Abstract
Rationale: Phagocytosis of apoptotic cells, also called efferocytosis, plays an essential role in the resolution of inflammation. Urokinase-type plasminogen activator (uPA) is a multifunctional protein that has been implicated in inflammatory conditions, including pneumonia and severe infection, which are often accompanied by the development of acute lung injury. However, the role of uPA in modulating efferocytosis of apoptotic neutrophils has not been defined.
Objectives: To characterize the role of uPA in regulation of efferocytosis and to delineate the underlying mechanisms involved in this process.
Methods: In vitro and in vivo phagocytosis, immunoprecipitation, and Western blotting assays.
Measurements and Main Results: The phagocytosis of apoptotic neutrophils by macrophages was significantly inhibited by uPA. Mutant uPA lacking the growth factor domain and catalytically inactive uPA had similar inhibitory effects on efferocytosis, as did wild-type uPA. In contrast, absence of the kringle domain abrogated the ability of uPA to diminish efferocytosis. Both the αVβ3 integrin and vitronectin seemed to be involved in the inhibition of efferocytosis by uPA. Incubation of macrophages with uPA also diminished activation of the small GTPase Rac-1, which normally occurs during ingestion of apoptotic neutrophils. Under in vivo conditions in the lungs, uPA decreased the uptake of apoptotic neutrophils by alveolar macrophages.
Conclusions: Our data demonstrate a novel role for uPA in which it is able to diminish the uptake of apoptotic neutrophils by macrophages under both in vitro and in vivo conditions.
Keywords: phagocytosis, integrin αvβ3, inflammation, acute lung injury
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Urokinase-type plasminogen activator (uPA) is involved in diverse physiologic and pathophysiologic processes, including fibrinolysis, cell migration and adhesion, and inflammation. However, the role of uPA in modulating efferocytosis of apoptotic neutrophils has not been defined.
What This Study Adds to the Field
The present results, by demonstrating that uPA inhibits the uptake of apoptotic neutrophils, suggest a novel mechanism by which elevated levels of uPA may participate in enhancing the duration and severity of inflammatory processes, such as acute lung injury, in which neutrophils play a major role.
Phagocytosis of apoptotic cells, also called efferocytosis, is an important feature of immune responses and is involved in normal tissue homeostasis and resolution of inflammation (1–5). Impaired efferocytosis occurs with autoimmune and inflammatory diseases, such as systemic lupus erythematosus and acute lung injury (ALI), and is associated with unfavorable outcome from inflammation (6–11).
Urokinase-type plasminogen activator (uPA) is involved in diverse physiologic and pathophysiologic processes, including fibrinolysis, cell migration and adhesion, and inflammation (12–15). uPA is initially secreted as a single-chain (scuPA) consisting of three structurally independent domains (16): (1) a N-terminal epidermal growth factor–like domain (GFD; aa 1–46) that is responsible for binding of uPA to its high-affinity receptor, CD87 (uPAR), and that participates in uPA-induced neutrophil chemotaxis; (2) a kringle domain (KD; aa 47–135), which mediates uPA binding to integrins, including αvβ3, and has been shown to enhance LPS-induced neutrophil activation (15, 17) and vascular contraction (18); and (3) a serine protease domain (aa 159–411) that includes the catalytically active site of uPA (19).
Elevated tissue and circulating levels of uPA are present for prolonged periods in patients with acute and chronic inflammatory conditions, including pneumonia and severe infection, which are often accompanied by the development of ALI (20–22). ALI is characterized by accumulation of activated neutrophils in the lungs, and clearance of neutrophils from the lungs occupies a major role in the resolution of this pathophysiologic process (23, 24). We have previously demonstrated that uPAR, the receptor of uPA, and plasminogen activator inhibitor 1 (PAI)-1, the natural inhibitor of uPA and tissue plasminogen activator, regulate the engulfment of both viable and apoptotic neutrophils (25, 26). Given the roles that uPAR and PAI-1 occupy in modulating the activity of uPA and the correlation between enhanced levels of uPA and the severity of acute inflammatory diseases, including ALI, we hypothesized that uPA might also participate in modulating efferocytosis. This study demonstrates a novel role for uPA in which it is able to diminish the uptake of apoptotic neutrophils by macrophages under both in vitro and in vivo conditions.
METHODS
Mice
C57BL/6, vitronectin (Vn)−/−, and uPA−/− mice were purchased from NCI-Frederick (Frederick, MD) and Jackson Laboratory (Bar Harbor, ME). The uPAR−/− mice were a gift from Margaret Gyetko (University of Michigan). The uPA−/−, uPAR−/−, and Vn−/− mice were all C57BL/6 background. Vn−/− mice were back-crossed to C57BL/6 for 12 generations. Mouse protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham and The University of Pennsylvania.
Materials
Custom antibody mixtures for bone marrow neutrophil purification were from Stem Cell Technologies (Vancouver, BC, Canada). Annexin V-FITC, propidium iodide, recombinant human integrin αvβ3, and recombinant mouse milk fat globule epidermal growth factor-8 (MFG-E8) were from R&D (Minneapolis, MN). Human serum albumin (HSA), carboxylate-modified beads, and protein G-agarose were from Sigma (St. Louis, MO). Anti-uPA antibodies were from Santa Cruz (Santa Cruz, CA). Anti–human-αvβ3 antibody, uPA activity assay kit, and Rac-1 activation assay kit were from Millipore (Billerica, MA). Mouse urokinase (low and high molecular weight) was from Molecular Innovations (Novi, MI). Human recombinant scuPA, uPA deletion mutants lacking GFD (ΔGFD) or KD (ΔKD), and the catalytically inactive uPA mutant (S356A), in which alanine 356 is substituted for serine, were previously described (27).
Purification and Culture of Mouse Bone Marrow Neutrophils
Purification and culture of mouse bone marrow neutrophils was performed as previously described (15, 28–30) (see online supplement).
Purification and Culture of Peritoneal Macrophages
Purification and culture of peritoneal macrophages was performed from thioglycollate peritoneal exudates as previously described (25, 26), and as detailed in the online supplement.
