Abstract
Pediatric practitioners face unique challenges when attempting to translate or adapt adult-derived evidence regarding ventilation practices for acute lung injury or acute respiratory distress syndrome into pediatric practice. Fortunately or unfortunately, there appears to be selective adoption of adult practices for pediatric mechanical ventilation, many of which pose considerable challenges or uncertainty when translated to pediatrics. These differences, combined with heterogeneous management strategies within pediatric critical care, can complicate clinical practice and make designing robust clinical trials in pediatric acute respiratory failure particularly difficult. These issues surround the lack of explicit ventilator protocols in pediatrics, either computer or paper based; differences in modes of conventional ventilation and perceived marked differences in the approach to high-frequency oscillatory ventilation; challenges with patient recruitment; the shortcomings of the definition of acute lung injury and acute respiratory distress syndrome; the more reliable yet still somewhat unpredictable relationship between lung injury severity and outcome; and the reliance on potentially biased surrogate outcome measures, such as ventilator-free days, for all pediatric trials. The purpose of this review is to highlight these challenges, discuss pertinent work that has begun to address them, and propose potential solutions or future investigations that may help facilitate comprehensive trials on pediatric mechanical ventilation and define clinical practice standards.
Keywords: positive pressure respiration, high-frequency ventilation, ventilator weaning, randomized controlled clinical trials
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Although many practices regarding mechanical ventilation of children with acute respiratory failure have been adopted from adult evidence, key differences between children and adults must be considered before adult-based practices are universally accepted in pediatric critical care.
What This Study Adds to the Field
This study reviews key differences between adult and pediatric mechanical ventilation practices for children with respiratory failure and acute lung injury, summarizes current evidence regarding these differences, and proposes a series of investigations to derive pediatric evidence and improve clinical practice.
By the end of the 20th century, pediatric intensivists had learned limited but important insights about mechanical ventilation. There was a philosophical shift in mechanical ventilation from normalizing arterial blood gases at any cost to embracing permissive hypercapnia (and hence the first part of lung-protective ventilation strategies) for the management of acute respiratory distress syndrome (ARDS) in adults (1). At about the same time, high-frequency oscillatory ventilation (HFOV) became a reality in pediatrics, as did further minimizing ventilator-induced lung injury by titrating conventional ventilator support to avoid atelectasis and inflammation by keeping positive end-expiratory pressure (PEEP) above the lower inflection point of the pressure–volume curve (2) and limiting tidal volume (Vt) or pressure to avoid overdistention above the upper inflection point (3). By the end of the century, the concept of breath-by-breath matching of the ventilator to the patient and the potential importance of different mechanical ventilation strategies (volume control vs. pressure control, high vs. low Vts, high vs. low PEEP) had become topics of urgent discussion. It had also become clear that children with ARDS had lower mortality than adults, and that the cause of lung injury affected outcome (e.g., lung-injured children with respiratory syncytial virus had much lower mortality than lung-injured immunosuppressed children).
TABLE 1.
SUMMARY OF CONSIDERATIONS WHEN DESIGNING FUTURE TRIALS ON PEDIATRIC ACUTE LUNG INJURY/ACUTE RESPIRATORY DISTRESS SYNDROME
| Careful consideration of the management of the lung-protective control group, particularly regarding targeted Vt based on lung injury severity |
| Optimization of lung-protective strategies for HFOV, NAVA, VDR, APRV |
| Determine the optimal body weight to target Vt |
| Determine circumstances in which proximal airway measurements are necessary for management |
| Develop explicit computerized protocols that are in line with current pediatric practice to optimize adherence |
| Embrace noninvasive oxygenation criteria for study recruitment |
| Design trials to minimize crossover and the use of rescue therapy |
| Use lung injury severity markers for initial stratification of risk and consider their use as entry criteria for studies |
| Validate measures of quality of life, long-term disability, neurodevelopment, and pulmonary function as outcome measures |
Definition of abbreviations: APRV = airway pressure release ventilation; HFOV = high-frequency oscillatory ventilation; NAVA = neurally adjusted ventilatory assist; VDR = Volume Diffusive Respirator.
Although it remains a catch phrase among pediatricians that “children are not little adults,” it has also become clear that medical care for children is often based on what works in adults. There are many reasons for this, not the least of which is that there are small numbers of pediatric intensive care unit (PICU) patients actually afflicted by any specific life-threatening disease. Hence, pediatric intensivists have selectively adopted practices from adult critical care. The reasons for this selective “cherry picking” are unclear. Interestingly, pediatric intensivists have adopted very few practices from neonatology regarding the management of acute lung injury (ALI) and ARDS, despite the original description of ARDS that noted similarities to infantile respiratory distress syndrome (4). The distinct pathophysiology related to prematurity and infantile respiratory distress syndrome makes extrapolating neonatal evidence particularly difficult. Given these distinctions, a detailed discussion of neonatal evidence for ventilator management is beyond the scope of this article. However, lessons from neonatology, particularly regarding fraction of inspired oxygen titration, may be relevant for pediatric ALI/ARDS.
With respect to ALI/ARDS, outcomes over the past 2 decades have improved for adults managed with lung-protective conventional mechanical ventilation (CMV). Specifically, the ARDS Network Vt study demonstrated that Vts of 6 ml/kg with limited plateau pressures were better than 12 ml/kg predicted body weight for adults with lung injury, using a volume-control (assist-control) mode of ventilation (5). In addition, for adults the application of PEEP for lung recruitment has improved outcomes (6–9), and specific ventilator protocols have helped standardize decision making (10), reduced practice variability (10), and improved outcomes (11, 12).
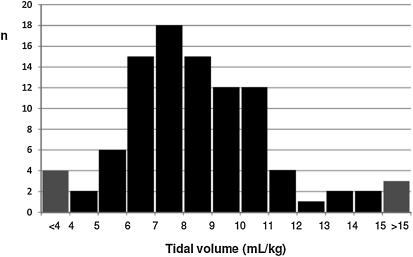
Much less is known about pediatric mechanical ventilation practice in ALI/ARDS, but the recent prospective, cross-sectional, observational Pediatric Acute Lung Injury VEntilation (PALIVE) study (13) highlights European and North American practices. In this point prevalence study, only 165 (4.3%) of 3,823 PICU patients met invasive (14) or noninvasive (15–17) blood gas criteria for ALI or ARDS, consistent with previous estimates (18–20). If conducting an interventional trial, it could be anticipated that only about 60% of these patients would be enrolled (20–23). Pediatric practitioners from 59 PICUs have embraced a “low” Vt (median 7 ml/kg; interquartile range [IQR], 6–9) strategy, although there was significant variability in management, with Vts available on less than half of the patients (Figure 1). Ventilator practices varied, with 44% of patients on pressure-control (PC) and 28% on pressure-regulated volume-control (PRVC) modes of ventilation. Almost 27% reported using the volume-control mode popular in adult ARDS management. The median Vt of 7 ml/kg was based on actual body weight rather than predicted body weight used in the ARDS Network study (5), and the site of measurement was not specified (vide infra). Attempts at creating a PEEP/FiO2 titration grid similar to the ARDS Network model were unsuccessful, as routine pediatric practice demonstrated great variability in the application of PEEP in relation to FiO2.
Figure 1.
