Abstract
Rationale: Chitin is a ubiquitous polysaccharide in fungi, insects, allergens, and parasites that is released at sites of infection. Its role in the generation of tissue inflammation, however, is not fully understood.
Objectives: We hypothesized that chitin is an important adjuvant for adaptive immunity.
Methods: Mice were injected with a solution of ovalbumin and chitin.
Measurements and Main Results: We used in vivo and ex vivo/in vitro approaches to characterize the ability of chitin fragments to foster adaptive immune responses against ovalbumin and compared these responses to those induced by aluminum hydroxide (alum). In vivo, ovalbumin challenge caused an eosinophil-rich pulmonary inflammatory response, Th2 cytokine elaboration, IgE induction, and mucus metaplasia in mice that had been sensitized with ovalbumin plus chitin or ovalbumin plus alum. Toll-like receptor-2, MyD88, and IL-17A played critical roles in the chitin-induced responses, and MyD88 and IL-17A played critical roles in the alum-induced responses. In vitro, CD4+ T cells from mice sensitized with ovalbumin plus chitin were incubated with ovalbumin-stimulated bone marrow–derived dendritic cells. In these experiments, CD4+ T-cell proliferation, IL-5, IL-13, IFN-γ, and IL-17A production were appreciated. Toll-like receptor-2, MyD88, and IL-17A played critical roles in these in vitro adjuvant properties of chitin. TLR-2 was required for cell proliferation, whereas IL-17 and TLR-2 were required for cytokine elaboration. IL-17A also inhibited the generation of adaptive Th1 responses.
Conclusions: These studies demonstrate that chitin is a potent multifaceted adjuvant that induces adaptive Th2, Th1, and Th17 immune responses. They also demonstrate that the adjuvant properties of chitin are mediated by a pathway(s) that involves and is regulated by TLR-2, MyD88, and IL-17A.
Keywords: chitin, adjuvant, ovalbumin, aluminum hydroxide, alum
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Chitin plays an important role in lung tissue inflammation; however, the mechanisms are not fully understood. By using in vitro and in vivo systems, we demonstrate that chitin is a potential multifaceted adjuvant that induces Th2, Th1, and Th17 immune responses in the lung.
What This Study Adds to the Field
These studies demonstrate that chitin is a potent adjuvant in Th2, Th1, and Th17 immune responses.
Chitin is a biopolymer of N-acetyl-β-D-glucosamine found in the walls of fungi; the exoskeleton of crabs, shrimp, and insects; the microfilarial sheath of parasitic nematodes; and the lining of the digestive tracts of many insects (1–4). As a consequence of its widespread distribution, chitin represents the second-most abundant polysaccharide in nature, after cellulose. During infections with parasites and other pathogens chitin is released and metabolized by chitinases, innate immune responses are activated, and, eventually, adaptive Th2 and other responses are elicited. The Th2 cytokines, in turn, stimulate chitinase production, which generates additional chitin fragmentation, which reinforces these responses (5). Previous studies from our laboratory demonstrated that chitin is a potent activator of innate immune responses in macrophages (6, 7), and studies by others (8) have demonstrated that chitin can elicit Type 2 innate immune responses. However, the ability of chitin to contribute to the pathogenesis of adaptive immune responses has not been adequately defined.
In an attempt to understand the processes that lead to adaptive immune responses, animal modeling has been undertaken. These studies demonstrated that immune-stimulating adjuvants like aluminum hydroxide (alum) are frequently required to elicit appropriate in vivo adaptive immunity. They also demonstrated that many of these adjuvants mediate their effects via activating innate immunity Toll-like receptors (TLRs) and NODs and inducing the production of proinflammatory cytokines (9–18). This can readily be appreciated in studies highlighting the important role(s) that TLRs plays in the pathogenesis of asthma-like Th2 adaptive immune responses (18) and studies that demonstrate that TLR-2 ligands such as Pam-3-cys are potent Th2 adjuvants in vivo (19, 20). Recent studies from our laboratory showed that chitin is a pathogen-associated molecular pattern (PAMP) that activates TLR-2 and regulates macrophage function and acute innate inflammation in vitro and in vivo (6). These studies also demonstrated that these chitin effects are mediated by an IL-17A–dependent mechanism. However, the ability of chitin particles to serve as adjuvants that contribute to the production of adaptive Th2 and other immune responses has not been defined. In addition, the role(s) of IL-17A in the pathogenesis of the adjuvant effects of chitin has not been investigated.
We hypothesized that chitin particles can act as adjuvants for the development of adaptive immunity. To test this hypothesis, we evaluated the ability of chitin fragments to act as adjuvants for adaptive immune responses elicited by the aeroallergen ovalbumin (OVA) and the effects of chitin in priming T cells in vitro. These studies demonstrate that chitin is a multifaceted adjuvant that contributes to the pathogenesis of allergen-induced Th2, Th1, and IL-17 responses. They also demonstrate that these adjuvant effects are mediated and regulated by pathways that involve TLR-2, MyD88, and IL-17A.
METHODS
Animals
Six-week-old TLR-2 and TLR-4 null mice (from Professor R. Medzhitov, Yale University), MyD88-null mice (from Professor S. Akira, Osaka University, Japan), and IL-17A null mice (from Professor Y. Iwakura, Tokyo) were bred at Yale University. Control C57BL/6 mice were from the National Cancer Institute (Bethesda, MD). All animals were kept under standard conditions in Yale Animal Resource Center at Yale University. All experiments were performed in accordance with University guidelines and protocols were approved by the Yale Institutional and Animal Care and Use Committee.