Induction of Neutrophil Apoptosis
Neutrophil apoptosis was induced by heating to 43°C for 1 hour, followed by culture at 37°C in 5% CO2 for 2.5 hours, as previously described (25, 26).
In Vitro Efferocytosis Assay
In vitro efferocytosis assays were performed as previously described (25, 26) and as detailed in the online supplement.
In Vivo Efferocytosis Assay
In vivo efferocytosis assays were performed as previously described (25, 26) and as detailed in the online supplement.
uPA Activity Assay
Protease activity of uPA and uPA mutants was determined using an activity kit (Millipore), and calculated as U/μg uPA protein.
Immunoprecipitation and Immunoblotting
Wild-type and mutant uPA were preincubated with soluble αvβ3 at 4°C for 1 hour. Anti-αvβ3 antibody (1 μg/ml) was added to each sample and incubated at 4°C overnight. Protein G-agarose beads were added to each sample and incubated for 2 hours. In designated experiments, uPA or MFG-E8 was preincubated with αvβ3 protein for 30 minutes followed by the addition of uPA or MFG-E8 for another 30 minutes. Finally, immunoprecipitation with anti-αvβ3 antibody was performed, followed by Western blotting with anti-uPA antibodies.
Rac-1 Activation Assay
Rac-1 activation was determined using a kit as described in detail in the online supplement.
Model for ALI
Age matched wild-type and uPA−/− mice were anesthetized with isoflurane. LPS (2 mg/kg body weight) in 50 μl phosphate-buffered saline was injected intratracheally. Mice were killed 24 hours after LPS administration and bronchoalveolar lavage (BAL) was performed using 1 ml phosphate-buffered saline.
Statistical Analysis
Macrophages and neutrophils for each experiment were isolated and pooled from three to five mice. Data are representative of two or three independent experiments. Means ± SD are shown. One-way analysis of variance followed by Tukey-Kramer analysis for comparison between multiple groups and Student t test for comparisons between two groups were used. Differences were considered statistically significant when P < 0.05.
RESULTS
uPA Inhibits Efferocytosis of Apoptotic Neutrophils
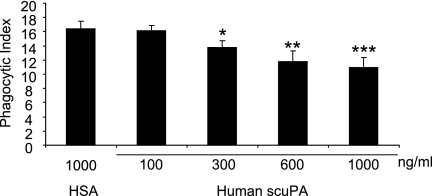
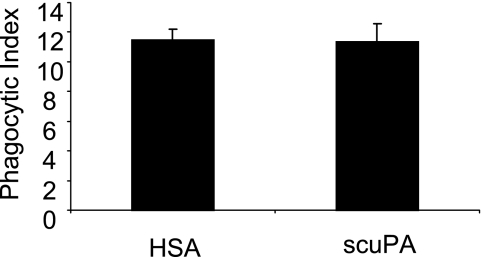
In initial experiments (Figure 1), we found that the uptake of apoptotic neutrophils by macrophages was significantly inhibited when human scuPA was added to cultures of apoptotic neutrophils and peritoneal macrophages at concentrations of 300–1,000 ng/ml. Levels of uPA similar to those that inhibited efferocytosis have been reported in the circulation and in tissue sites, such as the lungs, during acute inflammatory conditions induced by pneumonia and other severe infections (20, 21). Of note, incubation of macrophages with scuPA did not result in any increased release of tumor necrosis factor-α or IL-6 (see Figure E1 in the online supplement), indicating that the inhibitory effect of uPA on efferocytosis is a specific effect caused by uPA, but not by any potential contamination by pathogen-associated molecular patterns in the scuPA protein. Incubation of macrophages with scuPA did not affect their ability to ingest carboxylate-modified beads (Figure 2), a process that occurs through mechanisms distinct from those involved in apoptotic cell engulfment (5, 31, 32). Such results indicate that the inhibitory effects of uPA on efferocytosis are specific to this process and do not reflect more generalized actions on macrophage function.
Figure 1.
Urokinase-type plasminogen activator (uPA) inhibits efferocytosis of apoptotic neutrophils. A total of 100, 300, 600, or 1,000 ng/ml human single-chain plasminogen activator (scuPA) or 1,000 ng/ml human serum albumin (HSA) was added to cultures of macrophages and apoptotic neutrophils. Phagocytosis assays were performed as described in the Methods section. Each condition included two independent samples and each sample was counted four times by a blinded observer. Data are shown as SD of the eight counts from each condition. * P < 0.05, ** P < 0.01, *** P < 0.001 compared with the group treated with HSA.
Figure 2.
Urokinase-type plasminogen activator (uPA) does not affect macrophage ingestion of carboxylate-modified beads. Carboxylate-modified beads were incubated with peritoneal macrophages in the presence of 1 μg/ml human serum albumin (HSA) or single-chain plasminogen activator (scuPA) and then phagocytosis assays were performed. Representative data are shown. Two additional independent experiments provided similar results.
Suppression of Phagocytosis By uPA is Independent of Protease Activity
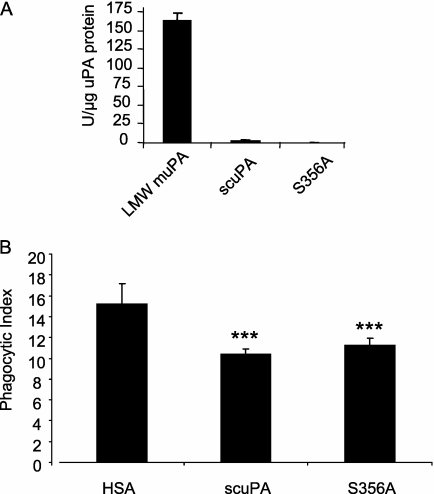
uPA consists of three domains: (1) GFD; (2) KD; and (3) the proteolytic domain, which is responsible for the proteolytic activity of the molecule (16). Although uPA was defined primarily as a protease that cleaves and activates plasminogen, recent studies have demonstrated that uPA has a variety of activities, such as regulation of adhesion and migration, which are independent of its protease activity (16, 33–35). To determine if protease activity is required for the inhibitory effect of uPA on efferocytosis, a catalytically inactive form of uPA in which alanine has been substituted for serine 356 (S356A) was used (27). As shown in Figure 3A, compared with catalytically active low-molecule-weight murine uPA, scuPA had minimal proteolytic activity and S356A had no detectable fibrinolytic activity. However, both scuPA and S356A inhibited phagocytosis of apoptotic neutrophils to a similar degree (Figure 3B). These data indicate that protease activity is not required for uPA to inhibit the engulfment of apoptotic neutrophils by macrophages.