Distribution of Vt in ml/kg of actual body weight from 75 patients with acute lung injury/acute respiratory distress syndrome across 59 pediatric intensive care units in Europe and North America. There was significant variability in management, with Vt available on less than half of the 165 patients. Pediatric intensivists embraced a “low” Vt (median, 7 ml/kg; interquartile range, 6–9) strategy. Reprinted by permission from Reference 12.
Although it is likely that future trials and practice for pediatric ALI/ARDS will embrace a “higher PEEP and lower Vt (or peak inspiratory pressure)” strategy, there are many unanswered questions in pediatric ALI, with key differences between adults and children, and unique challenges for pediatric critical care practitioners. These issues surround the lack of explicit ventilator protocols in pediatrics, either computer or paper based; the differences in modes of conventional ventilation; perceived marked differences in the approach to HFOV (24–26); challenges with patient recruitment; the shortcomings of the definition of ALI and ARDS; the more reliable yet still unpredictable relationship between lung injury severity and outcome; and the reliance on potentially biased composite outcome measures such as ventilator-free days (VFD).
KEY CONSIDERATIONS FOR THE DEVELOPMENT OF EXPLICIT VENTILATOR PROTOCOLS FOR PEDIATRIC ALI
Although some management protocols have been developed for pediatric mechanical ventilation (22, 27, 28), they have not been extensively validated, nor have they gained wide acceptance. Most have been translated from the adult-based ARDS Network guidelines for Vt (5, 22) without considering key differences between adult and pediatric practice. Given the variability in modes of ventilation (13, 18) not only between adult and pediatric practice but also within pediatrics, explicit protocols should be developed for different modes of ventilation.
Conventional Modes of Ventilation
Although there is limited evidence to support that one mode of ventilation is superior to another for ALI (29, 30), the ARDS Network volume-control, assist-control mode is infrequently used in pediatrics, with most pediatricians preferring the decelerating flow pattern of PC or PRVC (18). Despite its name, PRVC is volume targeted but still pressure limited. Although the benefits and drawbacks of PC versus PRVC can certainly be debated, there has been no pediatric study showing a benefit of one mode of ventilation over another, provided lung-protective techniques are used. For PC, this means limiting peak inspiratory pressures to 35 or 40 cm H2O and ventilator rates to less than 35/min (31). In PRVC, this means limiting Vts to be lower rather than higher, although no study in pediatrics has determined which Vt is optimal. In fact, even the ARDS Network lung-protective 6 ml/kg volume-control strategy would recommend decreasing Vt below 6 ml/kg if needed to limit plateau pressure to 30 cm H2O.
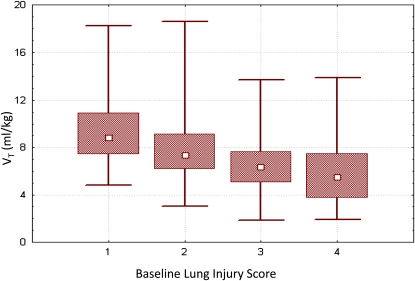
We recently addressed this issue with respect to PC ventilation (31). In a single institution, we demonstrated the association between oxygenation index (OI), pediatric lung injury score (LIS), dynamic compliance of the respiratory system, and PaO2/FiO2 (PF) ratio and mortality, for 398 patients with hypoxemic respiratory failure, of whom 192 met all ALI/ARDS criteria. The pediatric lung injury score (vide infra) is a modification (32) of the Murray lung injury score used in adults (33). All four measurements (OI, LIS, dynamic compliance of the respiratory system, PF ratio) were associated with mortality and the strength of association improved with each subsequent day of mechanical ventilation. There was a trend for higher mortality and fewer VFD with lower Vts throughout the first 3 days of ventilation. Most patients were ventilated with Vts measured at the mechanical ventilator of between 6 and 10 ml/kg actual body weight. Furthermore, patients with more severe lung disease, as measured by the lung injury score, had lower median Vts. In other words, using lung-protective pressure-control ventilation, patients with the sickest lungs received the lowest Vts, and patients with less sick lungs had better outcomes even when mechanically ventilated with Vts as high as 10 ml/kg. Advocates of a pressure-control strategy argue this approach is more physiologic, as the generated Vt will be a function of lung disease severity (Figure 2). In contrast, a “one Vt fits all” approach for ALI has met with previous controversy (34, 35) most notably in the aftermath of the ARDS Network Vt study (36, 37).
Figure 2.
Vt based on lung disease severity, using a lung-protective pressure-control strategy. As lung injury severity increases (as measured by increasing lung injury score), Vt is naturally limited, with patients with the most severe lung injury achieving median Vt just under 6 ml/kg. Data expressed as median, interquartile range, and actual range. Reprinted by permission from Reference 30.
HFOV
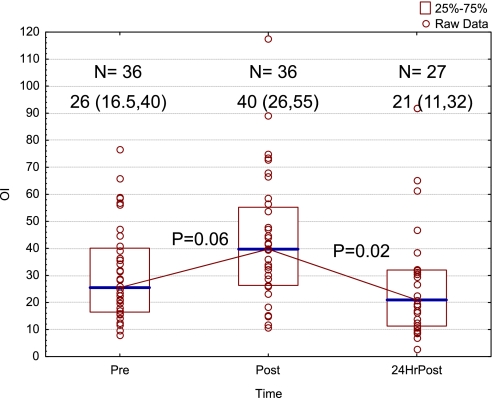
HFOV is used widely for pediatric ALI/ARDS. With the current understanding that excessive lung stretch, repeated opening and closing of distal bronchi and alveoli, and inadequate end-expiratory ventilator volume may be injurious to the lungs, HFOV would appear to be the ideal form of lung-protective ventilation in pediatric patients, and possibly adults, with tiny Vt excursions and high frequency at modest mean airway pressures. Animal studies have suggested that early institution of HFOV could limit ventilator-induced lung injury. Nonetheless, it appears that HFOV has largely become a rescue therapy when conventional management fails (38, 39). A decade ago, a report of 10 pediatric ICUs showed patients were started on HFOV after a mean of 3.3 days of ventilation at a mean OI of 30 (40). Our approach to HFOV appears to have changed little over the past 10 years. In our own institution, for children with severe ARDS or acute hypoxemic respiratory failure (AHRF), HFOV was implemented at a median of 3.5 (IQR, 1.25–7.5) days into the course of mechanical ventilation at a median OI of 26 (IQR, 16.5–40), similar to the multicenter experience. There was an initial increase in OI shortly after HFOV initiation, followed by a decrease in OI 24 hours later to pre-HFOV levels (Figure 3). Similar “rescue” use of HFOV has been reported in adults from the OSCILLATE pilot study (41). There has been only one randomized controlled trial of the efficacy of HFOV against conventional ventilation in children with predominantly ARDS (26) and two in adult ARDS (42, 43). All studies were underpowered to detect differences in important clinical outcomes, and the results were inconclusive. Moreover, the pediatric HFOV study enrolled patients 3 to 6 days into their course of mechanical ventilation, long after ventilator-induced lung injury could have developed. Patients were not analyzed in the group to which they were initially randomized using an intention-to-treat algorithm; 66% of patients in the conventional ventilation group were crossed over to HFOV, and 38% in the HFOV group were crossed over to the conventional group.
Figure 3.
Oxygenation index before initiation of high-frequency oscillatory ventilation (HFOV), shortly after initiation, and then 24 hours later. Data presented as median and interquartile range (IQR). Overall difference by Kruskal-Wallis analysis of variance, P = 0.009. Multiple comparisons by mean ranks. Pre and 24-hour post P = 0.67. Unpublished data from a single institution (Children's Hospital Los Angeles). HFOV implemented at a median of 3.5 (IQR, 1.25–7.5) days into mechanical ventilation for acute hypoxemic respiratory failure.
