waist-to-hip ratio; and MAP increase during rebreathing (P = 0.014). The β coefficient associated with the model indicates that for each 5% decrease in mean SaO2 during sleep, CCR was reduced by 0.4 cm per second per mm Hg (40% of the population SD for CCR). No two-way interactions were observed between the SaO2–CCR relationship and age, sex, or sleepiness. Adjustment of the statistical model for several categories of medications that are putative confounders of the SaO2–CCR relationship (antihypertensive agents, statins, estrogen, allopurinol, or cyclooxygenase inhibitors) did not affect β coefficients or P values, nor did further adjustment for insulin sensitivity (data not shown). No relationships were observed between mean SaO2 during sleep and increase in MAP during rebreathing (P = 0.782); change in heart rate during rebreathing (P = 0.995); or hypercapnic ventilatory response (P = 0.497).
TABLE 4.
BETA COEFFICIENTS AND P VALUES FOR UNADJUSTED AND ADJUSTED PREDICTION MODELS BASED ON CORRELATIONS BETWEEN MEAN SLEEP SaO2 AND THE PRIMARY OUTCOME MEASURE
| Outcome Measure: Cerebrovascular CO2 Reactivity (cm/s/mm Hg PETCO2) |
||||||
|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||
| Unadjusted | Adjusted for age, sex, body mass index, waist:hip ratio, current smoking, drinks per week, diabetes | Adjusted (Model 2 adjustments + increase in MAP) | ||||
| Mean sleep SaO2 | Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value |
| 0.08 (0.03) | 0.013 | 0.08 (0.04) | 0.019 | 0.09 (0.04) | 0.014 | |
Figure 2.
Cerebrovascular CO2 reactivity (CCR) plotted as a function of SaO2 during sleep. More severe sleep-disordered breathing, as indicated by lower mean sleep SaO2, was associated with reduced CCR. Values shown are means ± SE.
We also evaluated the association between CCR and AHI (Table 5). When AHI category was used as a trend variable, the association was nearly significant in the unadjusted analysis (P = 0.050); however, when alcohol consumption, smoking, diabetes, and waist-to-hip ratio were added to the model, the correlation was not significant (P = 0.111). The association between CCR and AHI category was also nonsignificant when MAP increase during rebreathing was added to the model (P = 0.179). Qualitatively similar results were found when using continuous AHI (log[AHI+1]) instead of categorical AHI. The unadjusted coefficient (SE) for CCR regressed on log(AHI+1) was −0.10 (0.05) (P = 0.033); with adjustment for age, sex, BMI, waist-to-hip ratio, current smoking, diabetes diagnosis, and alcohol consumption, the coefficient (SE) for log(AHI+1) was −0.10 (0.05) (P = 0.073). When the change in MAP during rebreathing was added to the model, the coefficient (SE) for log(AHI+1) was −0.08 (0.05) (P = 0.123). Further adjustment for insulin sensitivity did not affect β coefficients or P values; thus, we did not include insulin sensitivity in the final analysis. No two-way interactions were observed between the AHI–CCR relationship and age, sex, or sleepiness.
TABLE 5.
MEAN VALUES (SE) FOR CEREBROVASCULAR CO2 REACTIVITY IN THE FIVE SLEEP-DISORDERED BREATHING CATEGORIES
| Outcome Measure: Cerebrovascular CO2 Reactivity (cm/s/mm Hg PETCO2) |
|||
|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
|
| AHI | Unadjusted | Adjusted for Age, Sex, Body Mass Index, Waist:Hip Ratio, Current Smoking, Drinks Per Week, Diabetes | Adjusted (Model 2 adjustments + MAP Slope) |
| 1: AHI <1 | 2.39 (0.09) | 2.39 (0.10) | 2.37 (0.10) |
| 2: AHI 1 to <5 | 2.36 (0.10) | 2.34 (0.10) | 2.35 (0.10) |
| 3: AHI 5 to 14.9 | 2.10 (0.10) | 2.14 (0.11) | 2.15 (0.10) |
| 4: AHI 15 to 29.9 | 2.34 (0.19) | 2.29 (0.19) | 2.30 (0.19) |
| 5: AHI ≥ 30 | 1.96 (0.27) | 2.02 (0.27) | 2.07 (0.27) |
| P value for trend | 0.050 | 0.111 | 0.179 |
Definition of abbreviations: AHI = apnea–hypopnea index; MAP = mean arterial pressure.