Figure 3.
Inhibition of phagocytosis by urokinase-type plasminogen activator (uPA) does not require proteinase activity. (A) Proteolytic activities of murine low-molecular-weight uPA (LMW-muPA), single-chain plasminogen activator (scuPA), and mutant scuPA with substitution of serine 356 with alanine (S356A) were determined. The proteolytic activity of the samples is expressed as U/μg uPA protein. Results are from three independent assays of activity. (B) Catalytically inactive scuPA (S356A) exhibits similar inhibitory activity on the phagocytosis index as does scuPA. A representative experiment is shown. Two additional independent experiments provided similar results. *** P < 0.001 compared with human serum albumin (HSA).
Inhibition of Phagocytosis By uPA Requires the KD, but Not the GFD
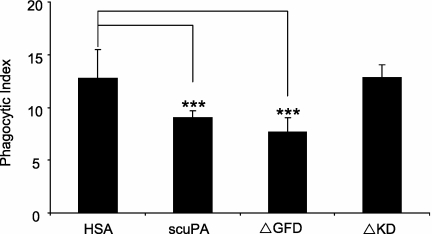
The GFD of uPA binds to uPAR, whereas the KD has been shown to interact with other cellular receptors, including lipoprotein-related receptor, αMβ2, and αvβ3 (36–38). To delineate the mechanisms by which uPA inhibits efferocytosis, we examined the role of the GFD and KD domains of uPA in this process. As shown in Figure 4, mutant uPA lacking GFD (ΔGFD) was as active as the wild-type molecule in diminishing the ability of macrophages to ingest apoptotic neutrophils. However, mutant uPA lacking KD (ΔKD) did not have any effect on macrophage uptake of apoptotic neutrophils. These data suggest that the KD, but not the GFD, is required for the inhibitory activity of uPA on phagocytosis of apoptotic neutrophils.
Figure 4.
The kringle domain (KD), but not the growth factor domain (GFD), of urokinase-type plasminogen activator (uPA), is required for inhibition of efferocytosis. Human serum albumin (HSA), single-chain plasminogen activator (scuPA), ΔGFD uPA, and ΔKD uPA, at 1 μg/ml, were added to cultures of macrophages and apoptotic neutrophils and phagocytosis assays were performed. Data shown are representative of three independent experiments. *** P < 0.001 compared with groups treated with HSA.
uPAR Is Not Required For uPA to Inhibit Phagocytosis of Apoptotic Neutrophils
Our data showing that the GFD, which binds to uPAR, is not required for inhibition of efferocytosis by uPA suggests that uPAR is not needed for this action of uPA. However, we have previously shown that uPAR itself regulates phagocytosis of both viable and apoptotic neutrophils (26). To further clarify if uPAR plays either a direct or indirect role in modulating the effect of uPA on efferocytosis, we exposed uPAR−/− macrophages to either human scuPA or mouse two-chain uPA, which binds to uPAR, during culture with apoptotic neutrophils. As shown in Figure 5, the absence of uPAR did not affect the activity of human scuPA or mouse uPA in inhibiting phagocytosis of apoptotic neutrophils by macrophages. Such findings indicate that uPAR is not required for uPA to affect efferocytosis, either directly or indirectly.
Figure 5.
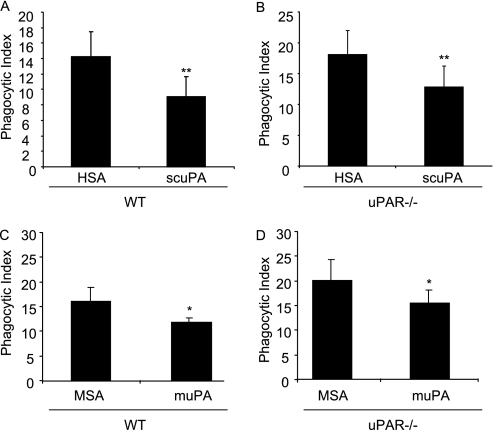
The urokinase-type plasminogen activator (uPA) receptor (uPAR) is not required for uPA to inhibit phagocytosis of apoptotic neutrophils. Apoptotic wild-type (WT) neutrophils were cultured with WT (A and C) or uPAR−/− (B and D) peritoneal macrophages in the presence of human serum albumin (HSA) or single-chain plasminogen activator (scuPA) (1 μg/ml) (A and B) or mouse serum albumin (MSA) or mouse two-chain uPA (muPA) (C and D) and phagocytosis assays were performed. Data shown are representative of two independent experiments. * P < 0.05, ** P < 0.01 compared with groups treated with HSA or MSA.
The αvβ3 Integrin Participates in the Inhibition of Efferocytosis By uPA
In addition to binding to uPAR, uPA can modulate cellular activation and signaling through uPAR-independent mechanisms, including interactions with integrins, particularly αvβ3, that occur through association with the KD (17, 38). Given the results showing that the KD was required for the ability of uPA to inhibit the ingestion of apoptotic neutrophils by macrophages, we hypothesized that interactions between αvβ3 and uPA might play a role in modulating efferocytosis. Of note, αvβ3 has previously been shown to participate in efferocytosis through facilitating interactions between apoptotic and phagocytic cells (39).
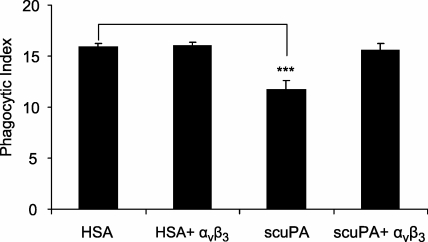
To examine the role that interactions between αvβ3 and uPA might play in affecting efferocytosis, scuPA or HSA were incubated with or without soluble αvβ3 before being added to macrophages that were then used in phagocytosis assays. As shown in Figure 6, coincubation of scuPA with soluble αvβ3 totally abrogated the decrease in phagocytosis produced by scuPA. Such findings suggest that binding of uPA to αvβ3 participates in modulating the inhibitory effects of uPA in efferocytosis.