In Vivo Th2 Inflammation
As described (21, 22), mice received two intraperitoneal injections (Day 0 and Day 5) of chitin (25 μg) plus OVA (20 μg) or alum (2 mg) plus OVA (20 μg). After 7 days, some animals were killed (time = 0) and others received aerosol challenge with OVA (1% w/v) or vehicle control. During these exposures the mice were placed for 40 minutes in a plastic chamber and the aerosol was generated via an Omron NE-U07 ultrasonic nebulizer (Omron Healthcare, Vernon Hills, IL). Mice were killed 24 hours later and bronchoalveolar lavage (BAL), blood samples, and tissue evaluations were collected. Total and differential cell recovery was evaluated after Diff-quick staining (Dade Behring, Dudingen, Switzerland). For histologic evaluation, the entire lung was inflated to 25 cm with Streck solution (Streck Laboratories, La Vista, NE). Hematoxylin and eosin and periodic acid-Schiff stains were performed in the Research Histology Laboratory in the Department of Pathology at Yale. Images of lung sections were captured at ×20 or ×40 final magnification on an Olympus (Tokyo, Japan) BH-2 microscope using a Sony DXC-760 MD camera. Each experimental group contained 10 mice.
In Vitro Cell Culture
Bone marrow–derived dendritic cells (BMDCs) and T cells were generated as described in the online supplement. Immature BMDCs were incubated with OVA (50 μg/ml) for 24 hours. Bovine serum albumin (BSA) or crude peanut extract (CPE) were used as antigen-specificity controls. The levels of endotoxin were assessed using the LAL assay and were less than 0.01 EU/ml (Cape Cod Inc., East Falmouth, MA). Primed CD4+ T cells were isolated from splenocytes and incubated with 105 OVA-stimulated BMDCs (ratio 1:10, 300 μl of Click medium) (Invitrogen, Carlsbad, CA). After 3 days, supernatants were collected and CD4+ T-cell proliferation was assessed at Day 5.
Cytokine Measurements
Concentrations of IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17A, TNFα, and IFN-γ were measured using multiplex kit (Upstate Inc., Temecula, CA) and a Bio-Plex system (Bio-Rad, Hercules, CA). The detection limit was 3.5 pg/ml.
Measurement of Serum Total and OVA-specific IgE
Total and OVA-specific IgE were measured by ELISA using BD OptEIA kit (BD Bioscience, San Jose, CA). Briefly, plates were coated with anti-mouse IgE mAb (clone R35-72; BD Biosciences) and mouse IgE (27-25; BD Biosciences) was used as a standard. Horseradish peroxidase–conjugated anti-mouse IgE (23G3; Southern Biotechnology, Birmingham, AL) (for total IgE) and horseradish peroxidase–labeled anti-biotin (Vector Laboratories, Burlingame, CA) followed with biotin-labeled OVA (for OVA-specific IgE) (provided by Dr. L. Cohn, Yale University) were added to the plates. Pooled sera with known concentrations of IgE (5,000 pg/ml) were used as OVA-specific IgE standards. Optical density was measured with a spectrophotometer.
Statistical Analysis
Results are expressed as means ± SEM. Statistical analysis was performed with the Student t test. Values of P less than 0.05 were considered significant.
RESULTS
Chitin Is an Adjuvant for OVA-induced Th2 Inflammation In Vivo
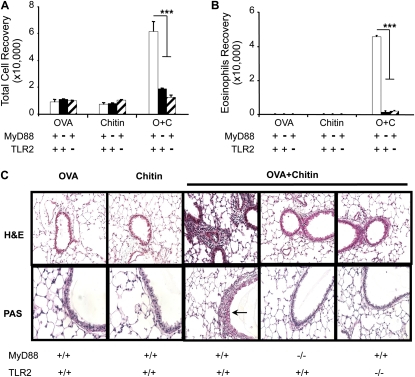
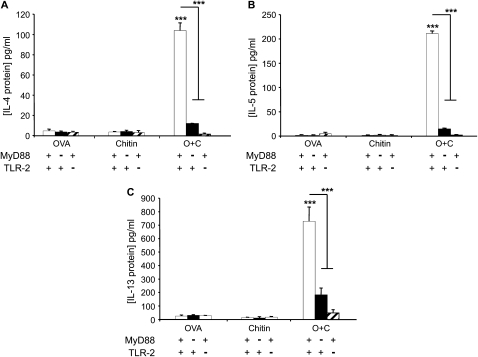
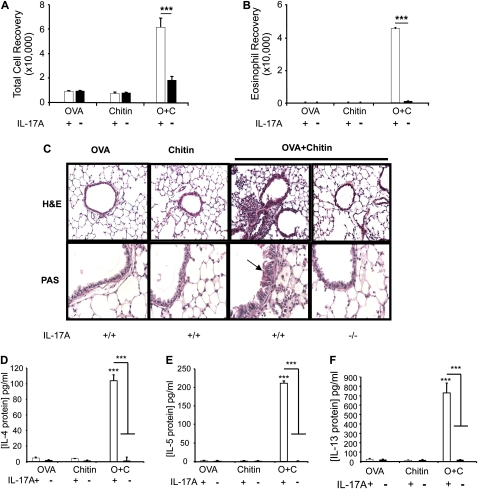
To test the hypothesis that chitin can serve as an adjuvant for adaptive pulmonary inflammation, we compared the effects of aerosol OVA challenge in mice that had been sensitized with OVA and chitin, alone and in combination. In these experiments, mice that were sensitized with OVA plus chitin manifested an impressive eosinophil-rich BAL and tissue inflammatory response (Figure 1) that contained rare neutrophils (< 2%, data not shown) and was associated with airway mucus metaplasia (Figure 1) and the induction of IL-4, IL-5, and IL-13 (Figure 2). This response was antigen-specific because similar reactions were not elicited when OVA plus chitin–sensitized mice were challenged with BSA or CPE (data not shown). BAL and tissue inflammation, mucus metaplasia, and Th2 cytokine production were also not seen in mice that were sensitized with OVA or chitin alone before OVA challenge (Figures 1 and 2). These studies demonstrate that chitin can serve as an adjuvant for aeroallergen-induced Th2 responses in the murine lung.
