Abstract
Rationale: Obstructive sleep apnea (OSA) is a risk factor for cardiovascular disease. We hypothesized that patients with OSA and no cardiovascular disease have oxidant-related microcirculatory endothelial dysfunction.
Objectives: To evaluate the microcirculation in OSA.
Methods: This study included seven patients with OSA and seven age- and weight-matched control subjects (mean age, 38 yr; mean body mass index, 32.5 kg/m2). All participants were free of cardiovascular risk factors. Participants received measurement of brachial artery flow-mediated dilation and forearm subcutaneous biopsy. Patients underwent repeated tests 12 weeks after treatment. Microcirculatory endothelial cells were isolated, and immunohistochemistry staining for peroxynitrite in the microcirculation was performed.
Measurements and Main Results: Flow-mediated dilation was lower in patients than in control subjects at baseline (mean ± SEM: 5.7 ± 0.5 vs. 9.5 ± 0.6; P = 0.02) and increased after treatment (5.7–7.3; change, 1.7 ± 0.6; P = 0.04). Microcirculatory peroxynitrite deposit was higher in patients compared with control subjects (44.0 ± 1.6 vs. 21.8 ± 1.9 stain density units; P < 0.001) and decreased after treatment from 44.0 to 30.5 stain density units (change, −13.5 ± 2.9; P = 0.009). In patients, transcription of endothelial nitric oxide synthase decreased from 5.2 to −1.3 after treatment (change, 6.5 ± 2.5; P = 0.05), and transcription of superoxide dismutase1 decreased from −4.0 to −12.3 after treatment (change, −8.3 ± 2.1; P = 0.01). These changes persisted after adjustment for weight and underlying severity of OSA.
Conclusions: This is the first direct evaluation of the microcirculation in OSA. Patients with OSA with low cardiovascular risk status had increased oxidant production in the microcirculation and endothelial dysfunction, both of which improved with treatment. Endothelial nitric oxide synthase transcription decreased with treatment.
Keywords: obstructive sleep apnea, endothelial function, microcirculation
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
This study evaluates the microcirculation in patients with obstructive sleep apnea (OSA). The techniques used enabled real-time quantification of expression of critical endothelial factors.
What This Study Adds to the Field
The findings of this study support that OSA leads to microcirculatory endothelial dysfunction by up-regulating eNOS and decreasing NO availability in the presence of oxidative stress.
Obstructive sleep apnea (OSA) is increasingly recognized as a cardiovascular risk factor (1–4). OSA is a cause of hypertension (5–7) and has strong association with atherosclerosis (8–10), coronary heart disease (11, 12), diabetes (13, 14), stroke (15–18), and fatal cardiovascular events (4). Endothelial dysfunction is a preclinical vascular abnormality that predicts subsequent development of vascular disease (19, 20). Patients with OSA demonstrate endothelial dysfunction in the absence of any manifested vascular disease (21, 22). The mechanism of endothelial dysfunction in OSA is largely unknown. Endothelial dysfunction in OSA is reversible with antioxidants (22–24), suggesting a role for oxidant overproduction in the decreased NO availability in OSA. This provides parallels to other cardiovascular diseases in which oxidative stress–induced endothelial dysfunction is important (25, 26).
Recent studies reported evidence of dysfunction or decreased expression of endothelial nitric oxide synthase (eNOS) in association with increased peroxynitrite in harvested venous endothelial cells of patients with OSA (27). We endeavored to perform the first direct quantification of microvascular endothelial genes from patients with OSA. We developed a method to directly isolate microvascular endothelial cells (MVECs) from patients with OSA (28). We hypothesized that patients with OSA who are free of cardiovascular disease have early functional changes in the microcirculatory endothelial cells that are associated with OSA and therefore would resolve with treatment. Given the role of oxidative stress in the vascular disease of OSA (22, 23), we expected to find evidence of superoxide overproduction in the microcirculatory vessels. We expected these functional changes to be reversible with treatment of OSA.