Erickson and coworkers (44) reported that 29% of children with ALI were ventilated with HFOV during the course of their disease, and Randolph and colleagues (27) reported the use of HFOV in 52% of children with physician-assessed severe ARDS. However, evidence supporting the use of HFOV in patients with ALI or ARDS is still scarce. A systematic review on HFOV for ALI and ARDS in both adults and children concluded there is not enough evidence that HFOV reduces mortality or long-term morbidity (45). Similar inconclusive results have been seen in neonates, although the pathophysiology for neonatal respiratory distress syndrome is quite unique from adult and pediatric ALI/ARDS (46). However, Hager and associates have made important observations concerning the relative contributions of oscillator frequency and amplitude (47) and argue that HFOV can be made even more lung protective if larger amplitudes are used for CO2 removal while prioritizing increases in frequency (and thus reducing the often considerable delivered Vt at lower hertz) (25). Adult intensivists have designed protocols incorporating these priorities (24) that are quite different from pediatric practice in which amplitude is limited and frequency is reduced with consequent larger Vts (48).
Other Modes of Ventilation
Other modes of ventilation have been applied to subpopulations of children but have failed to gain wide-scale acceptance in pediatric ALI/ARDS management. The Volume Diffusive Respirator (VDR) shares some theoretical benefits with HFOV. It is a high-frequency time-cycled pressure ventilator that allows for pneumatic control over the pressure/flow/volume relationship to optimize intrapulmonary gas distribution with a percussive burst, theoretically limiting barotrauma and overdistention. Unlike high-frequency oscillatory ventilation, wherein amplitude oscillates around a mean airway pressure, in VDR a high flow interrupter stacks oscillatory breaths on top of PEEP to a selected inspiratory pressure, followed by passive exhalation. The interrupter has also been reported to help with endobronchial secretion removal. This mode has primarily been applied to adults and children with burns, and there has only been one pediatric randomized control trial. Most children did not meet ALI or ARDS criteria, with mean PF ratios greater than 500. Ventilator support was targeted daily to maintain SpO2 greater than 90% and PaCO2 less than 55 mm Hg. PEEP was kept between 4 and 6 cm H2O in both groups. Children who received VDR had lower peak inspiratory pressures than those in the pressure-control group, and achieved higher PF ratios, although mean PF ratios were greater than 500 for both groups. There was no difference in survival, barotrauma, or ventilator days, although the study was underpowered for these outcomes (49).
Neurally adjusted ventilatory assist (NAVA) is a partial ventilator support mode wherein positive pressure is provided in response to diaphragmatic electrical activity, resulting in a variable breathing pattern. Animal models of ARDS have demonstrated similar degrees of ventilator-induced lung injury between NAVA and lung-protective volume-control ventilation (6 ml/kg with adequate PEEP) (50). NAVA's human clinical applications have to date revolved around ventilator weaning with comparison to supported modes of ventilation, such as pressure support (51). Compared with more conventional modes of ventilation, patients supported on NAVA typically achieve lower Vts with faster respiratory rates and more respiratory variation (52). There has yet to be a pediatric trial using NAVA for ALI or ARDS.
Airway pressure release ventilation (APRV) has theoretical advantages in improving short-term outcomes, such as length of mechanical ventilation, by preserving spontaneous breathing and requiring less sedation than more conventional modes of ventilation or HFOV. Of course, these theoretical advantages are challenging with noncooperative infants and children, for whom higher levels of sedation are often needed to guarantee patient safety. Although there have been several descriptions of APRV for adults with acute respiratory failure, the largest published randomized controlled trial (53) comparing APRV to conventional management failed to show a difference in ventilator-free days or mortality and was stopped for futility. This trial was conducted before the publication of the ARDS Network Vt study, so both groups had targeted Vts between 8 and 10 ml/kg. There have been no randomized controlled trials in children. More detailed reviews of APRV in ARDS have been previously published (54). Adjunctive therapies, such as heliox, nitric oxide, corticosteroids, fluid management, surfactant, and noninvasive ventilation have been described previously, with little new pediatric evidence for benefit in ALI/ARDS (55).
Oxygenation and Ventilation Targets
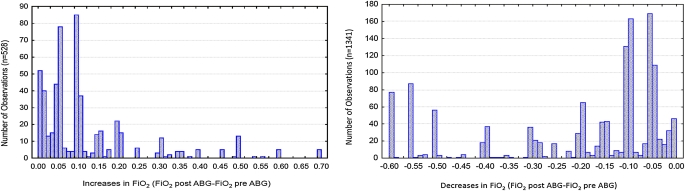
In addition to modes of ventilation, pediatric practitioners may behave differently than their adult counterparts regarding the management of PEEP and FiO2, their comfort with acceptable levels of permissive hypercapnia, and the frequency and degree of changes to parameters of mechanical ventilation (56). Data from a single institution of more than 6,000 blood gases and ventilator settings from more than 400 children with AHRF have demonstrated that pediatric practitioners make smaller changes in FiO2 (0.05 vs. 0.1) with higher target ranges for SpO2 and PaO2 than advocated in the adult ARDS Network management protocol (56) (Figure 4). Although this may be the reality of practice, pediatric practitioners may be willing to make larger changes to FiO2 and target lower ranges of SpO2 and PaO2, but this is unknown and is currently under investigation. This may be an area in which pediatricians can learn from neonatology, wherein nurse, respiratory therapist (RT), or closed-loop oxygen targeting protocols (57, 58) help minimize FiO2 exposure, largely to reduce the incidence of retinopathy of prematurity. Furthermore, it appears that pediatric intensivists may be more uncomfortable with the degree of permissive hypercapnia recommended in the ARDS Network management protocol, advocating tighter control of pH for children with ARDS. Single-institution data reinforce that without a protocol, practitioners are unlikely to behave in any consistent lung-protective manner with respect to pH/PaCO2 management (56).
Figure 4.
For 6,017 charted ventilator settings from 402 children with acute hypoxemic respiratory failure, FiO2 was changed 1,869 times. When practitioners change FiO2 they frequently make changes at intervals of 0.05, both for increases and decreases of FiO2. This is in contrast to the Acute Respiratory Distress Syndrome Network protocol, which implements changes in FiO2 at intervals of 0.1. Reprinted with permission from Reference 56.
Vt Measurements
Key physiologic and developmental considerations of children compared with adults make assessments of the impact of Vt on outcome challenging. Adult practice is to calculate Vt from predicted body weight for age, height, and sex using a set of readily available tables. The rationale is that although obesity is a major problem in adults, it is unlikely that the lungs are obese (i.e., larger), and therefore the predicted body weight should be the one used for calculation of Vt. Although pediatric practice is not entirely clear, it seems that actual body weight is most commonly used to calculate Vt. Obesity is also a large problem in pediatric practice, but so is failure to thrive, with low weight for age and height. In addition, contractures and spinal deformities are common in children, making direct measurement of length, or its usual surrogate, arm span, irrelevant. Formulae are now available using ulna length to determine height to predict body weight from birth to 18 years (59, 60). From growth grids, this height is used to find the ideal body weight to which Vt can be targeted.