Figure 1.
Adjuvant respiratory effects of chitin in vivo. MyD88 and Toll-like receptor 2 (TLR-2) sufficient (+/+; +) and deficient (−/−; −) mice received two intraperitoneal injections (Days 0 and 5) with a mixture containing ovalbumin (OVA) (20 μg), chitin (25 μg), OVA complexed with chitin, or its vehicle controls. One week later the mice received aerosolized OVA for 3 days, and 1 day after the last challenge the mice were killed, bronchoalveolar lavage (BAL) was undertaken, and lung sections were prepared. The effects of these treatments on (A) BAL total and (B) eosinophils recovery, and (C) tissue histology (hematoxylin and eosin and periodic acid-Schiff [PAS] stains, ×20 and ×40 of original magnification, respectively) were evaluated. Results are presented as the mean ± SEM of a minimum of six mice per group. ***P < 0.001. Arrow in C indicates PAS-positive airway mucus.
Figure 2.
Adjuvant effects of chitin on bronchoalveolar lavage (BAL) Th2 cytokines in vivo. MyD88 and Toll-like receptor 2 (TLR-2) sufficient (+/+; +) and deficient (−/−; −) mice were sensitized at Day 0 and boosted at Day 5 with intraperitoneal injections containing ovalbumin (OVA) (20 μg), chitin (25 μg), OVA complexed with chitin, or their vehicle controls. One week later the mice received aerosolized OVA for 3 days, and 1 day after the last challenge the mice were killed and BAL was undertaken. Concentrations of (A) IL-4, (B) IL-5, and (C) IL-13 in BAL were measured by ELISA. Results are presented as the mean ± SEM of a minimum of six mice per group. ***P < 0.001.
The Adjuvant Properties of Chitin Depend on TLR-2 and MyD88 Signaling
To define the mechanisms underlying the in vivo adjuvant effects of chitin, we compared the effects of aerosolized OVA in OVA plus chitin–sensitized wild-type (WT), MyD88−/−, and TLR-2−/− mice. As noted above, eosinophilic inflammation, mucus metaplasia, and Th2 cytokine elaboration were seen in OVA/chitin-sensitized and OVA-challenged WT mice. Importantly, this ability of chitin to serve as an adjuvant for adaptive Th2 immunity was almost completely abrogated in mice with null mutations of MyD88 or TLR-2 (Figures 1 and 2). This response, however, was TLR-2 specific and was not due to contaminating endotoxin because it was not altered by null mutations of TLR-4 (data not shown). When viewed in combination, these findings demonstrate that the adjuvant effects of chitin in adaptive Th2 immunity are mediated via an innate immune pathway that involves TLR-2 and MyD88.
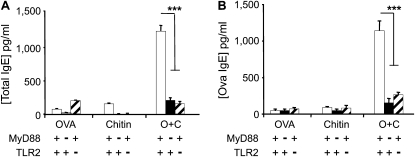
Chitin is an Adjuvant for OVA-dependent IgE Induction
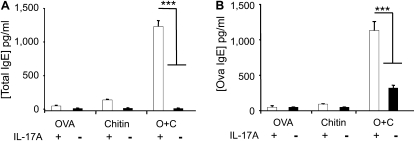
Studies were next undertaken to determine if chitin is an adjuvant for humoral immunity. This was accomplished by comparing the levels of total and antigen-specific IgE in the circulation of WT mice exposed to OVA and chitin, alone and in combination. As can be seen in Figure 3, significantly increased levels of total and antigen-specific IgE were seen in mice sensitized with chitin and OVA in combination. Similar induction was not seen in mice exposed to OVA, chitin, BSA, or CPE individually or their vehicle controls (Figure 3 and data not shown). To see if these IgE effects required TLR-2 and/or MyD88, we also compared the levels of total and antigen-specific IgE in WT, MyD88−/−, and TLR-2−/− mice. In these experiments, the IgE-inducing properties of chitin were almost completely abrogated by null mutations of TLR-2 or MyD88 (Figure 3). Thus, chitin can serve as an adjuvant for antigen-induced IgE responses and mediates these effects via a TLR-2– and MyD88-dependent pathway(s).
Figure 3.
Role of chitin in sensitization to ovalbumin (OVA) in vivo. MyD88 and Toll-like receptor 2 (TLR-2) sufficient (+/+; +) and deficient (−/−; −) mice were sensitized by two intraperitoneal injections (Days 0 and 5) of a mixture containing OVA (20 μg), chitin (25 μg), OVA complexed with chitin, or their vehicle controls. One week later the mice received aerosolized OVA for 3 days and 1 day after the last challenge were killed and serum was obtained. The levels of (A) total and (B) OVA-specific IgE were assessed by ELISA. Results are presented as the mean ± SEM of a minimum of six mice per group. ***P < 0.001.