We observed an inverse relationship between AHI category and increase in MAP during rebreathing that was of borderline significance (P = 0.079). In contrast, there was no significant relationship between AHI and change in heart rate during rebreathing (P = 0.208) or hypercapnic ventilatory response (P = 0.182).
Although we did not include data from current CPAP users in our statistical models, we did assess cerebrovascular reactivity in these individuals. In current CPAP users, the mean value for CCR (2.3 ± 0.2) was lower than in individuals with AHI less than five but higher than in those with AHI greater than 30 (Table 3).
DISCUSSION
The major finding of this study is that hypercapnic vasodilation in the cerebral circulation, an exquisitely sensitive physiologic mechanism responsible for minimizing changes in brain tissue Pco2 during fluctuations in arterial CO2, is diminished, in graded fashion, across the continuum of mild to severe SDB. We observed a significant positive correlation between the mean level of SaO2 during sleep and cerebrovascular CO2 reactivity. In contrast, AHI, regardless of whether it was represented as a categorical or continuous variable, was not statistically significantly associated with cerebrovascular CO2 reactivity. Although we cannot infer causation from our observational data, we interpret these findings as indirect evidence that nocturnal hypoxemia contributes importantly to impaired cerebrovascular function in individuals with SDB.
This conclusion is predicated on the assumption that reductions in SaO2 during sleep were caused by SDB. Alternatively, SaO2 may have been reduced secondary to lung disease. However, we believe this possibility was minimized because participants with a history of lung disease and those taking medications used to treat lung disease were excluded from this analysis. Also, our assumption that the reduction in CCR we observed in participants in the lower quartiles of nocturnal SaO2 represents impaired hypercapnic vasodilation is valid only if the rebreathing test evoked comparable increases in MAP across all subject groups. This is a reasonable assumption because there was no correlation between nocturnal SaO2 and MAP during rebreathing and because the SaO2–CCR relationship was statistically significant both before and after adjustment for the increase in MAP. In contrast, the AHI–CCR relationship was confounded by between-group differences in MAP: a negative correlation of borderline significance was observed between AHI and MAP during rebreathing. Thus, it is not possible to discern whether CCR was lower in participants with higher AHI because of blunted hypercapnic vasodilation or whether CCR was lower secondary to smaller increases in cerebral perfusion pressure during rebreathing. The finding that the AHI–CCR relationship became statistically nonsignificant after adjustment for the increase in MAP during rebreathing points to the latter possibility. Our data are consistent in demonstrating that, across a wide spectrum of SDB, the mean level of SaO2 during sleep better predicts the blunting of CO2 reactivity than does the frequency of events (i.e., AHI).
In contrast to the significant negative correlation between SDB and CCR in our subjects, ventilatory responses during rebreathing did not vary according to SaO2 or AHI category. In one previous study, enhanced ventilatory responses to CO2 were reported in OSA patients versus control subjects (40); however, several other studies have found no between-group differences (16, 41, 42). We speculate that much of the variability in ventilatory responses to CO2 is attributable to performance of these studies during wakefulness, when nonchemoreceptor, behavioral inputs have a substantial influence on respiratory output.