A portion of the results of this study has been previously reported in the form of an abstract (29).
METHODS
Participants
Patients with OSA were recruited from the Ohio State University (OSU) Sleep Disorders Center within 4 weeks of their diagnostic polysomnography. OSA was defined by an apnea/hypopnea index (AHI) > 15 events per hour of sleep. Healthy control subjects were recruited from the community or from the sleep center patients who had negative sleep studies. OSA was ruled out in one patient by the absence of sleepiness or risk factors of OSA as defined by negative Berlin questionnaire and negative Epworth Sleepiness Scale. Patients with OSA and healthy control subjects had low (< 10%) cardiovascular risk status as defined by Framingham risk score (30). None of the participants was on medications or supplements or had ever been a smoker. The research protocol was approved by the OSU Institutional Review Board (protocol number 2005H0221). The study was registered in the National Clinical Trials database (NCT00701441).
Procedures and Measurements
Patients with OSA provided all measurements at baseline before and 12 weeks after initiation of treatment with continuous positive airway pressure (CPAP). Control subjects provided measurements only once.
Endothelial function.
Endothelial function was evaluated by Doppler ultrasound of the brachial artery and measurement of flow-mediated dilation (FMD) (31, 32). Image acquisition was performed using a linear array transducer with frequency of 7 MHz and color spectral Doppler ultrasound (Vivid 7; GE Healthcare, Milwaukee, WI). This test was performed according to published guidelines (32).
Forearm subcutaneous biopsy.
Clinical skin punch biopsy techniques (33) were used to obtain a 6-mm-diameter cylinder of subcutaneous tissue from the forearms of volunteers.
Laser microdissection and pressure catapulting for procurement of MVECs.
The subcutaneous tissue was prepared according to our previously published protocol (28) with modifications as detailed in the online supplement. Frozen tissue blocks were cut into 12-μm cryostat sections. Two to three sections were mounted on each RNAZap-treated thermoplastic (polyethylene napthalate)-covered glass slide (PALM Technologies, Bernreid, Germany) and treated with RNA Later solution (Ambion, Austin, TX). The sections were stained with fluorescein-labeled UEA I (Vector Labs, Burlingame, CA). Figure 1 shows an arteriole with endothelial cells fluorescently stained that has been selected for RNA isolation via laser microdissection and pressure catapulting.
Figure 1.
Microcirculatory endothelial cell isolation. (A) In fluorescein-labeled UEA I–stained arterioles, localization of stain in microcirculatory endothelial cells is performed to enable cell selection by laser capture microdissection. (B) Area selected and marked for capture and RNA isolation for creation of cDNA library.
Quantitative immunohistochemistry staining for peroxynitrite.
Blocks of the subcutaneous tissue were cryosectioned into 10-μm sections. Staining with antinitrotyrosine polyclonal antibody (Upstate Biotechnology, Lake Placid, NY) was performed using an auto-stainer in the OSU core facility. A positive slide indicating the top level of stain density possible for a vessel was created. A negative slide indicating the lower limit possible for a skin biopsy stained for peroxynitrite was created by incubating the primary antibody with 10 mM nitrotyrosine in PBS for 1 hour at room temperature and using this in place of the primary antibody. Figure 2 shows an example of nitrotyrosine stain uptake in the microcirculation of a patient with OSA.
Figure 2.
Peroxynitrite deposit in a microcirculatory vessel of a patient with obstructive sleep apnea. Brown stain indicates nitrated tyrosine residues in microcirculation. (A) Section of pretreatment tissue stained with antinitrotyrosine antibody. (B) Section of post-treatment tissue stained with antinitrotyrosine antibody.
Quantitative rtPCR.
RNA isolation from the laser microdissection and pressure catapulting samples was performed using the PicoPure RNA Isolation Kit (Arcturus, Sunnyvale, CA). β-Actin and 18sRNA gene expression was measured to correct for differences in extraction efficiency between samples. Primers were from Super Array Bioscience Corporation (Frederick, MD).