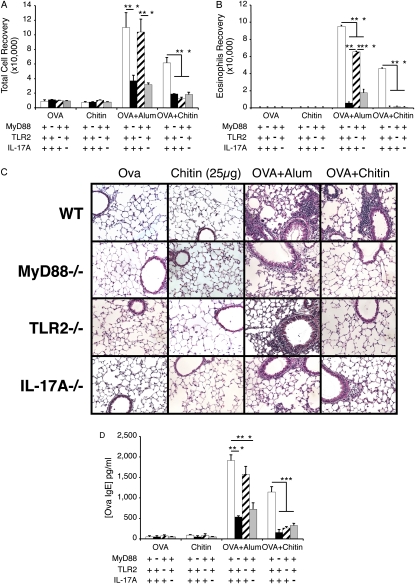
Similarities and Differences in Chitin- and Alum-induced In Vivo Responses
To further understand the properties of chitin, we compared the OVA-induced inflammatory and antibody responses that were seen in mice treated with chitin and alum. As can be seen in Figure 4, alum and chitin both served as adjuvants for OVA-induced BAL and tissue inflammation, eosinophilic infiltration, and OVA-specific IgE induction (Figures 4A–4D). In accord with what was seen with chitin, MyD88 and IL-17 played critical roles in these responses (Figure 4). In contrast, TLR-2 had a more limited role with only modest decreases in OVA plus alum-driven BAL and tissue inflammation, eosinophil accumulation, and IgE induction being noted in its absence (Figure 4). These studies demonstrate that chitin and alum are both adjuvants for antigen-induced eosinophilic inflammation and highlight similarities and differences in the pathways that they use in these inductive responses.
Figure 4.
Similarities and differences in chitin- and alum-induced in vivo responses. MyD88, Toll-like receptor 2 (TLR-2), and IL-17A sufficient (+/+; +) and deficient (−/−; −) mice received two intraperitoneal injections with a mixture containing ovalbumin (OVA) (20μg) plus chitin or alum or appropriate vehicle controls. One week later the mice received aerosolized OVA for 3 days, and 1 day after the last challenge the mice were killed. The effects of these treatments on (A) bronchoalveolar lavage (BAL) total and (B) eosinophils recovery, (C) tissue histology (hematoxylin and eosin stains, ×20 of original magnification), and (D) OVA-specific IgE were evaluated. Results in A, B, and D are presented as the mean ± SEM of a minimum of six mice per group. C is representative of three similar experiments. ***P < 0.001.
Chitin Contributes to Antigen-induced Lymphocyte Proliferation In Vitro
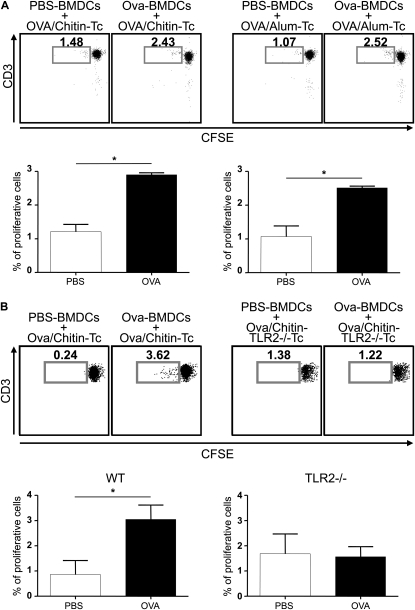
Dendritic cells are professional antigen-presenting cells and play a key role in the development of adaptive Th1 or Th2 immune responses. To further understand the adjuvant properties of chitin observed in vivo, studies were undertaken to determine if chitin induces T-cell proliferation in response to OVA. CD4+ T cells from WT mice sensitized with OVA and chitin were isolated from splenocytes and cocultured with OVA-stimulated BMDCs. As presented in Figure 5A, OVA induced antigen-specific proliferation of these T cells. As comparison, similar responses were observed with T cells from mice sensitized to OVA using alum (Figure 5A). In addition, proliferation was not observed in experiments that used CD4+ T cells isolated from mice sensitized with OVA alone, chitin alone, or alum alone (data not shown) and experiments that used CD4+ T cells isolated from TLR-2−/− mice sensitized with OVA plus chitin (Figure 5B). In combination, these studies demonstrate that chitin is an adjuvant for T-cell proliferation in vitro and that this effect is dependent on TLR-2.
Figure 5.
Effects of chitin in ovalbumin (OVA)-induced T-cell proliferation ex vivo. (A) Cocultures were prepared containing T cells (Tc) from wild-type (WT) mice immunized with OVA complexed to chitin (left pair) or alum (right pair) and OVA- or vehicle control–stimulated bone marrow–derived dendritic cells (BMDCs) from WT mice. (B) Identical cocultures were prepared using Tc from Toll-like receptor 2 (TLR-2)-sufficient and -deficient (TLR2−/−Tc) mice. Proliferation of carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled CD4+ T cells (vs. CD3 expression) was analyzed using flow cytometry at Day 5. Daughter cell generation is shown in the square box compared with undivided parental generation and is also represented as a percent of proliferative cells (histograms). One representative out of five independent experiments performed is shown. Results are presented as the mean ± SEM of a minimum of six mice per group. *P < 0.05.