The present findings in a population-based sample free of clinical selection biases are consistent with our previous observations of diminished CCR in patients with moderate to severe OSA (16). The magnitude of CCR decrement in the present subjects who fell within the lowest versus the highest quartiles of nocturnal SaO2 values was similar to that observed in our clinic-based sample (−17 versus −22%). Because the average CCR in CPAP users was enhanced relative to participants with untreated SDB and AHI greater than 30, the present findings suggest that SDB-related impairment in cerebrovascular function is reversible, at least in part, with treatment. This finding also agrees with our previous observations of patients with moderate to severe OSA (16). Nevertheless, because the average CCR in CPAP users was still somewhat diminished relative to that of participants with no SDB (AHI <5), the present data also suggest that CPAP does not provide full protection against this vascular consequence of SDB. A potential reason is that many individuals are not fully compliant with this treatment. Patients who are considered “CPAP compliant” use the device for as few as 4 hours on most nights (43–45). Thus, many of them remain exposed, for varying amounts of time, to the adverse effects of SDB and resultant intermittent hypoxemia.
The present findings also parallel two previous population-based studies that used flow-mediated dilation in the forearm to assess vascular function (21, 22). All three studies found associations between vascular dysfunction and SDB severity. Consistent with the present findings, one previous study found that nocturnal SaO2 was a more important predictor of vascular function than was AHI (21). The other previous study observed that SDB was associated with impaired vascular function in females, but not males (22). In the present study, the interaction between sex and SDB-associated vascular dysfunction was not statistically significant. Interestingly, a significant positive correlation was observed between vascular reactivity and alcohol consumption in one previous report (22). A similar correlation was not present in our study.
Mechanisms of SDB-induced Impairment in Cerebrovascular Reactivity
Hypercapnic vasodilation in the brain is a complex, endothelium-dependent process: nitric oxide, prostacyclin, and cytochrome P-450 metabolites have all been implicated (46–48). Endothelium-dependent dilation in the forearm is blunted in individuals with SDB (21, 49–51). The causes of this impairment are not well understood; however, oxidative stress (i.e., imbalance between production of reactive oxygen species and antioxidant defenses) and inflammation are putative contributors. Both processes have been observed in patients with OSA (4), are accompanied by decreased expression of endothelial nitric oxide synthase and increased expression of both nitrotyrosine and inducible nitric oxide synthase in venous endothelial cells (51), and are ameliorated by CPAP treatment (51). In addition, allopurinol treatment has been shown to normalize impaired flow-mediated dilation in patients with OSA (52), which suggests an important role for xanthine oxidase–derived superoxide. Excess superoxide would be expected to reduce the availability of nitric oxide by combining with it to form peroxynitrite. Peroxynitrite, in turn, could further limit nitric oxide via oxidation of tetrahydrobiopterin, a critical cofactor for endothelial nitric oxide synthase (53, 54). Peroxynitrite could also limit prostacyclin production by suppression of prostacyclin synthase (55). We speculate that the pathologic processes that interfere with endothelium-dependent vasodilation in the forearm also contribute to the observed blunting of hypercapnic vasodilation in the cerebral circulation.
Methodologic Considerations
Our conclusions regarding cerebrovascular responses are predicated on the assumption that Doppler measurements of flow velocity are reflective of volume flow, an assumption that is satisfied only when the cross-sectional area of the artery remains constant. We did not measure diameter; however, previous investigators have shown that middle cerebral artery diameter varies by less than or equal to 4% during changes in arterial pressure, CO2 tension (56), or gravitational stress (57). In addition, velocity and volume flow through the middle cerebral artery are highly correlated (58). Also, one of our measures of SDB severity, AHI, has limited reliability, especially over a single night of observation. Nevertheless, with 373 subjects, we believe our study is adequately powered considering the known night-to-night variability (59).
In the absence of SaO2 measurements during wakefulness and concurrent measurements of pulmonary function, individuals with lung disease were identified based on self-reported medical history and use of medications used to treat lung disease. We recognize that this imprecise method is a limitation of our study.
Finally, in this cross-sectional study, we cannot uncover the temporal nature of the relationship between SDB and blunted CCR. On one hand, SDB could cause impairment in reactivity via intermittent hypoxemia and attendant insults to vascular structure and function. However, diminished cerebrovascular CO2 reactivity could cause or exacerbate SDB. Answers to these questions await longitudinal observations in population-based studies.