Nonetheless, it is not known if the lungs fail to grow appropriately if the child fails to thrive (probable); nor is it known if the lung volumes are larger in obese children (unlikely). To approximate the “correct” Vt for mechanical ventilation, the best compromise at this time may be to use the actual body weight if the child's weight is less than the 50th percentile and ideal body weight (i.e., predicted from height or ulna length) if above the 50th percentile. Prospective investigation of measured lung volume compared with that predicted from actual versus ideal body weight in children is ongoing and should provide a more definitive answer to this question.
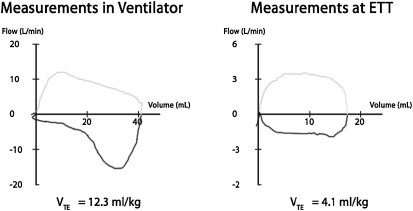
In addition to determining whether predicted or actual body weight should be used, the location of measurement of flow and Vt is important. Although most modern ventilators have built-in software to adjust for mechanical ventilator tubing compliance, Vts measured at the proximal airway with a pneumotachograph are still remarkably different from those measured at the mechanical ventilator. This problem is magnified with infants and smaller children, even when allowing for tubing compliance (61, 62), with Vts measured at the ventilator often being considerably higher than those at the endotracheal tube (ETT). In addition, the shape of the expiratory portion of the tidal flow–volume curve is often distorted to an obstructive pattern when acquired in the ventilator rather than at the ETT (Figure 5), which may lead to incorrect ventilator management choices. Future mechanical ventilation protocols must consider the location of the Vt measuring device. Furthermore, given the common use of uncuffed tubes in children, volume, resistance, and compliance measurements will not be accurate if there is a leak greater than 18% around the ETT (63).
Figure 5.
Measurement from a 4.0-kg infant with a cuffed endotracheal tube in pressure-control mode with tube compensation active. On the left, flow-volume measurements are made at the ventilator with compensation for tubing compliance. There is “overshoot” of flow measurements causing volumes to be larger and giving the expiratory portion of the flow-volume curve (below the horizontal axis) a pattern of obstructive airways disease. Vt is 12.3 ml/kg. On the right, measurements are made at the endotracheal tube connector within a minute of the left panel. Here, the flows and volumes are much lower with Vt now one-third at 4.1 ml/kg. The flow pattern on the expiratory limb now resembles that of normal airways. ETT = endotracheal tube; VTE = exhaled tidal volume.
Given the theoretical potential of HFOV to be more lung protective than CMV in both children and adults, and the current predilection to use it as rescue therapy (20, 41), it would appear logical to undertake a study of early institution of HFOV. This is likely feasible in children given that most pediatric intensivists are comfortable with the mode and have experience using it, and it has been demonstrated to be possible in adults by the OSCILLATE pilot study (64). Because of the previously discussed difficulties in Vt assessment and targeting, a lung protective pressure-control strategy may be the most logical choice for the CMV arm. Given the current use role of HFOV in ALI/ARDS, key consideration must be given to its use as rescue therapy for the conventional ventilation arm in a clinical trial. As has recently been demonstrated, a trial incorporating rescue therapy cannot definitively asses the overall efficacy of a therapy but can only assess the effects of delayed versus immediate provision of the treatment (65). Although less commonly used modes of ventilation (VDR, APRV, NAVA) should be compared with conventional therapies, more early-stage evidence regarding their use in pediatric ALI and ARDS is needed.
RECRUITMENT OF PATIENTS FOR PEDIATRIC ALI TRIALS
Pediatric ALI trials have suffered from poor recruitment and enrollment. The relative infrequency of ALI/ARDS in pediatrics (13, 18, 27, 44) has meant that meaningful ALI trials have required 12 to 16 different performance sites, oftentimes enrolling patients over a 4- to 5-year period (22, 23). Given the importance of standardization and the relative infrequency of ALI at any site, maintaining equal study standards across institutions is challenging. This is compounded by the 4 to 5 years needed to complete a study, making fatigue and drift in clinical practice over the study period legitimate challenges that will likely impact results. The use of computerized decision support tools may help minimize some of this variability and drift by standardizing decisions about mechanical ventilation for similar clinical states and recording protocol adherence in an automated fashion.
As is evident from PALIVE (13) and also from investigations by Curley and colleagues (21), Thomas and colleagues (17), and our group (66), pediatric ALI/ARDS trials have been hampered by requiring invasive arterial blood gas criteria (PF ratio) for patient inclusion. The SpO2/FiO2 (SF) ratio and oxygenation saturation index (OSI), rather than their invasive counterparts of PF ratio and OI, have been developed and validated for pediatrics. This has not yet been done for the pediatric LIS (which includes the PF ratio) but is ongoing. By using noninvasive criteria rather than arterial blood gases, patient screening and eligibility for studies can be improved by nearly 35% (66), which is particularly important given the relatively low (typically ∼ 60%) enrollment in such trials (20).
We have retrospectively validated the SF ratio using blood gas data banks at two children's hospitals (15). There were 3,143 observations in the derivation and validation samples. The relevant ARDS and ALI definition values for the SF ratio were 201 and 263, respectively, when using linear regression. Thomas and colleagues have demonstrated similar results with secondary analysis of the calfactant and prone positioning studies, showing ARDS and ALI PF ratio equivalent values of 212 and 253, respectively (17). Prospective validation is ongoing in a multicenter trial. These values can be compared with those obtained by Rice and coworkers (16) in adults. From secondary analysis of more than 5,000 observations from two ARDS Network studies, they found ARDS and ALI comparable SF values of 235 and 315, considerably higher than those reported in both pediatric studies. Potential reasons for this difference may include the presence of fetal hemoglobin, a difference in saturation probes based on age, and more children in the higher (96–97%) SpO2 range, where there is more variability in the relationship between PaO2 and SpO2.
Enrollment in pediatric trials is frequently hampered by high parental refusal rates, generally ranging from 27 to 53% (21). Although there may be some institutional variability based on ICU or investigator-specific characteristics, this poor enrollment combined with the infrequent occurrence of many diseases in pediatric critical care makes many clinical trials impossible to conduct in a timely manner before interventions undergo natural selection in the PICU. In and of itself, this area is worthy of focused research within the pediatric community. We must learn from past studies in pediatric critical care, understand the reasons for parental refusal (including the roles of how and by whom consent is approached), and address these concerns in a systematic and thorough fashion (67).
DEFINITION OF ALI/ARDS
The Murray Lung Injury score (33) was initially created to gauge the severity of ALI in adults. A score of 2.5 or higher (out of a possible 4) was defined as severe ARDS and intended to characterize a particularly high-risk group appropriate for interventional trials. The American-European Consensus Conference (AECC) definitions (14) sought to simplify the diagnosis of ALI for bedside clinicians and create a distinction, albeit arbitrary, between ALI and ARDS for clinical trials. The AECC definitions for ALI/ARDS are now widely embraced in both adult and pediatric critical care, but they are limited by their simplicity and imprecision. Distinct disadvantages of the guidelines revolve around the potential manipulation of the PF ratio, which can be “artificially” lowered if a patient has inadequate lung recruitment. This limitation can be lessened by incorporating some measure of ventilator support into the predictive equation, a concept that was embraced early on by pediatric researchers (68). For this reason, OI and the LIS incorporate mean airway pressure (MAP) and PEEP, respectively, to define lung injury severity. In addition, the requirement for an arterial PaO2 greatly hampers the recognition of the disease, as many patients without arterial blood gases would fulfill the oxygenation criteria for ALI or ARDS. Embracing noninvasive oxygenation criteria can overcome this.