Chitin Contributes to Antigen-induced Th2 Cytokine Production In Vitro
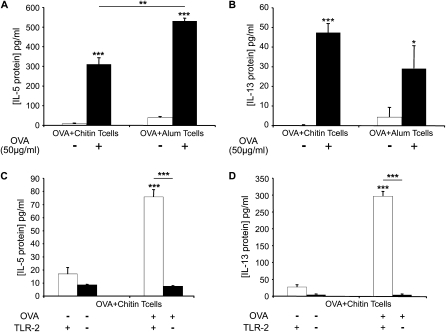
The ability of chitin to induce a Type 2 immune response was assessed using the same in vitro system. In these experiments, OVA stimulated the production of IL-4, IL-5, and IL-13 by OVA/chitin-primed T cells (Figures 6A and 6B and data not shown). In comparison, similar responses were observed with T cells from mice sensitized to OVA using alum (Figures 6A and 6B). In addition, augmented cytokine elaboration was not seen in OVA-stimulated cocultures that contained T cells from mice that were sensitized with OVA alone or chitin or alum alone (data not shown). To clarify the mechanism(s) of this response, we also performed experiments using CD4+ T cells isolated from OVA plus chitin–sensitized WT and TLR-2−/− mice. These studies demonstrated that TLR-2 plays a critical role in the production of IL-5 and IL-13 in this OVA plus chitin–driven system (Figures 6C and 6D). In combination, these studies demonstrate that chitin is a Th2 adjuvant that mediates its effect(s) via a TLR-2–dependent mechanism(s).
Figure 6.
Effects of chitin on ovalbumin (OVA)-induced cytokine production by T cells ex vivo. Cocultures were prepared that contained CD4+ T cells from wild-type (WT) mice (A and B) immunized with OVA complexed to chitin (OVA+Chitin T cells) or alum (OVA+ Alum T cells) and OVA (50 μg/ml; OVA+) or vehicle control–stimulated (OVA−) bone marrow–derived dendritic cells. Supernatants were collected after incubation for 3 days. CD4+ T cells from OVA/chitin-stimulated TLR-2 sufficient (+/+) and deficient (−/−) mice (C and D) were also used. The levels of IL-5 (A and C) and IL-13 (B and D) were measured by Bioplex ELISA. Results are presented as the mean ± SEM of a minimum of six mice per group. *P < 0.05, **P < 0.01, ***P < 0.001.
IL-17A Plays an Important Role in the In Vivo Adjuvant Effects of Chitin
Previous studies from our laboratory demonstrated that IL-17A plays a critical role in chitin-induced acute innate inflammation in vivo (6). We therefore used WT and IL-17A−/− mice to evaluate the role(s) of IL-17A in the adjuvant effects of chitin in adaptive immune responses. As noted above, BAL and tissue eosinophilic inflammation, airway mucus metaplasia, IL-4, IL-5, and IL-13 production, and increased levels of total and OVA-specific IgE were seen in OVA/chitin sensitized and OVA-challenged WT mice (Figures 7 and 8). Importantly, the ability of chitin to serve as an adjuvant for these responses was almost completely abrogated in IL-17A null mice (Figures 7 and 8). These findings demonstrate that a TLR-2–dependent pathway involving IL-17A mediates the adjuvant effects of chitin.
Figure 7.
Roles of IL-17A in the adjuvant effects of chitin in vivo. Wild-type and IL-17A−/− mice were sensitized with OVA and chitin and then challenged with ovalbumin (OVA). (A) Total bronchoalveolar lavage (BAL) cell and (B) eosinophil recovery, (C) tissue inflammation (hematoxylin and eosin, ×20 final magnification), and mucus metaplasia (periodic acid-Schiff [PAS], ×40 final magnification), and the levels of (D) BAL IL-4, (E) IL-5, and (F) IL-13 were evaluated. Supernatant cytokines were measured using ELISA. The results are expressed as the mean ± SEM of a minimum of six mice per group. ***P < 0.001. The arrow in C indicates PAS-positive airway mucus.
Figure 8.
Roles of IL-17A in the humoral adjuvant effects of chitin in vivo. Wild-type and IL-17A−/− mice were sensitized with ovalbumin (OVA) and chitin and then challenged with OVA. (A) Total and (B) OVA-specific IgE were evaluated. The results are expressed as the mean ± SEM of a minimum of six mice per group. ***P < 0.001.
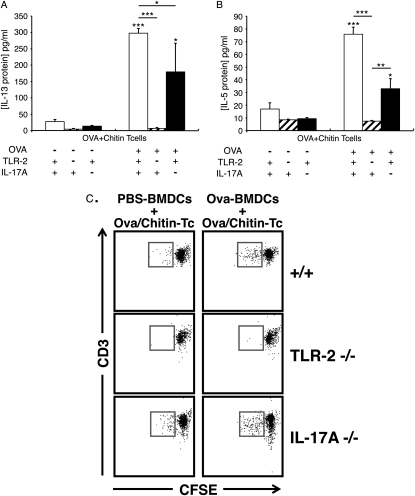
IL-17A and TLR-2 Play Different Roles in the In Vitro Adjuvant Effects of Chitin
To further understand the mechanism(s) of the in vitro Th2 adjuvant response, we evaluated the cytokine production and proliferation of CD4+ T cells isolated from OVA plus chitin–sensitized WT mice and mice with null mutations of TLR-2 or IL-17A. These studies demonstrated that TLR-2 plays a major, and IL-17A plays a significant, but lesser, role in the production of IL-5 and IL-13 in this OVA plus chitin–driven system (Figures 9A and 9B) Interestingly, TLR-2 also played a critical role in T-cell proliferation, whereas IL-17A did not (Figure 9C). Overall, these studies demonstrate that TLR-2 and IL-17A contribute to the in vitro Th2 adjuvant effects of chitin and do so via different mechanisms.
Figure 9.