Clinical Significance of the Present Findings
Several previous reports suggest that the observed impairments in CCR may be clinically relevant. In patients with essential hypertension and diabetes mellitus, impaired CCR was correlated with endothelial dysfunction in the forearm (17), a strong predictor of cardiovascular disease risk (18, 20). Because hypercapnic vasodilation in the cerebral circulation is evoked by substances produced in endothelial cells (46–48), we believe that CCR is a proxy measure of endothelial function and therefore may also be a predictor of cardiovascular risk. Diminished cerebrovascular responsiveness to CO2 has been observed in patients with ischemic stroke (60) and those with multiple subcortical infarctions (61). Therefore, this functional impairment may play a pathogenetic role in cerebrovascular disease. Further, in patients with congestive heart failure, CCR was correlated with ejection fraction, prompting the speculation that depressed cerebrovascular reactivity may be responsible for cognitive impairments in patients with severe ventricular dysfunction (62). We have shown that CCR is reduced in patients with heart failure and central sleep apnea relative to patients with similar cardiac dysfunction but without central sleep apnea (63). Because CO2 reactivity in the cerebral circulation minimizes changes in brain Pco2 during fluctuations in arterial Pco2, the observed compromise in cerebrovascular regulation may disturb breathing stability during sleep. Reductions in CCR could exacerbate breathing instability during sleep by exaggerating the accumulation and also the washout of CO2 from central chemoreceptors during fluctuations in ventilation and arterial Pco2.
Acknowledgments
The authors are grateful to their colleague, the late James B. Skatrud, M.D., for his invaluable contributions to study design. We also appreciate the assistance of the following individuals: Dominic S. Puleo, Ailiang Xie, M.D., Ph.D., K. Mae Hla, M.D., Maryan Stubbs, Diane Austin, Amanda Rasmuson, Kathryn Pluff, Katherine Stanback, and Linda Evans.
Supported by National Institutes of Health grants R01 HL075035, R01 HL062252, R01 AG14124, and T32 HL07654 and by 1UL1RR025011 from the Clinical and Translational Science Award Program of the National Center for Research Resources, National Institutes of Health. This work was also supported by the Office of Research and Development, Clinical Science R&D Service, Department of Veterans Affairs.
Originally Published in Press as DOI: 10.1164/rccm.201002-0313OC on July 16, 2010
Author Disclosure: B.J.M. received $1,001–$5,000 from Respironics in consultancy fees and $50,001–$100,000 from the National Heart, Lung, and Blood Institute in sponsored grants. K.J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.E.P. received more than $100,001 from the National Institutes of Health in sponsored grants as an investigator with salary support on multiple National Institutes of Health awards. L.F. received up to $1,000 from Respironics for statistical consulting. S.R.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 2.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol 2008;52:686–717. [DOI] [PubMed] [Google Scholar]
- 4.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J 2009;33:1467–1484. [DOI] [PubMed] [Google Scholar]
- 5.Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J 2000;16:648–652. [DOI] [PubMed] [Google Scholar]
- 6.von Kanel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: treatment effects of nasal continuous positive airway pressure. Sleep Breath 2006;10:139–146. [DOI] [PubMed] [Google Scholar]