Second, the AECC requirement for “bilateral pulmonary infiltrates” on chest radiograph is open to considerable interpretation and has very poor interobserver variability in both pediatric and adult critical care (69, 70). The presence of such infiltrates was meant to help distinguish the distinct pathophysiologic processes of ALI/ARDS from lobar pneumonia, atelectasis, or simply radiographic technique. However, the lack of bilateral infiltrates can exclude close to half of all eligible patients for ALI studies (31, 66). Although the characteristic pathology and pathophysiology of ALI is distinct from, for example, lobar pneumonia, distinguishing these two entities based on the nonstandardized interpretation of a chest radiograph seems an oversimplification. The LIS may perform better in this realm, as quadrants of alveolar consolidation are equally weighted with PF ratio, PEEP, and compliance of the respiratory system, as part of a four-point scale.
Finally, given the heterogeneous conditions that lead to ALI/ARDS, the AECC criteria do not distinguish the cause of ALI. Although this may not be necessary for the definition of the syndrome, the cause of ALI certainly affects outcome, as has been noted by Willson and colleagues (23) and by our group (31). Clearly, we would like to examine lung injury severity measures that exhibit the potential for generalizability to a large cohort of ICU patients. Nonetheless, given the importance of a multitude of other factors on outcome for ALI, we must ensure an equal distribution of these high-risk confounding variables in a randomized trial.
LUNG INJURY SEVERITY MEASURES
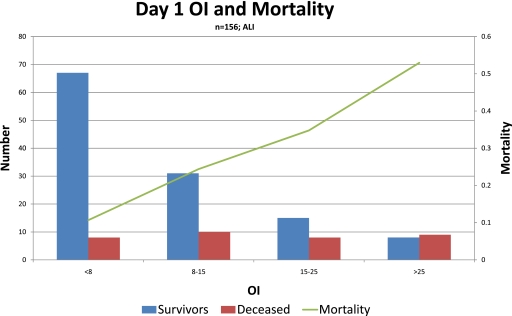
The performance of meaningful interventional studies on mechanically ventilated children requires that the interventions proposed have a sound basis for benefit on a defined cohort of children. For this reason, many have viewed children with ALI/ARDS differently than those with AHRF. Nevertheless, the response to a particular intervention or therapy, such as HFOV, may be more reliant on the severity of lung disease, rather than the presence or absence of bilateral pulmonary infiltrates. In our examination of the course of 398 children with AHRF, 192 of whom had bilateral infiltrates on chest radiograph, severity of lung injury markers such as OI, dynamic compliance, PF ratio, or LIS performed equally well in predicting VFD or mortality, regardless of the presence or absence of such infiltrates. Moreover, it is clear that the risk for mortality increases in a near linear fashion based on further impairments in PF ratio, OI, or LIS (Figure 6). As such, it may be more beneficial to stratify the enrollment in a multicenter trial based on the degree of lung injury severity, rather than the absence or presence of ALI criteria. Unfortunately, although the association between measures of lung disease severity and outcome are stronger in pediatrics than adults, they are by no means perfect. Even at their best, the area under the receiver operator curve plots for OI, PF ratio, LIS, and mortality are approximately 0.7, which most would deem an acceptable but not outstanding way to characterize risk (31). This is likely explained by the fact that mortality and VFD are clearly influenced by other metrics, such as other organ dysfunction, the cause of lung injury, and other comorbidities including immunosuppression and bone marrow transplantation. Even further, the inciting cause of lung injury impacts outcomes, as evident by numerous investigations on RSV showing that not only is it important to discriminate between RSV-induced bronchiolitis and RSV-induced pneumonia, but RSV-induced ALI/ARDS has much lower mortality than other causes of ARDS (32).
Figure 6.
Mortality stratified by time-weighted average oxygenation index (OI) on the first day of mechanical ventilation after meeting criteria for acute lung injury (n = 156 children). Note the stepwise increase in mortality as oxygenation index increases. Unpublished data from a single institution (Children's Hospital Los Angeles).
ALTERNATIVE OUTCOME MEASURES
In contrast to the debatably unchanging (71, 72) high mortality rate in adult ARDS (35–45%) (73) over the past decade, mortality for pediatric ALI/ARDS has fallen to close to 20% (19, 23, 31). Although some estimates are higher (44), with explicit protocols in certain populations of children with ALI/ARDS mortality can be as low as 8% (22). Nonetheless, patients with ALI and ARDS continue to be among those at the highest risk in PICUs, with longer lengths of mechanical ventilation, higher risk for nosocomial infections, and unknown long-term neurodevelopmental and respiratory morbidity.
Following adult examples, most pediatric mechanical ventilation trials now have primary outcome measures related to a combined mortality and length of mechanical ventilation metric, such as VFD. Although most pediatric mechanical ventilation trials would not be feasible without some similar outcome measure, they have limited objectivity. Any variable that prolongs the length of mechanical ventilation may impact VFD: sedation, fluid balance, post-extubation subglottic edema requiring reintubation, institutional practices regarding weaning, and the use of noninvasive ventilation after extubation. As such, trials that use VFD as an outcome must be adequately explicit to control for these potential confounding variables. Although one would hope that these variables equalize with adequate randomization, individual ICU management practices can potentially have a large impact on VFD. There are randomization strategies to minimize this effect. However, with the infrequency of ALI, some participating institutions may enroll only a handful of patients over the study period. Under these circumstances, such pediatric center-specific clustering would be very challenging to control for prospectively with a block randomization design or post hoc with center-specific multivariable modeling.
Creation of an explicit protocol for all aspects of care related to a study would certainly benefit from a comprehensive computer-based decision support tool. Nonetheless, it is unrealistic to protocolize everything to guarantee equal practice across multiple institutions. As such, for the sake of a study it may be beneficial to select a more specific outcome measure, subject to less bias. This might include marking the end of mechanical ventilation (for the purposes of analysis) at successful passage of an extubation readiness test (ERT), regardless of whether extubation was successful. Unfortunately, even this is not perfect, as readiness for such a trial depends on sedation, fluid status, and neuromuscular strength, and there must be an explicit weaning protocol to trigger initial evaluation with an extubation readiness test (74).
Aside from the imprecision of the estimate of length of mechanical ventilation, composite outcome metrics such as VFDs require relatively equal importance for each of the components of the outcome (75)—in this case mortality and length of mechanical ventilation. Although the length of ventilation may affect ICU length of stay, cost, and additional morbidities, these are clearly not as important as mortality. As has been previously demonstrated, trials that show differences (increases or decreases) in mortality may not demonstrate differences in VFDs (23). Therefore, pediatric studies on ALI/ARDS should not strictly rely on VFDs as an outcome, but must also report mortality and other outcomes that measure morbidity. Unfortunately, given the low incidence of mortality, sample size calculations for these outcomes will be extremely disparate.
Given overall improvements in mortality, the imprecision of composite outcome measures, and impressions of high morbidity for many children in the PICUs of children's hospitals, longer-term measures of function should be considered as primary outcome measures of pediatric clinical trials. Although this adds complexity and significant cost to a study because of the need for long-term follow-up, it is imperative that we not only determine whether our therapies allow a child to be liberated from the mechanical ventilator a day or two earlier than anticipated but also assess whether a child returns to his or her pre-ICU cognitive and pulmonary function in a reasonably timely fashion. Follow-up studies have demonstrated diminished functional outcome and quality of life after PICU admission (76), and several studies have demonstrated persistent impairments in pulmonary function of unknown long-term significance for children who required mechanical ventilation in PICUs for respiratory failure (77–81).