Analysis
For FMD, all diameter measurements were obtained at end-diastole (peak R wave). At least three baseline measurements were obtained and averaged for each participant. FMD was defined as the maximum dilation between 30 to 60 seconds after deflation of the forearm cuff. The three highest dilation measurements were averaged. The formula used for calculation is:
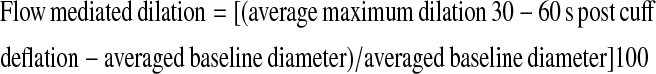 |
For nitrotyrosine staining, the slides were scanned, and all subcutaneous vessels were marked by a technician who was blinded to the origin of the slides. The technician digitally dissected vessels that were less than 300 μm in diameter for all slides. A pathologist confirmed that the dissected vessels were arterioles. Another blinded observer used image analysis software (BioImagene, Cupertino, CA) to quantify stain densities in the deidentified vessels. The stain density was defined as the amount of total of brown stain present in microvasculature from tissue sections compared with the blue counterstain in the tissue.
For qPCR, we used ΔCt values in analyzing the PCR results, correcting to an average of the two house-keeping genes. Because these were measures of the mechanism behind the effectiveness of treatment, we considered the comparison of gene transcription between pre- and post-treatment our focus. The qPCR experiments and analysis were not designed for the comparison between patients and control subjects, as was planned for FMD. The hypothesis was that if peroxynitrite deposition in the microcirculation is due to increased eNOS activity and superoxide overproduction, then eNOS and superoxide dismutase (SOD1) transcription should decrease with treatment as peroxynitrite production is decreased. All genes were tested in triplicate.
Statistical Analysis
For comparing pre- versus post-treatment, paired t tests were used. For comparing pretreatment with control subjects, we used linear models that adjusted for body mass index (BMI), age, and sex to avoid confounding bias that might be due to these variables. For each variable and comparison, we tested at the 0.05 level with double-sided P values. We only consider tests in the hypothesized direction conclusive, so testing is conservative from that perspective. However, we used no correction for multiple testing when declaring significance.
RESULTS
Characteristics of Participants
Table 1 lists the baseline characteristics of patients and control subjects. There was no significant difference in BMI, age, or sex. For patients with OSA, average CPAP use on device download was (mean ± SEM) 5.05 ± 0.83 hours. Residual AHI was 3.3 ± 0.7 events per hour.
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS AND CONTROL SUBJECTS
| Control |
OSA Group (Pretreatment) |
Control vs. Pretreatment |
||||
|---|---|---|---|---|---|---|
| Mean (SEM) | n | Mean (SEM) | n | Mean Difference (SEM) | P Value | |
| Age, yr | 36.4 (2.3) | 7 | 39.4 (5.4) | 7 | −3.0 (5.9) | 0.6 |
| BMI, kg/m2 | 30 (2) | 7 | 35 (2) | 7 | −5 (3) | 0.17 |
| AHI | 3 (1) | 6 | 35 (10) | 7 | −32 (12) | 0.018 |
| Sex, % male | 71 (18) | 7 | 71 (18) | 7 | 0 (0.3) | 1.00 |
Definition of abbreviations: AHI = apnea/hypopnea index; BMI = body mass index; OSA = obstructive sleep apnea.
Endothelial Function
FMD increased in patients after 12 weeks of CPAP treatment from 5.7 ± 0.5 to 7.3 ± 0.9%, with a difference of 1.7 ± 0.6% (P < 0.04). Patients with OSA had lower FMD at baseline as compared with control subjects (5.7 ± 0.5% vs. 9.7 ± 0.6%, with a difference of 3.8 ± 0.7%; P < 0.001). After adjustment for BMI, age, and sex, the difference between OSA and control remained significant (difference, 3.2 ± 1.1; P = 0.02). We also explored relationships between change in FMD after treatment with BMI, AHI, and compliance, and no clear associations were found.