- 7.Peled N, Kassirer M, Kramer MR, Rogowski O, Shlomi D, Fox B, Berliner AS, Shitrit D. Increased erythrocyte adhesiveness and aggregation in obstructive sleep apnea syndrome. Thromb Res 2007;121:631–636. [DOI] [PubMed] [Google Scholar]
- 8.Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589–2594. [DOI] [PubMed] [Google Scholar]
- 9.Beelke M, Angeli S, Del Sette M, De Carli F, Canovaro P, Nobili L, Ferrillo F. Obstructive sleep apnea can be provocative for right-to-left shunting through a patent foramen ovale. Sleep 2002;25:856–862. [PubMed] [Google Scholar]
- 10.Lee SA, Amis TC, Byth K, Larcos G, Kairaitis K, Robinson TD, Wheatley JR. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep 2008;31:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 11.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172:625–630. [DOI] [PubMed] [Google Scholar]
- 12.Tanriverdi H, Evrengul H, Kara CO, Kuru O, Tanriverdi S, Ozkurt S, Kaftan A, Kilic M. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration 2006;73:741–750. [DOI] [PubMed] [Google Scholar]
- 13.Protogerou AD, Laaban JP, Czernichow S, Kostopoulos C, Lekakis J, Safar ME, Blacher J. Structural and functional arterial properties in patients with obstructive sleep apnoea syndrome and cardiovascular comorbidities. J Hum Hypertens 2008;22:415–422. [DOI] [PubMed] [Google Scholar]
- 14.Loeppky JA, Miranda FG, Eldridge MW. Abnormal cerebrovascular responses to CO2 in sleep apnea patients. Sleep 1984;7:97–109. [DOI] [PubMed] [Google Scholar]
- 15.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology 1998;51:1051–1056. [DOI] [PubMed] [Google Scholar]
- 16.Reichmuth KJ, Dopp JM, Barczi SR, Skatrud JB, Wojdyla P, Hayes D Jr, Morgan BJ. Impaired vascular regulation in patients with obstructive sleep apnea: effects of continuous positive airway pressure treatment. Am J Respir Crit Care Med 2009;180:1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol 2006;291:H1856–H1861. [DOI] [PubMed] [Google Scholar]
- 18.Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 19.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 1991;84:1589–1596. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima Y, Takase B, Uehata A, Kawano H, Yano K, Ohsuzu F, Ishihara M, Kurita A. Comparative predictive and diagnostic value of flow-mediated vasodilation in the brachial artery and intima media thickness of the carotid artery for assessment of coronary artery disease severity. Int J Cardiol 2007;117:165–172. [DOI] [PubMed] [Google Scholar]
- 21.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med 2004;169:354–360. [DOI] [PubMed] [Google Scholar]
- 22.Faulx MD, Larkin EK, Hoit BD, Aylor JE, Wright AT, Redline S. Sex influences endothelial function in sleep-disordered breathing. Sleep 2004;27:1113–1120. [DOI] [PubMed] [Google Scholar]
- 23.Reichmuth K, Austin D, Peppard P, Nieto J, Young T, Barczi S, Skatrud J, Morgan B. Sleep disordered breathing and cerebral vasoreactivity to CO2. Sleep 2007;30:A157. [Google Scholar]
- 24.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 25.Lohman TG, Roche A, Martorel R. Measurement description and techniques. In: Lohman TG, Roche AF, Martorell R, eds. Anthropomentric standardization reference manual. Champaign, IL: Human Kinetics Publishers; 1988. pp. 1–55.
- 26.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992;15:376–381. [DOI] [PubMed] [Google Scholar]
- 28.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: National Institutes of Health; 1968.
- 30.Otis SM, Ringelstein EB. The transcranial Doppler examination: principles and applications of transcranial Doppler sonography. In: Tegeler CH, Babikian VL, Gomez CR, editors. Neurosonology. St. Louis, MO; Mosby; 1996. pp. 113–128.