Unfortunately, no tool has been specifically validated to assess long-term neurodevelopmental or pulmonary outcome for children admitted to PICUs with ALI or ARDS. This is complicated by the fact that many children in ALI/ARDS trials have significant preexisting morbidities, so outcomes must be adjusted for baseline dysfunction. Already developed tools (82) and telephone questionnaires may adequately characterize quality of life (83) or respiratory symptoms (84), and this methodology is currently being used in pediatric critical care trials such as the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials, and has been proposed for others (85, 86). However, deficits in performance IQ, memory, motor, attention, language, academic achievement, and pulmonary function will require more extensive testing and follow-up. Such studies can be extraordinarily expensive to conduct. Unfortunately, without tools that characterize quality of life and long-term morbidity, other outcomes (such as economic ones) cannot be considered. As such, validation of long-term quality-of-life measures deserve extensive scrutiny as outcome measures for pediatric critical care investigations (87).
CONCLUSIONS
Differences between adult and pediatric mechanical ventilation practices for ALI/ARDS, as well as heterogeneous practices within pediatric critical care, pose significant challenges for designing pediatric mechanical ventilation trials and patient management. Future comprehensive ventilation trials for pediatric AHRF and its subsets of ALI and ARDS will need explicit management protocols. Given historical problems with protocol adherence, particularly for more complicated algorithms, computer-based interfaces are a natural fit for clinical trials. However, clinical trials using algorithms for ventilation management must also address the different modes of ventilation and granularity of ventilator changes in pediatric practice, overcome challenges with patient recruitment, address the shortcomings of the AECC definitions of ALI and ARDS, and consider enrollment based on the more reliable yet still unpredictable relationship between lung injury severity and outcome. Finally, we must find ways for more objective assessment of potentially biased alternative outcome measures and validate existing or develop new measures of long-term morbidity, given improvements in mortality (Table 1).
Supported by a grant from the National Library of Medicine, Advanced Computational Framework for Decision Support in Children, 1RC1LM010639–01 (R.G.K.), a VPICU Fellowship, Advanced Statistical and Database Techniques for Pediatric ICU Severity of Illness scoring, from the Whittier Foundation (R.G.K.), a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Collaborative Pediatric Critical Care Research Network), 2 U10HD050012–06 (C.J.L.N.), a grant from the National Heart, Lung and Blood Institute (Therapeutic Hypothermia after Pediatric Cardiac Arrest), 1 U01HL094345–01 (C.J.L.N.), and Hospira for a dexmedetomidine pharmacodynamic study in ventilated infants and young children (C.J.L.N.).
Originally Published in Press as DOI: 10.1164/rccm.201004-0606CI on August 23, 2010
Author Disclosure: C.J.L.N. received a sponsored grant from Hospira for $50,001–$100,000 for a Dexmedetomidine pharmacokinetic study. R.G.K. received sponsored grants from the National Institutes of Health for $50,001–$100,000 and from the Whittier Foundation for $10,001–$50,000.
References
- 1.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med 1990;16:372–377. [DOI] [PubMed] [Google Scholar]
- 2.Albaiceta GM, Luyando LH, Parra D, Menendez R, Calvo J, Pedreira PR, Taboada F. Inspiratory vs. expiratory pressure-volume curves to set end-expiratory pressure in acute lung injury. Intensive Care Med 2005;31:1370–1378. [DOI] [PubMed] [Google Scholar]
- 3.Sondergaard S, Karason S, Wiklund J, Lundin S, Stenqvist O. Alveolar pressure monitoring: an evaluation in a lung model and in patients with acute lung injury. Intensive Care Med 2003;29:955–962. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319–323. [DOI] [PubMed] [Google Scholar]
- 5.ARDSnet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308. [DOI] [PubMed] [Google Scholar]
- 6.Chelucci GL, Dall'Ava-Santucci J, Dhainaut JF, Chelucci A, Allegra A, Lockhart A, Zin WA, Milic-Emili J. Association of PEEP with two different inflation volumes in ARDS patients: effects on passive lung deflation and alveolar recruitment. Intensive Care Med 2000;26:870–877. [DOI] [PubMed] [Google Scholar]
- 7.Burns D, West TA, Hawkins K, O'Keefe GE. Immediate effects of positive end-expiratory pressure and low and high tidal volume ventilation upon gas exchange and compliance in patients with acute lung injury. J Trauma Inj Infect Crit Care 2001;51:1177–1181. [DOI] [PubMed] [Google Scholar]
- 8.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299:646–655. [DOI] [PubMed] [Google Scholar]
- 9.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311–1318. [DOI] [PubMed] [Google Scholar]
- 10.Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M, Cabello B, Bouadma L, Rodriguez P, Maggiore S, et al. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med 2006;174:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 2000;132:373–383. [DOI] [PubMed] [Google Scholar]
- 12.Blaser R, Schnabel M, Biber C, Baumlein M, Heger O, Beyer M, Opitz E, Lenz R, Kuhn KA. Improving pathway compliance and clinician performance by using information technology. Int J Med Inform 2007;76:151–156. [DOI] [PubMed] [Google Scholar]
- 13.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll C, Flori H, Tasker RC, Rimensberger P, Randolph A, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med (In press) [DOI] [PubMed]
- 14.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The consensus committee. Intensive Care Med 1994;20:225–232. [DOI] [PubMed] [Google Scholar]
- 15.Khemani RG, Patel NR, Bart RD III, Newth CJ. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest 2009;135:662–668. [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB, for the National Institutes of Health NHLaBIAN. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132:410–417. [DOI] [PubMed] [Google Scholar]
- 17.Thomas N, Shaffer ML, Willson D, Shih M, Curley M. Defining acute lung disease in children with the oxygen saturation index. Pediatr Crit Care Med 2010;11:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farias JA, Frutos F, Esteban A, Flores JC, Retta A, Baltodano A, Alia I, Hatzis T, Olazarri F, Petros A, et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med 2004;30:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171:995–1001. [DOI] [PubMed] [Google Scholar]
- 20.Randolph AG, Meert KL, O'Neil ME, Hanson JH, Luckett PM, Arnold JH, Gedeit RG, Cox PN, Roberts JS, Venkataraman ST, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med 2003;167:1334–1340. [DOI] [PubMed] [Google Scholar]