Peroxynitrite Deposit in the Microcirculation
Tissue was available for immunohistochemistry staining on five patients with OSA and five control subjects. Peroxynitrite stain density in the microcirculation decreased with CPAP treatment from 44.0 ± 1.6 stain density units (SDU) to 30.50 ± 2.3 SDU, with a difference of −13.5 ± 2.9 SDU (P = 0.009). Microcirculatory peroxynitrite stain density was increased in patients with OSA compared with control subjects (44.0 ± 1.6 SDU vs. 21.8 ± 1.9 SDU, with a difference of −22.3 ± 2.6 SDU; P < 0.001). Figure 3 is an individual value line plot showing the peroxynitrite deposit relationship in the microvasculature between patients and control subjects. We also adjusted for BMI, age, and sex for the comparison between patients and control subjects, and the adjusted difference remained significant (difference, −23.1 ± 6.4; P = 0.02).
Figure 3.
Peroxynitrite deposits in the microvascular walls of patients with obstructive sleep apnea and control subjects. Peroxynitrite stain density in the microcirculation decreased with continuous positive airway pressure treatment from 44.0 ± 1.6 stain density units (SDU) to 30.5 ± 2.3 SDU, with a difference of −13.5 ± 2.9 SDU (P < 0.01). Microcirculatory peroxynitrite stain density was increased in patients with obstructive sleep apnea compared with control subjects (44.0 ± 1.6 SDU vs. 21.8 ± 1.9 SDU, with a difference of −22.3 ± 2.6 SDU; P < 0.001).
Quantitative PCR Studies
Transcription of zinc and copper SOD-1.
Quantitative PCR measurement of SOD-1 revealed a decrease in expression with treatment of OSA from −4.0 to −12.1 ΔCt, with a difference of −8.3 ± 2.1 ΔCt (P = 0.011). Figure 4 is an individual value line plot showing the relationship between transcription of SOD-1 in patients before and after treatment.
Figure 4.
Superoxide dismutase transcription in microvascular endothelial cells of patients and matched control subjects. Quantitative PCR measurement of superoxide dismutase 1 revealed a decrease in expression with treatment of obstructive sleep apnea from −4.0 to −12.1 ΔCt, with a difference of −8.3 ± 2.1 ΔCt (P = 0.011).
Transcription of eNOS.
There was a decrease in eNOS transcription with CPAP treatment from 5.2 to −1.3 ΔCt, with a difference of −6.5 ± 2.5 ΔCt (P = 0.05). Figure 5 provides the individual changes with CPAP treatment and control subjects.
Figure 5.
Endothelial nitric oxide transcription in microvascular endothelial cells of patients and matched control subjects. There was a decrease in eNOS transcription with continuous positive airway pressure treatment from 5.2 to −1.3 ΔCt, with a difference of −6.5 ± (2.5) ΔCt (P = 0.05).
Transcription of inducible nitric oxide synthase.
There was no significant difference change in inducible nitric oxide synthase RNA with CPAP treatment (−4.3 ± 5.7 ΔCt at baseline to 0.6 ± 2.4 ΔCt after treatment, with a difference of −4.8 ± 6.1 ΔCt; P = 0.46).
DISCUSSION
This study is the first direct evaluation of the microcirculation in OSA. We examined the subcutaneous vascular tissue from patients with OSA and low cardiovascular risk status. We found increased peroxynitrite production in the microvascular walls of patients with OSA, indicating overproduction of NO and superoxide in the endothelial environment. A novel endothelial cell isolation technique (28) enabled real-time quantification of transcription of critical endothelial genes. The uptake of NO by superoxide explains the decreased NO availability and endothelial dysfunction in OSA. Additionally, the up-regulation of SOD-1 in the MVECs confirms oxidant overproduction in the endothelium. The finding of up-regulated eNOS at baseline in patients with OSA provides a potential source for the overproduction of NO and superoxide. The findings of this study persisted after adjustment to BMI, AHI, age, sex, and hours of CPAP use.