Figure 6.
Association of urokinase-type plasminogen activator (uPA) with the αvβ3 integrin abrogates the inhibitory effects of uPA on efferocytosis. Human serum albumin (HSA) or single-chain plasminogen activator (scuPA) (1 μg/ml) was preincubated with or without soluble αvβ3 (0.5 μg/ml) for 1 hour after which the mixtures were added to peritoneal macrophages and incubated for 1 hour. The macrophages were washed three times with RPMI, then apoptotic neutrophils were added and phagocytosis assays were performed. Data shown are representative of three independent experiments. *** P < 0.001 compared with groups treated with HSA.
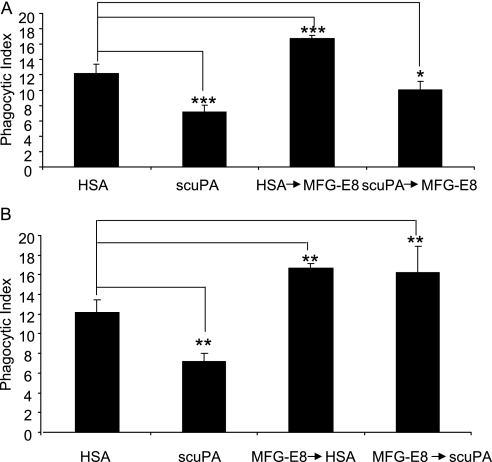
To confirm the importance that interactions between uPA and αVβ3 play in modulating efferocytosis, we examined the ability of uPA to affect the ability of MFG-E8 to enhance the engulfment of apoptotic neutrophils by macrophages. MFG-E8 functions as an opsonin, bridging phosphatidylserine (PtSer) on the apoptotic neutrophil with αvβ3 on the macrophage surface, thereby increasing the uptake of apoptotic neutrophils by phagocytes (40, 41). Given the ability of MFG-E8 to increase phagocytosis of apoptotic neutrophils through binding to αvβ3, we hypothesized that uPA might block this effect through competitively interacting with the same integrin. To examine this question, we pretreated macrophages with MFG-E8 followed by incubation with scuPA or pretreated macrophages with scuPA followed by incubation with MFG-E8. As shown in Figure 7A, when macrophages were incubated with MFG-E8 before scuPA, scuPA lost its ability to inhibit efferocytosis. In contrast, exposure of macrophages to scuPA before MFG-E8 not only resulted in a significant decrease in phagocytosis of apoptotic neutrophils, but also prevented MFG-E8 from increasing efferocytosis (Figure 7B). Such results suggest that uPA and MFG-E8 compete for the same receptor, presumably αvβ3, on the macrophage surface and can modulate efferocytosis in opposite directions through interaction with αvβ3.
Figure 7.
Urokinase-type plasminogen activator (uPA) and milk fat globule epidermal growth factor-8 (MFG-E8) have competitive effects on efferocytosis. (A) uPA diminishes the enhanced effect of MFG-E8 on phagocytosis of apoptotic neutrophils. Macrophages were preincubated with human serum albumin (HSA) or single-chain plasminogen activator (scuPA) (1 μg/ml) for 1 hour at 37°C in RPMI medium. The cells were then washed three times and incubated with HSA or MFG-E8 (1 μg/ml) for an additional 1 hour at 37°C. After washing three times, apoptotic neutrophils were added to the cultures and phagocytosis assays were performed. (B) The enhancement of phagocytosis induced by preincubation of macrophages with MFG-E8 is not affected by the subsequent addition of uPA. Macrophages were preincubated with HSA or MFG-E8 (1 μg/ml) for 1 hour at 37°C in RPMI medium. The cells were then washed three times and incubated with HSA or uPA (1 μg/ml) for an additional 1 hour at 37°C. After washing three times, apoptotic neutrophils were added to the cultures and phagocytosis assays were performed. Data shown are representative of three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001.
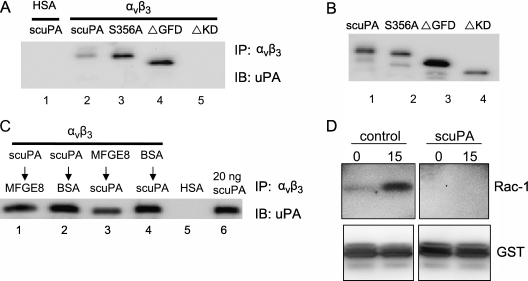
To demonstrate directly that binding between αvβ3 and uPA is responsible for the inhibitory effect of uPA on efferocytosis, we examined the interaction of αvβ3 with wild-type uPA, uPA lacking proteolytic activity (S356A), and uPA mutants lacking the GFD or KD. As shown in Figure 8A, wild-type uPA and ΔGFD uPA and S356A uPA were able to bind to αvβ3. However, ΔKD uPA did not interact with αvβ3. Of note, the anti-uPA antibody used in Figure 8A was able to recognize all forms of uPA (Figure 8B). These results are consistent with our findings that uPA lacking the KD (i.e., ΔKD uPA) does not inhibit efferocytosis, and suggest that the interaction between αvβ3 and uPA is responsible for the inhibitory effects of uPA on efferocytosis.
Figure 8.