- 21.Curley MA, Arnold JH, Thompson JE, Fackler JC, Grant MJ, Fineman LD, Cvijanovich N, Barr FE, Molitor-Kirsch S, Steinhorn DM, et al. Clinical trial design–effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care 2006;21:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, Grant MJ, Barr FE, Cvijanovich NZ, Sorce L, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 2005;294:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA 2005;293:470–476. [DOI] [PubMed] [Google Scholar]
- 24.Fessler HE, Derdak S, Ferguson ND, Hager DN, Kacmarek RM, Thompson BT, Brower RG. A protocol for high-frequency oscillatory ventilation in adults: results from a roundtable discussion. Crit Care Med 2007;35:1649–1654. [DOI] [PubMed] [Google Scholar]
- 25.Fessler HE, Hager DN, Brower RG. Feasibility of very high-frequency ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2008;36:1043–1048. [DOI] [PubMed] [Google Scholar]
- 26.Arnold JH, Hanson JH, Toro-Figuero LO, Gutierrez J, Berens RJ, Anglin DL. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med 1994;22:1530–1539. [PubMed] [Google Scholar]
- 27.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, et al. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA 2002;288:2561–2568. [DOI] [PubMed] [Google Scholar]
- 28.Jouvet P, Farges C, Hatzakis G, Monir A, Lesage F, Dupic L, Brochard L, Hubert P. Weaning children from mechanical ventilation with a computer-driven system (closed-loop protocol): a pilot study. Pediatr Crit Care Med 2007;8:425–432. [DOI] [PubMed] [Google Scholar]
- 29.Yang YM, Huang WD, Shen MY, Xu ZR. Comparative study of pressure-control ventilation and volume-control ventilation in treating traumatic acute respiratory distress syndrome. Chin J Traumatol 2005;8:36–38. [PubMed] [Google Scholar]
- 30.Prella M, Feihl F, Domenighetti G, Prella M, Feihl F, Domenighetti G. Effects of short-term pressure-controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS: comparison with volume-controlled ventilation. Chest 2002;122:1382–1388. [DOI] [PubMed] [Google Scholar]
- 31.Khemani RG, Conti D, Alonzo TA, Bart RD, Newth CJ. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med 2009;35:1428–1437. [DOI] [PubMed] [Google Scholar]
- 32.Hammer J, Numa A, Newth CJ. Acute respiratory distress syndrome caused by respiratory syncytial virus. Pediatr Pulmonol 1997;23:176–183. [DOI] [PubMed] [Google Scholar]
- 33.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis 1988;138:720–723. [DOI] [PubMed] [Google Scholar]
- 34.Deans KJ, Minneci PC, Cui X, Banks SM, Natanson C, Eichacker PQ. Mechanical ventilation in ARDS: one size does not fit all. Crit Care Med 2005;33:1141–1143. [DOI] [PubMed] [Google Scholar]
- 35.Tobin MJ. Culmination of an era in research on the acute respiratory distress syndrome. N Engl J Med 2000;342:1360–1361. [DOI] [PubMed] [Google Scholar]
- 36.Silverman HJ. The acute respiratory distress syndrome network controversy: lessons and legacy. Curr Opin Crit Care 2004;10:560–564. [DOI] [PubMed] [Google Scholar]
- 37.Thompson T, Schoenfeld D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc 2007;4:577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta NM, Arnold JH. Mechanical ventilation in children with acute respiratory failure. Curr Opin Crit Care 2004;10:7–12. [DOI] [PubMed] [Google Scholar]
- 39.Ben Jaballah N, Khaldi A, Mnif K, Bouziri A, Belhadj S, Hamdi A, Kchaou W. High-frequency oscillatory ventilation in pediatric patients with acute respiratory failure. Pediatr Crit Care Med 2006;7:362–367. [DOI] [PubMed] [Google Scholar]
- 40.Arnold JH, Anas NG, Luckett P, Cheifetz IM, Reyes G, Newth CJ, Kocis KC, Heidemann SM, Hanson JH, Brogan TV, et al. High-frequency oscillatory ventilation in pediatric respiratory failure: a multicenter experience. Crit Care Med 2000;28:3913–3919. [DOI] [PubMed] [Google Scholar]
- 41.Adhikari NK, Ferguson ND, Mehta S, Freitag A, Friedrich JO, Granton JT, Zhou Q, Stewart TE, Meade MO. Current high frequency oscillation (HFO) utilization in Ontario. Am J Respir Crit Care Med 2009;179:A3072. [Google Scholar]
- 42.Bollen CW, van Well GT, Sherry T, Beale RJ, Shah S, Findlay G, Monchi M, Chiche JD, Weiler N, Uiterwaal CS, et al. High frequency oscillatory ventilation compared with conventional mechanical ventilation in adult respiratory distress syndrome: a randomized controlled trial. Crit Care 2005;9:R430–R439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, Carlin B, Lowson S, Granton J, Multicenter Oscillatory Ventilation For Acute Respiratory Distress Syndrome Trial Study Investigators. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med 2002;166:801–808. [DOI] [PubMed] [Google Scholar]
- 44.Erickson S, Schibler A, Numa A, Nuthall G, Yung M, Pascoe E, Wilkins B. Society. PSGAaNZIC. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med 2007;8:317–323. [DOI] [PubMed] [Google Scholar]
- 45.Wunsch H, Mapstone J. High-frequency ventilation versus conventional ventilation for treatment of acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev 2004;1:CD004085. [DOI] [PubMed] [Google Scholar]
- 46.Henderson-Smart DJ, De Paoli AG, Clark RH, Bhuta T, Henderson-Smart DJ, De Paoli AG, Clark RH, Bhuta T. High frequency oscillatory ventilation versus conventional ventilation for infants with severe pulmonary dysfunction born at or near term. Cochrane Database Syst Rev 2009;CD002974. [DOI] [PMC free article] [PubMed]
- 47.Hager DN, Fessler HE, Kaczka DW, Shanholtz CB, Fuld MK, Simon BA, Brower RG. Tidal volume delivery during high-frequency oscillatory ventilation in adults with acute respiratory distress syndrome. Crit Care Med 2007;35:1522–1529. [DOI] [PubMed] [Google Scholar]
- 48.Priebe G, Arnold J. High-frequency oscillatory ventilation in pediatric patients. Respir Care Clin N Am 2001;7:633–646. [DOI] [PubMed] [Google Scholar]
- 49.Carman B, Cahill T, Warden G, McCall J. A prospective, randomized comparison of the volume diffusive respirator vs conventional ventilation for ventilation of burned children. J Burn Care Rehabil 2002;23:444–448. [DOI] [PubMed] [Google Scholar]
- 50.Brander L, Sinderby C, Lecomte F, Leong-Poi H, Bell D, Beck J, Tsoporis JN, Vaschetto R, Schultz MJ, Parker TG, et al. Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and non-pulmonary organ dysfunction in rabbits with acute lung injury. Intensive Care Med 2009;35:1979–1989. [DOI] [PubMed] [Google Scholar]
- 51.Schmidt M, Demoule A, Cracco C, Gharbi A, Fiamma MN, Straus C, Duguet A, Gottfried SB, Similowski T. Neurally adjusted ventilatory assist increases respiratory variability and complexity in acute respiratory failure. Anesthesiology 2010;112:670–681. [DOI] [PubMed] [Google Scholar]
- 52.Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, Sinderby C, Brander L, Leong-Poi H, Beck J, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest 2009;135:695–703. [DOI] [PubMed] [Google Scholar]
- 53.Varpula T, Valta P, Niemi R, Takkunen O, Hynynen M, Pettila VV. Airway pressure release ventilation as a primary ventilatory mode in acute respiratory distress syndrome. Acta Anaesthesiol Scand 2004;48:722–731. [DOI] [PubMed] [Google Scholar]