Previous studies reported that endothelial dysfunction in patients with OSA was reversible with administration of antioxidants (22, 24). In this study, we found direct evidence of increased peroxynitrite production in the microcirculation of patients with OSA, providing an explanation for the role of antioxidants. Only one previous study reported increased circulating levels of peroxynitrite, but the researchers did not localize the abnormality to the microcirculation (27). Oxidative stress has been demonstrated in patients with OSA (34, 35). Oxidant overproduction in OSA may be a manifestation of systemic inflammatory response and increased activated circulating leukocytes and oxidative burst (36, 37). This study supports increased oxidant production directly in the microcirculatory endothelium of patients with OSA. This study was not designed to evaluate the effects of obesity or other demographic factors on the response to treatment in OSA. However, underlying BMI and AHI were not associated with the change in endothelial function or peroxynitrite in the OSA group. Evaluation of a possible obesity-specific effect of CPAP on the vascular abnormality profile of OSA would require a larger study that compares treatment effects in obese with nonobese patients with OSA.
Peroxynitrite is the product of NO and superoxide. There are several potential sources for superoxide in the endothelium (38–40). However, increased transcription of eNOS in the endothelial cells of patients with OSA supports that eNOS may be a source of superoxide overproduction in this setting (41, 42). Up-regulation of eNOS occurs in several cardiovascular diseases in the presence of decreased NO availability (43, 41, 44). Although overexpression of eNOS by gene transfer can increase NO activity in the vessel wall, constitutive overexpression of eNOS is associated with accelerated atherosclerosis and increased oxidative stress (45). A recent study reported decreased eNOS protein in harvested venous endothelial cells of patients with OSA (27). The technique we used provides absolute quantification of transcription of eNOS in mainly arteriolar endothelial cells. The explanation for the different findings may be related to the method and site of isolation. Our findings also support possible functional suppression of eNOS or “uncoupling” of eNOS from critical cofactors (42). Superoxide has very high rate constant for the reaction with NO, producing peroxynitrite (46). In turn, peroxynitrite has strong predilection of oxidizing tetrahydrobiopterin (47, 48), a critical eNOS cofactor, resulting in eNOS uncoupling and production of superoxide directly. It has been established that vascular disease occurs in the presence of eNOS overexpression and superoxide overproduction (41, 45, 49). Superoxide generation by eNOS has been implicated in a variety of experimental and clinical vascular disease states, including diabetes (43, 50), hypertension (51), and atherosclerosis (52). Studies evaluating eNOS function and superoxide production in the endothelial cells of patients with OSA are needed to confirm our findings.
Microcirculation, and particularly the arterioles, is critical for the peripheral vascular resistance in hypertension and vascular disease. Our functional studies were done on the brachial artery, a conduit vessel, and the structural and PCR studies were done on subcutaneous arterioles. Correlation between endothelial dysfunction in the two beds has been confirmed (53, 54). We did not compare the gene expression between control subjects and patients at baseline. We planned the gene testing to explain the treatment effect on peroxynitrite production in the microcirculation. Once we established the difference from control subjects for clinical variables, we only wanted to show that treatment affected mechanistic variables in the hypothesized pathway.
In this direct examination of the microcirculation, we found evidence of increased oxidant production in the microcirculation of patients with OSA who are free of cardiovascular disease or risk factors. These microcirculatory changes were independent of age, weight, or sex. WE concluded that these changes were only related to OSA because they reversed with treatment. The presence of peroxynitrite makes it highly likely that superoxide is overproduced directly in the microcirculation of patients with OSA. This is further supported by the findings of up-regulated SOD. Superoxide is likely to be generated directly in the endothelial cell, but this must be verified with direct measurement of superoxide production in the endothelium. This study supports that endothelial dysfunction in OSA is not related to decreased transcription of eNOS. This study also reports the first direct quantification of critical genetic markers of endothelial function in humans with OSA, providing a novel method of further studying vascular disease.