Urokinase-type plasminogen activator (uPA) and milk fat globule epidermal growth factor-8 (MFG-E8) competitively bind to αvβ3. (A) Single-chain plasminogen activator (scuPA), ΔGFD uPA, and S356A uPA, but not ΔKD uPA bind to αvβ3. In these experiments, 100-ng recombinant αvβ3 extracellular domain was incubated with scuPA, ΔGFD, S356A, or ΔKD (100 ng) at 4°C overnight with rotation. Antibodies to αvβ3 were then added and the mixtures incubated for 1 hour. Immunocomplexes were precipitated with Protein G agarose and resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). uPA interacting with αvβ3 was detected by anti-uPA antibodies. uPA incubated with human serum albumin (HSA) was used as a negative control and showed no pull-down by anti-αvβ3 antibodies (lane 1). (B) The inputs of scuPA, ΔGFD, S356A, and ΔKD used in (A). A total of 20 ng of each protein was resolved by SDS-PAGE and blotted with anti-uPA antibodies. (C) uPA diminishes binding of MFG-E8 to αvβ3. A 100-ng recombinant αvβ3 extracellular domain was incubated with 100 ng scuPA (lanes 1 and 2), MFG-E8 (lane 3), or bovine serum albumin (BSA) (lane 4) at 4°C overnight with rotation. MFG-E8 (lane 1), BSA (lane 2), or scuPA (lanes 1 and 2) was then added and incubated for another 1 hour, followed by the addition of anti-αvβ3 antibodies and incubation for 1 hour. The immunocomplexes were precipitated with Protein G agarose and resolved by SDS-PAGE. uPA interacting with αvβ3 was detected by anti-uPA antibodies. A total of 20 ng uPA was used as input. (D) uPA diminishes Rac-1 activation induced by efferocytosis. Macrophages were pretreated with HSA or scuPA (1 μg/ml) for 1 hour. The medium was then aspirated and 10×106 apoptotic thymocytes were added to the macrophages for 0 and 30 minutes. The cells were washed five times with cold phosphate-buffered saline and lysed in Mg2+ lysis buffer. Activated Rac-1 that bound to GST-tagged PAK-1 PBD was assayed as described in the Methods section (top panels). The membrane was then stripped and blotted with anti-GST antibody to demonstrate equal pull-down of GST tagged PAK-1 PBD (bottom panels). Top panels or bottom panels were from the same blot with deletion of unrelated experiments in the middle. Data shown are representative of two to three independent experiments.
To further demonstrate that competition between uPA and MFG-E8 for binding to αvβ3 is involved in the modulation of efferocytosis in opposite directions by these two proteins, we performed coimmunoprecipitation studies. As shown in Figure 8C, preincubation of uPA with αvβ3 diminished binding of αvβ3 to MFG-E8, whereas the interaction of MFG-E8 and αvβ3 was unaffected by subsequent exposure of αvβ3 to uPA. These results are consistent with the findings that preincubation of macrophages with uPA diminished phagocytosis even if the macrophages were subsequently exposed to MFG-E8, whereas the ability of MFG-E8 to enhance efferocytosis was unaffected by the subsequent incubation of macrophages with uPA.
To determine if uPA diminishes integrin-associated downstream signaling events that are activated during efferocytosis, we examined Rac-1 activation in macrophages incubated with either uPA or HSA followed by exposure to apoptotic thymocytes. As shown in Figure 8D, resting macrophages had only minimum activation of Rac-1. Addition of apoptotic cells to macrophages dramatically activated Rac-1. However, preincubation of macrophages with uPA attenuated such efferocytosis-associated Rac-1 activation (Figure 8D). Because activation of Rac-1 is involved in facilitating alterations in macrophage morphology required for ingestion of apoptotic cells (3–5, 42), these results suggest that a mechanism by which uPA inhibits macrophages from engulfing apoptotic cells is through preventing αvβ3-induced activation of intracellular pathways, including those involving Rac-1, required for optimal phagocytic function during efferocytosis.
Inhibition of Efferocytosis By uPA Requires Vn
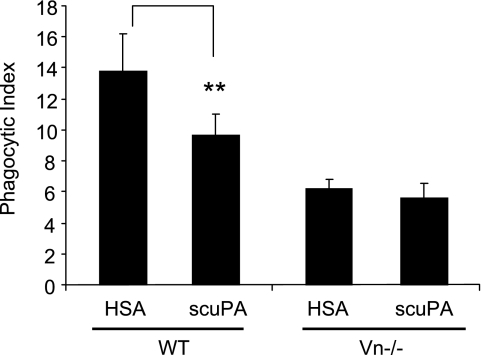
Vn is a ligand for integrins, including αvβ3, and also promotes neutrophil adhesion and migration (43, 44). Interactions between Vn, uPA, and PAI-1 modulate the proteolytic activity of uPA and its binding to uPAR and other receptors (45–47). Given the ability of Vn to associate with αvβ3, we hypothesized that Vn might also play a role in modulating the ability of uPA to affect efferocytosis. To examine this issue, we used neutrophils, macrophages, and serum from wild-type and transgenic mice lacking Vn (Vn−/−) in phagocytosis assays. As shown in Figure 9, in the absence of Vn, scuPA lost its ability to inhibit phagocytosis of apoptotic neutrophils by macrophages, indicating that interactions between uPA and Vn are necessary for the inhibitory effects of uPA on efferocytosis.
Figure 9.
The inhibitory effect of urokinase-type plasminogen activator (uPA) on efferocytosis requires vitronectin (Vn). Human serum albumin (HSA) or single-chain plasminogen activator (scuPA) (1 μg/ml) was added to cultures of wild-type apoptotic neutrophils and macrophages including wild-type mouse serum (A) or to cultures of Vn−/− apoptotic neutrophils and macrophages containing Vn−/−serum (B) and phagocytosis assays were performed. Data from a representative experiment are shown (triplicates for each condition). Two additional independent experiments provided similar results. ** P < 0.01 compared with the group treated with HSA.
uPA Inhibits Efferocytosis In Vivo
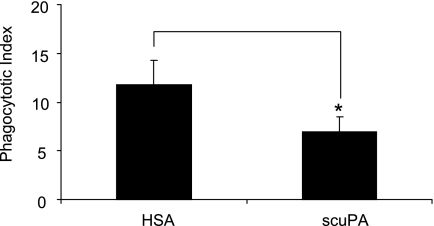
To investigate whether uPA can inhibit efferocytosis under in vivo conditions, apoptotic wild-type neutrophils were injected intratracheally with scuPA or HSA into wild-type mice. As shown in Figure 10, the phagocytic index was significantly decreased when scuPA, but not HSA, was included with the apoptotic neutrophils. Such results demonstrate that uPA inhibits efferocytosis in vivo in a fashion similar to that observed under in vitro conditions.
Figure 10.