Roles of IL-17A and Toll-like receptor 2 (TLR-2) in the in vitro adjuvant effects of chitin. Cocultures were prepared that contained CD4+ T cells from wild-type, IL-17A−/−, and TLR-2−/− mice immunized with ovalbumin (OVA) plus chitin (OVA+Chitin T cells) and OVA (50 μg/ml; OVA+) or vehicle control–stimulated (OVA−) bone marrow–derived dendritic cells (BMDCs). Supernatants were collected after incubation for 3 days and the levels of (A) IL-13 and (B) IL-5 were assessed by ELISA. (C) Proliferation of CFSE-labeled CD4+ T cells (vs. CD3 expression) was analyzed using flow cytometry at Day 5. Daughter cell generation is shown in the square box compared with undivided parental generation. Results in A and B are the mean ± SEM of a minimum of six mice per group. In C, one representative out of three independent experiments performed is shown. *P < 0.05, **P < 0.01, ***P < 0.001.
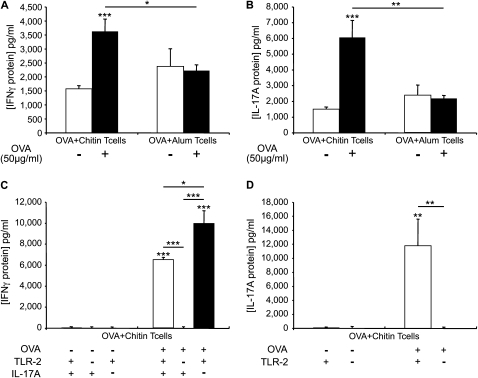
Chitin Is an Adjuvant for Antigen-induced IFN-γ and IL-17A Production
To understand the spectrum of the adjuvant effects of chitin, studies were undertaken to determine if chitin is an adjuvant for Th1 and Th17 immune responses. To accomplish this, we evaluated the levels of IFN-γ and IL-17A in supernatants from cocultures of OVA-stimulated BMDCs and WT OVA/chitin or OVA/alum-primed CD4+ T cells. T cells from mice sensitized to OVA in the presence of chitin produced elevated levels of IFN-γ and IL-17A when restimulated with OVA in vitro (Figures 10A and 10B). They were also chitin specific because similar responses were not seen when alum was used as an adjuvant (Figures 10A and 10B). These studies demonstrate that chitin is also a strong adjuvant for both Th1 and Th17 immune responses.
Figure 10.
Roles of Toll-like receptor 2 (TLR-2) and IL-17A in ovalbumin (OVA) plus chitin-induced T cell IFN-γ and IL-17A production ex vivo. Cocultures were prepared that contained CD4+ T cells from wild-type mice (A and B) immunized with OVA complexed to chitin (OVA+Chitin T cells) or alum (OVA +Alum T cells) and OVA (50 μg/ml; OVA+) or vehicle control–stimulated (OVA−) bone marrow–derived dendritic cells. Supernatants were collected after incubation for 3 days. CD4+ T cells from OVA/chitin-stimulated TLR-2 sufficient (+/+) and deficient (−/−) and IL-17A sufficient (+/+) and deficient (−/−) mice (C and D) were also used. The levels of IFN-γ (A and C) and IL-17A (B and D) were assessed by ELISA. The results are expressed as the mean ± SEM of a minimum of six mice per group. *P < 0.05, **P < 0.01, ***P < 0.001.
TLR-2 and IL-17A Play Important Roles in the Th1 and Th17 Adjuvant Effects of Chitin
To define the roles of TLR-2 and IL-17 in the Th1 and Th17 priming induced by chitin, we compared the production of IFN-γ and IL-17 by cocultures of antigen-stimulated BMDCs and CD4+ T cells from WT, TLR-2−/−, and IL-17A−/− mice that had been immunized with chitin and OVA. Compared with WT cells, cells from TLR-2 null mice produced significantly less IFN-γ and IL-17 (Figures 10C and 10D). In contrast, CD4+ T cells from IL-17A−/− mice produced significantly greater amounts of IFN-γ than cells from similarly treated WT animals (Figure 10C). When viewed in combination, these studies demonstrate that the Th1 and Th17 priming effects of chitin are directly linked to TLR-2 signaling. They also demonstrate that IL-17A down-regulates the production of IFN-γ, which can favor the development of Th2 immune responses.
DISCUSSION
The response of CD4+ T cells to protein antigens in vivo can be dramatically enhanced by the administration of antigen in adjuvant. This increases T-cell expansion and prevents tolerance induction. Potent adjuvants enhance T-cell function by augmenting the clonal expansion of antigen-stimulated T cells by producing growth and/or survival signals (23, 24). They also regulate intracellular response pathways such as that controlled by nuclear factor-κB (NF-κB) and the Nalp3-containing inflammasome (25). As a result, adjuvants have been frequently used in infectious and malignant vaccination strategies, attempts to ameliorate and/or augment immune responses in immunocompromised individuals and the elderly (23), and attempts to use animals to model antigen-mediated chronic inflammatory human disorders. Previous studies from our laboratory and others have demonstrated that appropriately sized chitin fragments can induce innate inflammatory responses (6–8). In these studies we add to our knowledge of the biology of chitin, adaptive immunity, adjuvants, and adjuvant responses by demonstrating that chitin is also a potent multifaceted adjuvant that induces adaptive Th2, Th1, and Th17 immune responses in the murine lung. These studies also demonstrate that the adjuvant properties of chitin are mediated by a pathway(s) that involves TLR-2, MyD88, and IL-17A and that the IL-17A response feeds back to inhibit IFN-γ production in this antigen-driven experimental system.