Supplementary Material
Supported by National Institutes of Health grant R21HL092480-01 (R.K. and D.J.), Center for Clinical and Translational Research grant UL1RR025755 from the National Center for Research Resources (R.N.K. and D.J.), NIDDK R01 DK076566 (S.R.), and NIGMS GM069589 and GM077185 (C.K.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0162OC on July 23, 2010
Author Disclosure: B.T.P. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.J. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.N.H. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.K.S. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.R. has received industry-sponsored grants from Glycotex Inc. (over $100,000), Carotech (over $100,000), and Cascade ($10,001–$50,000); he owns stock in Redox Technologies (up to $1,000) and NutrimiR (up to $1,000); and he has received sponsored grants from NIH, NIDDK (over $100,000). N.A.F. has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.N.K. has received consultancy fees from Cardiac Concepts, Inc. ($1,001–$5,000); he has received lecture fees from Phillip Respironics ($1,001–$5,000); he has received industry-sponsored grants from Respironics ($10,001–$50,000); and he has received sponsored grants from NIH (over $100,000).
References
- 1.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156–178. [DOI] [PubMed] [Google Scholar]
- 2.Campos-Rodriguez F, Pena-Grinan N, Reyes-Nunez N, De la Cruz-Moron I, Perez-Ronchel J, De la Vega-Gallardo F, Fernandez-Palacin A. Mortality in obstructive sleep apnea-hypopnea patients treated with positive airway pressure. Chest 2005;128:624–633. [DOI] [PubMed] [Google Scholar]
- 3.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 2005;127(6):2076–2084. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046–1053. [DOI] [PubMed] [Google Scholar]
- 5.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension: Evidence from a canine model. J Clin Invest 1997;99(1):106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 1997;157(16):1746–1752. [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 2000;283(14):1829–1836. [DOI] [PubMed] [Google Scholar]
- 8.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2007;176(7):706–712. [DOI] [PubMed] [Google Scholar]
- 9.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172(5):625–630. [DOI] [PubMed] [Google Scholar]
- 10.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172(5):613–618. [DOI] [PubMed] [Google Scholar]
- 11.Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am J Cardiol 2007;99(1):26–30. [DOI] [PubMed] [Google Scholar]
- 12.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med 2000;162(1):81–86. [DOI] [PubMed] [Google Scholar]
- 13.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of Untreated Obstructive Sleep Apnea on Glucose Control in Type 2 Diabetes. Am J Respir Crit Care Med 2009. [DOI] [PMC free article] [PubMed]
- 14.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62(11):969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med 2005;172(11):1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353(19):2034–2041. [DOI] [PubMed] [Google Scholar]
- 17.Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006;37(4):967–972. [DOI] [PubMed] [Google Scholar]
- 18.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke 1996;27(2):252–259. [DOI] [PubMed] [Google Scholar]
- 19.Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003;108(17):2093–2098. [DOI] [PubMed] [Google Scholar]
- 20.Bugiardini R, Manfrini O, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 2004;109(21):2518–2523. [DOI] [PubMed] [Google Scholar]
- 21.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169(3):348–353. [DOI] [PubMed] [Google Scholar]
- 22.Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R. Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med 2006;173(8):897–901. [DOI] [PubMed] [Google Scholar]
- 23.Phillips SA, Olson EB, Lombard JH, Morgan BJ. Chronic intermittent hypoxia alters NE reactivity and mechanics of skeletal muscle resistance arteries. J Appl Physiol 2006;100(4):1117–1123. [DOI] [PubMed] [Google Scholar]
- 24.El Solh AA, Saliba R, Bosinski T, Grant BJ, Berbary E, Miller N. Allopurinol improves endothelial function in sleep apnoea: a randomised controlled study. Eur Respir J 2006;27:997–1002. [DOI] [PubMed] [Google Scholar]
- 25.Pieper GM, Dembny K, Siebeneich W. Long-term treatment in vivo with NOX-101, a scavenger of nitric oxide, prevents diabetes-induced endothelial dysfunction. Diabetologia 1998;41:1220–1226. [DOI] [PubMed] [Google Scholar]
- 26.Katayama Y, Shige H, Yamamoto A, Hirata F, Yasuda H. Oral vitamin C ameliorates smoking-induced arterial wall stiffness in healthy volunteers. J Atheroscler Thromb 2004;11:354–357. [DOI] [PubMed] [Google Scholar]
- 27.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci USA 2007;104:14472–14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad D, Patt B, Yearsley K, Roy S, Sen C, Khayat R. Oxidative stress in an obstructive sleep apnea population. In: Association of professional sleep societies, vol. 32. Seattle: 2009; p. 1235.