Urokinase-type plasminogen activator (uPA) diminishes efferocytosis in vivo. Apoptotic neutrophils (10×106) and 2 μg single-chain plasminogen activator (scuPA) or human serum albumin (HSA) in 50 μl phosphate-buffered saline were injected intratracheally. Two hours later, bronchoalveolar lavages were obtained and phagocytic indices determined. Data from a representative experiment are shown. A second independent experiment provided similar results. * P < 0.05 compared with the group injected intratracheally with HSA.
Levels of uPA are elevated in the lungs of mice with ALI (48). To determine if the presence of uPA in BAL fluid during ALI modulates the ability of macrophages to phagocytose apoptotic neutrophils, we included BAL fluid from wild-type or uPA−/− mice with LPS-induced ALI in efferocytosis assays. As shown in Figure E2, uptake of apoptotic neutrophils was reduced when macrophages were treated with BAL fluid from wild-type mice with LPS-induced ALI as compared with when BAL fluid from uPA−/− mice with LPS-induced ALI was included in the cultures. These data suggest that the presence of uPA in the lungs of mice with ALI is associated with diminished clearance of apoptotic neutrophils.
DISCUSSION
uPA is involved in diverse physiologic and pathophysiologic processes, including fibrinolysis, cell migration and adhesion, and inflammation (12–15). In this study, we identified a novel function for uPA in inhibiting the phagocytosis of apoptotic neutrophils. This activity of uPA seemed to involve interaction between uPA and the αvβ3 integrin, and is specific for apoptotic cell engulfment because uPA had no effect on the uptake of carboxylate modified beads by macrophages, a process that involves distinct mechanisms from those implicated in efferocytosis (5, 31, 32).
A classic activity of uPA is participation in fibrinolysis, in which it cleaves plasminogen to generate plasmin (12–15). The proteolytic activity of uPA is also involved in modulating cellular function through cleavage of receptors, such as the α6 integrin (49, 50). However, the proteolytic activity of uPA does not seem to contribute to its inhibitory effects on neutrophil phagocytosis. This conclusion is supported by several lines of evidence. Single-chain uPA, which has minimal proteolytic activity, inhibited phagocytosis of apoptotic neutrophils. Furthermore, a proteolytically inactive mutant of uPA, S536A, was as effective as wild-type scuPA in diminishing engulfment of apoptotic neutrophils. Our data therefore add to the accumulating evidence showing that uPA possesses a variety of important biologic functions relating to inflammation and vascular reactivity that are independent of its proteolytic activity (12–15).
We previously demonstrated that uPAR, a major receptor for scuPA and proteolytically active two-chain uPA, regulates phagocytosis of both viable and apoptotic neutrophils through integrin-dependent mechanisms (26). However, as shown in the present studies, the inhibitory effect of uPA on efferocytosis is unrelated to uPAR. This conclusion is supported by evidence showing that human scuPA, which is unable to bind to mouse uPAR (51), effectively blocked the engulfment of apoptotic neutrophils by mouse peritoneal macrophages. In addition, we found that mouse two-chain uPA, which binds to mouse uPAR, inhibits the engulfment of apoptotic neutrophils by uPAR−/− macrophages to an extent comparable with that found with wild-type macrophages. Our findings therefore indicate that uPAR does not participate in the regulation of phagocytosis by uPA.
In addition to binding to uPAR, uPA also interacts with other cellular receptors, including integrins and the low-density lipoprotein-related receptor, through which uPA is able to activate intracellular signaling events (18, 37, 38). Integrins, particularly αvβ3, are among the most important receptors that mediate engulfment of apoptotic cells (3–5, 42). Recent evidence has shown that αvβ3 does not directly bind to PtSer exposed on the surface of apoptotic cells, but rather participates in modulating efferocytosis through binding to extracellular proteins, such as MFG-E8, that serve as bridging molecules between phagocytes and PtSer on apoptotic cells (40, 41). In our experiments, we demonstrated that uPA directly binds to αvβ3 and that association between uPA and soluble αvβ3 abrogated the ability of uPA to inhibit the phagocytic index. These data suggest that uPA inhibits phagocytosis through regulation of αvβ3-dependent engulfment of apoptotic cells.
MFG-E8 bridges αvβ3 on the surface of macrophages with PtSer on the surface of apoptotic cells, thereby promoting phagocytosis of apoptotic cells (40, 41). We found that preincubation of macrophages with uPA abrogated the enhanced phagocytosis normally seen after addition of MFG-E8 to cultures of macrophages and apoptotic neutrophils. However, subsequent addition of uPA to macrophages precultured with MFG-E8 had no effect on the enhancement of phagocytic index induced by MFG-E8. These data suggest that binding of αvβ3 to either MFG-E8 or uPA blocks subsequent interactions between αvβ3 and the other molecule.
The KD of uPA interacts with lipoprotein-related receptor and integrins, such as αMβ2 and αvβ3, and directly participates in uPA-regulated cell migration and vascular contraction (36–38). Our previous studies demonstrated that the KD, through interactions with αvβ3, is responsible for the ability of uPA to enhance TLR4-induced inflammatory responses in neutrophils (15, 17). In the present experiments, we found that the uPA KD was required for the inhibitory effects of uPA on efferocytosis. In particular, mutants of uPA lacking the KD had no effect on the ingestion of apoptotic neutrophils by macrophages. Given the ability of the uPA KD to bind to αvβ3, such results support the hypothesis that inhibition of efferocytosis by uPA is mediated by interactions between uPA and αvβ3.
Vn is an extracellular protein, present in high concentrations in the circulation, which participates in cell migration and adhesion and in the maintenance of the extracellular matrix (45–47). In the present experiments, we found that uPA had no effect on the uptake of apoptotic neutrophils when added to phagocytic assays in which no Vn was present, thereby demonstrating that Vn is required for the inhibitory activity of uPA on efferocytosis. The major receptor of Vn on the cell surface is αvβ3 (52). Previous studies have shown that binding of Vn to αvβ3 plays an important role in regulating the activity of αvβ3, including association with uPAR (53–55). Given that uPA inhibits efferocytosis through binding to αvβ3, it is possible that Vn modulates such interactions through affecting the structure of αvβ3 or its availability for binding to uPA. Of note, Vn−/− macrophages demonstrate diminished ability to ingest apoptotic neutrophils compared with wild-type macrophages (Figure 9), suggesting that Vn may regulate mechanisms for efferocytosis in addition to those involving αvβ3. We are presently investigating this issue.