Innate immune activation is a critical step in the initiation of an adaptive immune response. As a result, activation of a class of innate pathogen receptors called pattern recognition receptors is a central feature of many adjuvant systems (26). This can be readily appreciated in prior studies, which demonstrated that TLR activators, such as LPS, and NOD-like receptor activators, such as aluminum hydroxide, are effective immune adjuvants (15, 17, 20, 27–29). Our studies demonstrate that chitin is also an effective immune adjuvant and that the adjuvant properties of chitin are dependent on TLR-2 signaling. These findings are in accord with prior studies from our laboratory demonstrating that chitin is a PAMP that activates macrophages via TLR-2 (6) and studies from others that highlight the ability of TLR-2 agonists to serve as effective adjuvants for Th2 responses (16, 19, 20).
A number of lines of evidence suggest that adjuvants augment adaptive immune responses by stimulating the production of key proinflammatory cytokines. TNF and IL-1 enhance the expansion, persistence, and differentiation of responding CD4+ T cells. IL-1 and IL-6 also exhibit costimulatory effects on CD4+ cells (25, 27), and IL-1 enhances the expression of CD40L and OX40, which are potent stimulators of CD4 cognate helper function (30). Our studies demonstrate that chitin is a potent stimulator of the production of IL-17A. They also demonstrate that IL-17A plays an important role in the pathogenesis of the Th2 adjuvant effects of this polysaccharide. The finding that chitin is a potent adjuvant for IL-17A production is in accord with studies with a variety of other adjuvants, including poly (I:C) (31), cholera toxin (32), class I restricted peptides (33), and Dectin-1 agonists (34). They are also in accord with studies from our laboratory that demonstrated that chitin is a PAMP that activates IL-17A via TLR-2 (6). The present studies, however, are the first to demonstrate that IL-17A plays a critical role in the adjuvant effects of chitin (or any other adjuvant) and thus plays a critical role in the pathogenesis of adaptive Th2 responses while inhibiting Th1 adaptive immunity. These findings add to studies from a number of other investigators highlighting the important role of IL-17A in Th2 responses and the ability of IL-17 to regulate Th1 cell differentiation (35–37).
Interestingly, although IL-17A production was largely TLR-2 dependent, the contributions that each makes to the adjuvant effects of chitin were not identical. In the absence of TLR-2, both T-cell proliferation and cytokine elaboration were markedly diminished, suggesting the absence of T-cell priming to OVA. In contrast, in the absence of IL-17A, only cytokine production was affected. Th2 cytokines such as IL-5 and IL-13 were decreased, whereas the level of IFN-γ was augmented. In addition, T-cell proliferation was not altered. These results suggest that in IL-17 null mice, the priming of CD4+ T cells is still occurring; however, the polarization toward a Th2 profile is impaired.
Allergens comprise a vast collection of nonreplicating entities with diverse immunogenic potential capable of inducing specific immune-inflammatory responses (38). How they accomplish these tasks is a subject of intense research. A great deal has been learned using OVA as a surrogate allergen. Because it is an archetypical innocuous antigen, conventional modeling has used aluminum hydroxide as an adjuvant. In contrast, it is now known that other antigens are immunogenic. This can be readily seen with house dust mite (HDM), which can induce sensitization and acute and chronic inflammation in the absence of an exogenous adjuvant (39, 40). HDM also allows sensitization to OVA antigen to occur through its “bystander” effect (38). It is clear from these studies that some allergens have intrinsic adjuvant activity. The mechanisms that underlie these responses, however, are poorly defined. It is important to point out that chitin is a component of many antigen preparations, including HDM and cockroach. Because our data demonstrate that chitin is a potent multifaceted antigen, it is tempting to speculate that, in some cases, powerful antigens induce allergic sensitization via the adjuvant properties of this associated chitin.
Aluminum hydroxide is the most commonly used adjuvant in vaccines and models of human diseases (24, 25). This is nicely illustrated in the commonly used OVA mouse model of asthma-like Th2 inflammation. The present studies demonstrate that chitin is an important adjuvant, which, like aluminum hydroxide, induces adaptive Th2 immunity. Interestingly, in vivo, the Th2 inflammatory responses that were induced by chitin and aluminum hydroxide were quantitatively and qualitatively similar. However, chitin and aluminum hydroxide also differed in a number of interesting ways. One of the most striking was the ability of chitin and the relative inability of aluminum hydroxide to stimulate IL-17A and Th1 responses. Another is the impressive dependence of chitin on TLR-2 versus the reported LPS TLR-4–dependence of aluminum hydroxide (25) and the lesser role of TLR-2 in alum-driven responses. These observations suggest that chitin and aluminum hydroxide do not mediate their adjuvant effects via identical mechanisms. Recent studies from our laboratory demonstrated that chitin is a size-dependent PAMP that can also bind to Dectin-1 and activate Syk (6). Interestingly, Dectin-1 agonists have been demonstrated to be adjuvants that augment Th1 and IL-17 responses (34). This allows for the interesting hypothesis that the multifaceted adjuvant properties of chitin are the result of its ability to activate more than one innate immune receptor. It is also tempting to speculate that the adjuvant properties of chitin will vary with size and thus may vary after interaction with chitinases or metabolism via other pathways. Additional investigations will be required to address these possibilities.
In summary, our studies demonstrate that chitin is a multifaceted adjuvant that augments Th2, Th1, humoral, and IL-17 responses in vivo and in vitro. These novel effects were mediated by pathways involving TLR-2, MyD88, and IL-17A. It also shows that the induced IL-17A inhibits the Th1 stimulatory effects of this polysaccharide. These studies provide insights that are relevant to the pathogenesis of allergic and parasitic sensitization and help to clarify the relationships between Th2, Th1, and IL-17 antigen-induced tissue responses in diseases such as asthma, allergy, and parasitic infestation. Additional investigations of the mechanisms of these adjuvant effects and the consequences of interventions that alter them in asthma, allergy, and related disorders are warranted.