- 31.Read DJ. A clinical method for assessing the ventilatory response to carbon dioxide. Australas Ann Med 1967;16:20–32. [DOI] [PubMed] [Google Scholar]
- 32.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 33.Puddey IB, Zilkens RR, Croft KD, Beilin LJ. Alcohol and endothelial function: a brief review. Clin Exp Pharmacol Physiol 2001;28:1020–1024. [DOI] [PubMed] [Google Scholar]
- 34.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 2004;286:R233–R249. [DOI] [PubMed] [Google Scholar]
- 35.Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol 2008;35:458–463. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 2009;120:1266–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol 2009;106:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Widlansky ME. Lifestyle choices and endothelial function: risk and relevance. Curr Vasc Pharmacol 2009;7:209–224. [DOI] [PubMed] [Google Scholar]
- 39.Przybylowski T, Bangash MF, Reichmuth K, Morgan BJ, Skatrud JB, Dempsey JA. Mechanisms of the cerebrovascular response to apnoea in humans. J Physiol 2003;548:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Grunstein RR, Teichtahl H. Association between ventilatory response to hypercapnia and obstructive sleep apnea-hypopnea index in asymptomatic subjects. Sleep Breath 2007;11:103–108. [DOI] [PubMed] [Google Scholar]
- 41.Narkiewicz K, van de Borne PJH, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 1999;99:1183–1189. [DOI] [PubMed] [Google Scholar]
- 42.Foster GE, Hanly PJ, Ostrowski M, Poulin MJ. Ventilatory and cerebrovascular responses to hypercapnia in patients with obstructive sleep apnoea: effect of CPAP therapy. Respir Physiol Neurobiol 2009;165:73–81. [DOI] [PubMed] [Google Scholar]
- 43.Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887–895. [DOI] [PubMed] [Google Scholar]
- 44.Pepin JL, Krieger J, Rodenstein D, Cornette A, Sforza E, Delguste P, Deschaux C, Grillier V, Levy P. Effective compliance during the first 3 months of continuous positive airway pressure. A European prospective study of 121 patients. Am J Respir Crit Care Med 1999;160:1124–1129. [DOI] [PubMed] [Google Scholar]
- 45.Lewis KE, Seale L, Bartle IE, Watkins AJ, Ebden P. Early predictors of CPAP use for the treatment of obstructive sleep apnea. Sleep 2004;27:134–138. [DOI] [PubMed] [Google Scholar]
- 46.Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol 1994;266:R546–R552. [DOI] [PubMed] [Google Scholar]
- 47.Pelligrino DA, Santizo RA, Wang Q. Miconazole represses CO(2)-induced pial arteriolar dilation only under selected circumstances. Am J Physiol 1999;277:H1484–H1490. [DOI] [PubMed] [Google Scholar]
- 48.Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res 2001;88:600–608. [DOI] [PubMed] [Google Scholar]
- 49.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation 2000;102:2607–2610. [DOI] [PubMed] [Google Scholar]
- 50.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169:348–353. [DOI] [PubMed] [Google Scholar]
- 51.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J 2006;27:997–1002. [DOI] [PubMed] [Google Scholar]
- 53.Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol 2001;281:H981–H986. [DOI] [PubMed] [Google Scholar]
- 54.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 2000;20:1430–1442. [DOI] [PubMed] [Google Scholar]
- 56.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 1993;32:737–741. [PubMed] [Google Scholar]
- 57.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000;31:1672–1678. [DOI] [PubMed] [Google Scholar]
- 58.Kirkham FJ, Padayachee TS, Parsons S, Seargeant LS, House FR, Gosling RG. Transcranial measurement of blood velocities in the basal cerebral arteries using pulsed Doppler ultrasound: velocity as an index of flow. Ultrasound Med Biol 1986;12:15–21. [DOI] [PubMed] [Google Scholar]
- 59.Levendowski DJ, Zack N, Rao S, Wong K, Gendreau M, Kranzler J, Zavora T, Westbrook PR. Assessment of the test-retest reliability of laboratory polysomnography. Sleep Breath 2009;13:163–167. [DOI] [PubMed] [Google Scholar]
- 60.Maeda H, Matsumoto M, Handa N, Hougaku H, Ogawa S, Itoh T, Tsukamoto Y, Kamada T. Reactivity of cerebral blood flow to carbon dioxide in various types of ischemic cerebrovascular disease: evaluation by the transcranial Doppler method. Stroke 1993;24:670–675. [DOI] [PubMed] [Google Scholar]
- 61.Cupini LM, Diomedi M, Placidi F, Silvestrini M, Giacomini P. Cerebrovascular reactivity and subcortical infarctions. Arch Neurol 2001;58:577–581. [DOI] [PubMed] [Google Scholar]
- 62.Georgiadis D, Sievert M, Cencetti S, Uhlmann F, Krivokuca M, Zierz S, Werdan K. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur Heart J 2000;21:407–413. [DOI] [PubMed] [Google Scholar]
- 63.Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med 2005;172:371–378. [DOI] [PubMed] [Google Scholar]