Tissue and circulating levels of uPA are up-regulated in a number of inflammatory settings, including sepsis and pneumonia, with the degree of elevation in uPA concentrations being closely associated with disease progression (20, 21). In this study, we found that uPA inhibited the clearance of apoptotic neutrophils by alveolar macrophages under in vivo conditions in the lungs. Neutrophils are primary effectors that contribute to ALI (23, 24), and delayed clearance of apoptotic neutrophils may promote lung inflammation and injury. The present results, by demonstrating that uPA inhibits the uptake of apoptotic neutrophils, suggest a novel mechanism by which elevated levels of uPA may participate in enhancing the duration and severity of inflammatory processes, such as ALI, in which neutrophils play a major role.
Supplementary Material
Supported by National Institutes of Health grants HL76206 and GM87748 (E.A.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201003-0452OC on July 23, 2010
Author Disclosure: Y.Y. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B.C. received more than $100,001 from the National Institutes of Health as a research grant. G.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.A. holds a PCT Patent Application No. PCT/US2005/39108 for “Antibodies that Bind Urokinase-Type Plasminogen Activator and Epitopes Therefor”; US Patent No. 6,057,328, “Method for Treating Hyperoxia,” Jack W. Singer and Edward Abraham, issued May 2, 2000; US Patent No. 5,973,122, “Serum Immunoregulatory Polypeptides and Uses Therefor,” Yi-Han Chang and Edward Abraham, issued October 26, 1999; and US Patent No. 5,650,487, “Serum Immunoregulatory Polypeptides and Uses Therefor,” Yi-Han Chang and Edward Abraham, issued July 22, 1997; and received more than $100,001 from the National Institutes of Health in sponsored grants.
References
- 1.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005;123:321–334. [DOI] [PubMed] [Google Scholar]
- 2.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 1999;160:S5–S11. [DOI] [PubMed] [Google Scholar]
- 3.Henson PM, Tuder RM. Apoptosis in the lung: induction, clearance and detection. Am J Physiol Lung Cell Mol Physiol 2008;294:L601–L611. [DOI] [PubMed] [Google Scholar]
- 4.Kinchen JM, Ravichandran KS. Journey to the grave: signaling events regulating removal of apoptotic cells. J Cell Sci 2007;120:2143–2149. [DOI] [PubMed] [Google Scholar]
- 5.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol 2007;7:964–974. [DOI] [PubMed] [Google Scholar]
- 6.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol 2001;11:R795–R805. [DOI] [PubMed] [Google Scholar]
- 7.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care 2003;7:355–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002;2:965–975. [DOI] [PubMed] [Google Scholar]
- 9.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 2001;411:207–211. [DOI] [PubMed] [Google Scholar]
- 10.Tas SW, Quartier P, Botto M, Fossati-Jimack L. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann Rheum Dis 2006;65:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor PR, Carugati A, Fadok VA, Cook HT, Andrews M, Carroll MC, Savill JS, Henson PM, Botto M, Walport MJ. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 2000;192:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rijneveld AW, Levi M, Florquin S, Speelman P, Carmeliet P, van Der Poll T. Urokinase receptor is necessary for adequate host defense against pneumococcal pneumonia. J Immunol 2002;168:3507–3511. [DOI] [PubMed] [Google Scholar]
- 13.May AE, Kanse SM, Lund LR, Gisler RH, Imhof BA, Preissner KT. Urokinase receptor (CD87) regulates leukocyte recruitment via beta 2 integrins in vivo. J Exp Med 1998;188:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen AP, Petros AM, Meadows RP, Nettesheim DG, Mazar AP, Olejniczak ET, Xu RX, Pederson TM, Henkin J, Fesik SW. Solution structure of the amino-terminal fragment of urokinase-type plasminogen activator. Biochemistry 1994;33:4847–4864. [DOI] [PubMed] [Google Scholar]
- 15.Wang XQ, Bdeir K, Yarovoi S, Cines DB, Fang W, Abraham E. Involvement of the urokinase kringle domain in lipopolysaccharide-induced acute lung injury. J Immunol 2006;177:5550–5557. [DOI] [PubMed] [Google Scholar]
- 16.Mondino A, Blasi F. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol 2004;25:450–455. [DOI] [PubMed] [Google Scholar]
- 17.Kwak SH, Mitra S, Bdeir K, Strassheim D, Park JS, Kim JY, Idell S, Cines D, Abraham E. The kringle domain of urokinase-type plasminogen activator potentiates LPS-induced neutrophil activation through interaction with {alpha}V{beta}3 integrins. J Leukoc Biol 2005;78:937–945. [DOI] [PubMed] [Google Scholar]
- 18.Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, Cines DB, Higazi AA. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem 2002;277:40499–40504. [DOI] [PubMed] [Google Scholar]
- 19.Myohanen H, Vaheri A. Regulation and interactions in the activation of cell-associated plasminogen. Cell Mol Life Sci 2004;61:2840–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robbie LA, Dummer S, Booth NA, Adey GD, Bennett B. Plasminogen activator inhibitor 2 and urokinase-type plasminogen activator in plasma and leucocytes in patients with severe sepsis. Br J Haematol 2000;109:342–348. [DOI] [PubMed] [Google Scholar]
- 21.Choi G, Schultz MJ, van Till JW, Bresser P, van der Zee JS, Boermeester MA, Levi M, van der Poll T. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J 2004;24:786–789. [DOI] [PubMed] [Google Scholar]
- 22.Levi M, Schultz MJ, Rijneveld AW, van der Poll T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit Care Med 2003;31:S238–S242. [DOI] [PubMed] [Google Scholar]