Supplementary Material
Supported by National Institutes of Health Grant HL-081639 (J.A.E.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200912-1877OC on July 23, 2010
Author Disclosure: C.A.D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.G.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.E. received $5,001–$10,000 from Promedior Inc. for consulting on pulmonary fibrosis (ended years ago) and $1,001–$5,000 from Intermune Inc. for serving on a scientific advisory board; holds patents from Yale University on chitinases in lung inflammation, mir-1 in VEGF tissue responses, IL-18 in COPD, and VEGF in asthma; and holds $1,001–$5,000 from Merck and $5,001–$10,000 from Intermune in stock ownership or options.
References
- 1.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci 1976;21:73. [DOI] [PubMed] [Google Scholar]
- 2.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol 1985;17:93. [DOI] [PubMed] [Google Scholar]
- 3.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem 1993;41:571. [DOI] [PubMed] [Google Scholar]
- 4.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol 1994;48:471. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678. [DOI] [PubMed] [Google Scholar]
- 6.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol 2008;181:4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol 2009;182:3573. [DOI] [PubMed] [Google Scholar]
- 8.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007;447:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redecke V, Dalhoff K, Bohnet S, Braun J, Maass M. Interaction of Chlamydia pneumoniae and human alveolar macrophages: infection and inflammatory response. Am J Respir Cell Mol Biol 1998;19:721. [DOI] [PubMed] [Google Scholar]
- 10.Schroder NW, Opitz B, Lamping N, Michelsen KS, Zahringer U, Gobel UB, Schumann RR. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J Immunol 2000;165:2683. [DOI] [PubMed] [Google Scholar]
- 11.Horner AA, Raz E. Do microbes influence the pathogenesis of allergic diseases? Building the case for Toll-like receptor ligands. Curr Opin Immunol 2003;15:614. [DOI] [PubMed] [Google Scholar]
- 12.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol 2004;164:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr 2004;16:659. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. J Immunol 2004;172:4527. [DOI] [PubMed] [Google Scholar]
- 15.Horner AA, Redecke V, Raz E. Toll-like receptor ligands: hygiene, atopy and therapeutic implications. Curr Opin Allergy Clin Immunol 2004;4:555. [DOI] [PubMed] [Google Scholar]
- 16.Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol 2004;172:4425. [DOI] [PubMed] [Google Scholar]
- 17.Duez C, Gosset P, Tonnel AB. Dendritic cells and toll-like receptors in allergy and asthma. Eur J Dermatol 2006;16:12. [PubMed] [Google Scholar]
- 18.Schroder NW, Arditi M. The role of innate immunity in the pathogenesis of asthma: evidence for the involvement of Toll-like receptor signaling. J Endotoxin Res 2007;13:305. [DOI] [PubMed] [Google Scholar]
- 19.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol 2004;172:4733. [DOI] [PubMed] [Google Scholar]
- 20.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol 2004;172:2739. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Homer RJ, Chen Q, Elias JA. Endogenous and exogenous IL-6 inhibit aeroallergen-induced Th2 inflammation. J Immunol 2000;165:4051. [DOI] [PubMed] [Google Scholar]
- 22.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med 1998;188:1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol 2009;182:6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenbarth SC. Use and limitations of alum-based models of allergy. Clin Exp Allergy 2008;38:1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 2008;453:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahiri A, Das P, Chakravortty D. Engagement of TLR signaling as adjuvant: towards smarter vaccine and beyond. Vaccine 2008;26:6777. [DOI] [PubMed] [Google Scholar]
- 27.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med 2002;196:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol 2003;15:620. [DOI] [PubMed] [Google Scholar]
- 29.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maue AC, Waters WR, Palmer MV, Whipple DL, Minion FC, Brown WC, Estes DM. CD80 and CD86, but not CD154, augment DNA vaccine-induced protection in experimental bovine tuberculosis. Vaccine 2004;23:769. [DOI] [PubMed] [Google Scholar]
- 31.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 2009;206:1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS ONE 2009;4:e5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tigno-Aranjuez JT, Lehmann PV, Tary-Lehmann M. Dissociated induction of cytotoxicity and DTH by CFA and CpG. J Immunother 2009;32:389. [DOI] [PubMed] [Google Scholar]
- 34.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood 2008;112:4971. [DOI] [PubMed] [Google Scholar]
- 35.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002;17:375. [DOI] [PubMed] [Google Scholar]
- 36.Song C, Luo L, Lei Z, Li B, Liang Z, Liu G, Li D, Zhang G, Huang B, Feng ZH. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol 2008;181:6117. [DOI] [PubMed] [Google Scholar]
- 37.Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation. Curr Opin Immunol 2008;20:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattouh R, Pouladi MA, Alvarez D, Johnson JR, Walker TD, Goncharova S, Inman MD, Jordana M. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med 2005;172:314. [DOI] [PubMed] [Google Scholar]
- 39.Hammad H, Charbonnier AS, Duez C, Jacquet A, Stewart GA, Tonnel AB, Pestel J. Th2 polarization by Der p 1–pulsed monocyte-derived dendritic cells is due to the allergic status of the donors. Blood 2001;98:1135. [DOI] [PubMed] [Google Scholar]
- 40.Hammad H, Lambrecht BN, Pochard P, Gosset P, Marquillies P, Tonnel AB, Pestel J. Monocyte-derived dendritic cells induce a house dust mite-specific Th2 allergic inflammation in the lung of humanized SCID mice: involvement of CCR7. J Immunol 2002;169:1524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.