- 54.Siau C, Stewart TE. Current role of high frequency oscillatory ventilation and airway pressure release ventilation in acute lung injury and acute respiratory distress syndrome. Clin Chest Med 2008;29:265–275. [DOI] [PubMed] [Google Scholar]
- 55.Randolph AG. Management of acute lung injury and acute respiratory distress syndrome in children. Crit Care Med 2009;37:2448–2454. [DOI] [PubMed] [Google Scholar]
- 56.Khemani R, Sward K, Newth C. Adaptation of an adult based mechanical ventilation protocol for application in pediatric ALI/ARDS. Am J Respir Crit Care Med 2010;181:A3903. [Google Scholar]
- 57.Claure N, Gerhardt T, Everett R, Musante G, Herrera C, Bancalari E. Closed-loop controlled inspired oxygen concentration for mechanically ventilated very low birth weight infants with frequent episodes of hypoxemia. Pediatrics 2001;107:1120–1124. [DOI] [PubMed] [Google Scholar]
- 58.Urschitz MS, Horn W, Seyfang A, Hallenberger A, Herberts T, Miksch S, Popow C, Muller-Hansen I, Poets CF, Urschitz MS, et al. Automatic control of the inspired oxygen fraction in preterm infants: a randomized crossover trial. Am J Respir Crit Care Med 2004;170:1095–1100. [DOI] [PubMed] [Google Scholar]
- 59.von Ungern-Sternberg BS, Trachsel D, Erb TO, Hammer J, von Ungern-Sternberg BS, Trachsel D, Erb TO, Hammer J. Forced expiratory flows and volumes in intubated and paralyzed infants and children: normative data up to 5 years of age. J Appl Physiol 2009;107:105–111. [DOI] [PubMed] [Google Scholar]
- 60.Gauld LM, Kappers J, Carlin JB, Robertson CF. Height prediction from ulna length. Dev Med Child Neurol 2004;46:475–480. [DOI] [PubMed] [Google Scholar]
- 61.Heulitt MJ, Thurman TL, Holt SJ, Jo CH, Simpson P. Reliability of displayed tidal volume in infants and children during dual-controlled ventilation. Pediatr Crit Care Med 2009;10:661–667. [DOI] [PubMed] [Google Scholar]
- 62.Al-Majed SI, Thompson JE, Watson KF, Randolph AG. Effect of lung compliance and endotracheal tube leakage on measurement of tidal volume. Crit Care 2004;8:r398–r402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Main E, Castle R, Stocks J, James I, Hatch D. The influence of endotracheal tube leak on the assessment of respiratory function in ventilated children. Intensive Care Med 2001;27:1788–1797. [DOI] [PubMed] [Google Scholar]
- 64.Meade MO, Cook DJ, Mehta S, Arabi YM, Keenan S, Ronco JJ, Adhikari NK, Jacka M, Freitag A, Friedrich A, et al. A multicentre pilot randomized trial of high frequency oscillation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2009;179:A1559. [Google Scholar]
- 65.Holubkov R, Dean JM, Berger J, Anand KJ, Carcillo J, Meert K, Zimmerman J, Newth C, Harrison R, Willson DF, et al. Is “rescue” therapy ethical in randomized controlled trials? Pediatr Crit Care Med 2009;10:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khemani RG, Markovitz BP, Curley MA. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest 2009;136:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kodish E, Eder M, Noll RB, Ruccione K, Lange B, Angiolillo A, Pentz R, Zyzanski S, Siminoff LA, Drotar D. Communication of randomization in childhood leukemia trials. JAMA 2004;291:470–475. [DOI] [PubMed] [Google Scholar]
- 68.Pfenninger J, Gerber AC. High-frequency ventilation (HFV) in hyaline membrane disease–a preliminary report. Intensive Care Med 1987;13:71–75. [DOI] [PubMed] [Google Scholar]
- 69.Angoulvant F, Llor J, Alberti C, Kheniche A, Zaccaria I, Garel C, Dauger S. Inter-observer variability in chest radiograph reading for diagnosing acute lung injury in children. Pediatr Pulmonol 2008;43:987–991. [DOI] [PubMed] [Google Scholar]
- 70.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest 1999;116:1347–1353. [DOI] [PubMed] [Google Scholar]
- 71.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: a systematic review. Am J Respir Crit Care Med 2009;179:220–227. [DOI] [PubMed] [Google Scholar]
- 72.Brochard L, Rouby JJ. Changing mortality in acute respiratory distress syndrome? Yes, we can! Am J Respir Crit Care Med 2009;179:177–178. [DOI] [PubMed] [Google Scholar]
- 73.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009;151:566–576. [DOI] [PubMed] [Google Scholar]
- 74.Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 2009;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guyatt G, Montori M, Ferreira-Gonzalez I, Busse J, Schunemann H, Jaeschke R, Permanyer-Miralda G. Composite endpoints. In: Guyatt G, editor. Users' guides to the medical literature. Colombus OH: McGraw-Hill; 2008. pp. 237–238.
- 76.Taylor A, Butt W, Ciardulli M. The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med 2003;29:795–800. [DOI] [PubMed] [Google Scholar]
- 77.Ben Abraham R, Weinbroum AA, Roizin H, Efrati O, Augarten A, Harel R, Moreh O, Barzilay Z, Paret G. Long-term assessment of pulmonary function tests in pediatric survivors of acute respiratory distress syndrome. Med Sci Monit 2002;8:CR153–CR157. [PubMed] [Google Scholar]
- 78.Fanconi S, Kraemer R, Weber J, Tschaeppeler H, Pfenninger J. Long-term sequelae in children surviving adult respiratory distress syndrome. J Pediatr 1985;106:218–222. [DOI] [PubMed] [Google Scholar]
- 79.Kraemer R, Fanconi S, Meister B, Pfenninger J. Residual lung function changes following adult respiratory distress syndrome (ARDS) in children. Schweiz Med Wochenschr 1985;115:96–99. [PubMed] [Google Scholar]
- 80.Laughlin JJ, Eigen H. Pulmonary function abnormalities in survivors of near drowning. J Pediatr 1982;100:26–30. [DOI] [PubMed] [Google Scholar]
- 81.Weiss I, Ushay HM, DeBruin W, O'Loughlin J, Rosner I, Notterman D. Respiratory and cardiac function in children after acute hypoxemic respiratory failure. Crit Care Med 1996;24:148–154. [DOI] [PubMed] [Google Scholar]
- 82.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, Anand KJ, Carcillo J, Newth CJ, Harrison R, et al. Functional status scale: new pediatric outcome measure. Pediatrics 2009;124:e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varni JW, Limbers CA. The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents. Pediatr Clin North Am 2009;56:843–863. [DOI] [PubMed] [Google Scholar]
- 84.Seid M, Limbers CA, Driscoll KA, Opipari-Arrigan LA, Gelhard LR, Varni JW. Reliability, validity, and responsiveness of the pediatric quality of life inventory (PedsQL) generic core scales and asthma symptoms scale in vulnerable children with asthma. J Asthma 2010;47:170–177. [DOI] [PubMed] [Google Scholar]
- 85.Ottenbacher KJ, Msall ME, Lyon N, Duffy LC, Ziviani J, Granger CV, Braun S. Functional assessment and care of children with neurodevelopmental disabilities. Am J Phys Med Rehabil 2000;79:114–123. [DOI] [PubMed] [Google Scholar]
- 86.Raggio DJ, Massingale TW. Comparison of the Vineland Social Maturity Scale, the Vineland Adaptive Behavior Scales–Survey Form, and the Bayley Scales of Infant Development with infants evaluated for developmental delay. Percept Mot Skills 1993;77:931–937. [DOI] [PubMed] [Google Scholar]
- 87.Curley MA, Zimmerman JJ. Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med 2005;6:S150–S156. [DOI] [PubMed] [Google Scholar]