- 23.Bianchi SM, Prince LR, McPhillips K, Allen L, Marriott HM, Taylor GW, Hellewell PG, Sabroe I, Dockrell DH, Henson PW, et al. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am J Respir Crit Care Med 2008;177:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax 2005;60:659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci USA 2008;105:11784–11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park YJ, Liu G, Tsuruta Y, Lorne E, Abraham E. Participation of the urokinase receptor in neutrophil efferocytosis. Blood 2009;114:860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepanova V, Lebedeva T, Kuo A, Yarovoi S, Tkachuk S, Zaitsev S, Bdeir K, Dumler I, Marks MS, Parfyonova Y, et al. Nuclear translocation of urokinase-type plasminogen activator. Blood 2008;112:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak SH, Wang XQ, He Q, Fang WF, Mitra S, Bdeir K, Ploplis VA, Xu Z, Idell S, Cines D, et al. Plasminogen activator inhibitor-1 potentiates LPS-induced neutrophil activation through a JNK-mediated pathway. Thromb Haemost 2006;95:829–835. [PubMed] [Google Scholar]
- 29.Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol 2008;181:4240–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 2003;284:C870–C879. [DOI] [PubMed] [Google Scholar]
- 31.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med 2009;15:1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science 2004;304:1014–1018. [DOI] [PubMed] [Google Scholar]
- 33.Maupas-Schwalm F, Bedel A, Auge N, Grazide MH, Mucher E, Thiers JC, Salvayre R, Negre-Salvayre A. Integrin alpha(v)beta(3), metalloproteinases, and sphingomyelinase-2 mediate urokinase mitogenic effect. Cell Signal 2009;21:1925–1934. [DOI] [PubMed] [Google Scholar]
- 34.Czekay RP, Loskutoff DJ. Plasminogen activator inhibitors regulate cell adhesion through a uPAR-dependent mechanism. J Cell Physiol 2009;220:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang ML, Vararattanavech A, Tan SM. Urokinase-type plasminogen activator receptor induces conformational changes in the integrin alphaMbeta2 headpiece and reorientation of its transmembrane domains. J Biol Chem 2008;283:25392–25403. [DOI] [PubMed] [Google Scholar]
- 36.Pluskota E, Soloviev DA, Plow EF. Convergence of the adhesive and fibrinolytic systems: recognition of urokinase by integrin alpha Mbeta 2 as well as by the urokinase receptor regulates cell adhesion and migration. Blood 2003;101:1582–1590. [DOI] [PubMed] [Google Scholar]
- 37.Abraham E. Alterations in transcriptional regulation of proinflammatory and immunoregulatory cytokine expression by hemorrhage, injury, and critical illness. New Horiz 1996;4:184–193. [PubMed] [Google Scholar]
- 38.Tarui T, Akakura N, Majumdar M, Andronicos N, Takagi J, Mazar AP, Bdeir K, Kuo A, Yarovoi SV, Cines DB, et al. Direct interaction of the kringle domain of urokinase-type plasminogen activator (uPA) and integrin alpha v beta 3 induces signal transduction and enhances plasminogen activation. Thromb Haemost 2006;95:524–534. [DOI] [PubMed] [Google Scholar]
- 39.Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci 2008;121:1773–1783. [DOI] [PubMed] [Google Scholar]
- 40.Hanayama R, Miyasaka K, Nakaya M, Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr Dir Autoimmun 2006;9:162–172. [DOI] [PubMed] [Google Scholar]
- 41.Akakura S, Singh S, Spataro M, Akakura R, Kim JI, Albert ML, Birge RB. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp Cell Res 2004;292:403–416. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 2006;16:189–197. [DOI] [PubMed] [Google Scholar]
- 43.Kanse SM, Matz RL, Preissner KT, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the beta2 integrin Mac-1 (alphaMbeta2, CD11b/CD18). Arterioscler Thromb Vasc Biol 2004;24:2251–2256. [DOI] [PubMed] [Google Scholar]
- 44.Tsuruta Y, Park YJ, Siegal GP, Liu G, Abraham E. Involvement of vitronectin in lipopolysaccaride-induced acute lung injury. J Immunol 2007;179:7079–7086. [DOI] [PubMed] [Google Scholar]
- 45.Gardsvoll H, Ploug M. Mapping of the vitronectin-binding site on the urokinase receptor: involvement of a coherent receptor interface consisting of residues from both domain I and the flanking interdomain linker region. J Biol Chem 2007;282:13561–13572. [DOI] [PubMed] [Google Scholar]
- 46.Lazar MH, Christensen PJ, Du M, Yu B, Subbotina NM, Hanson KE, Hansen JM, White ES, Simon RH, Sisson TH. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol 2004;31:672–678. [DOI] [PubMed] [Google Scholar]
- 47.Madsen CD, Ferraris GM, Andolfo A, Cunningham O, Sidenius N. uPAR-induced cell adhesion and migration: vitronectin provides the key. J Cell Biol 2007;177:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q, Dziarski R, Kirschning CJ, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NF-kappaB signal transduction pathway that induces transcription of interleukin-8. Infect Immun 2001;69:2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demetriou MC, Pennington ME, Nagle RB, Cress AE. Extracellular alpha 6 integrin cleavage by urokinase-type plasminogen activator in human prostate cancer. Exp Cell Res 2004;294:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.King TE, Pawar SC, Majuta L, Sroka IC, Wynn D, Demetriou MC, Nagle RB, Porreca F, Cress AE. The role of alpha 6 integrin in prostate cancer migration and bone pain in a novel xenograft model. PLoS ONE 2008;3:e3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estreicher A, Wohlwend A, Belin D, Schleuning WD, Vassalli JD. Characterization of the cellular binding site for the urokinase-type plasminogen activator. J Biol Chem 1989;264:1180–1189. [PubMed] [Google Scholar]
- 52.Preissner KT, Jenne D. Vitronectin: a new molecular connection in haemostasis. Thromb Haemost 1991;66:189–194. [PubMed] [Google Scholar]
- 53.Ragno P. The urokinase receptor: a ligand or a receptor? Story of a sociable molecule. Cell Mol Life Sci 2006;63:1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillig T, Engelholm LH, Ingvarsen S, Madsen DH, Gardsvoll H, Larsen JK, Ploug M, Dano K, Kjoller L, Behrendt N. A composite role of vitronectin and urokinase in the modulation of cell morphology upon expression of the urokinase receptor. J Biol Chem 2008;283:15217–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 2010;11:23–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.