- 30.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 31.Geny B, Rouyer O, Doutreleau S, Piquard F. Comments on Point-Counterpoint “Flow-mediated dilation does/does not reflect nitric oxide-mediated endothelial function.” J Appl Physiol 2006;100:362. [PubMed] [Google Scholar]
- 32.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 33.Alguire PC, Mathes BM. Skin biopsy techniques for the internist. J Gen Intern Med 1998;13:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest 2003;124:1386–1392. [DOI] [PubMed] [Google Scholar]
- 35.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep 2004;27:123–128. [PubMed] [Google Scholar]
- 36.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 2002;165:934–939. [DOI] [PubMed] [Google Scholar]
- 37.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, Seeger W, Grimminger F. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med 2000;162:566–570. [DOI] [PubMed] [Google Scholar]
- 38.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 2000;86:494–501. [DOI] [PubMed] [Google Scholar]
- 39.Mugge A, Elwell JH, Peterson TE, Hofmeyer TG, Heistad DD, Harrison DG. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res 1991;69:1293–1300. [DOI] [PubMed] [Google Scholar]
- 40.Ito K, Akita H, Kanazawa K, Yamada S, Terashima M, Matsuda Y, Yokoyama M. Comparison of effects of ascorbic acid on endothelium-dependent vasodilation in patients with chronic congestive heart failure secondary to idiopathic dilated cardiomyopathy versus patients with effort angina pectoris secondary to coronary artery disease. Am J Cardiol 1998;82:762–767. [DOI] [PubMed] [Google Scholar]
- 41.Bouloumie A, Bauersachs J, Linz W, Scholkens BA, Wiemer G, Fleming I, Busse R. Endothelial dysfunction coincides with an enhanced nitric oxide synthase expression and superoxide anion production. Hypertension 1997;30:934–941. [DOI] [PubMed] [Google Scholar]
- 42.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA, Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 1997;96:25–28. [DOI] [PubMed] [Google Scholar]
- 44.Bauersachs J, Bouloumie A, Fraccarollo D, Hu K, Busse R, Ertl G. Endothelial dysfunction in chronic myocardial infarction despite increased vascular endothelial nitric oxide synthase and soluble guanylate cyclase expression: role of enhanced vascular superoxide production. Circulation 1999;100:292–298. [DOI] [PubMed] [Google Scholar]
- 45.Ozaki M, Kawashima S, Yamashita T, Hirase T, Namiki M, Inoue N, Hirata K, Yasui H, Sakurai H, Yoshida Y, et al. Overexpression of endothelial nitric oxide synthase accelerates atherosclerotic lesion formation in apoE-deficient mice. J Clin Invest 2002;110:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun 1993;18:195–199. [DOI] [PubMed] [Google Scholar]
- 47.Vasquez-Vivar J, Whitsett J, Martasek P, Hogg N, Kalyanaraman B. Reaction of tetrahydrobiopterin with superoxide: EPR-kinetic analysis and characterization of the pteridine radical. Free Radic Biol Med 2001;31:975–985. [DOI] [PubMed] [Google Scholar]
- 48.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 2003;278:22546–22554. [DOI] [PubMed] [Google Scholar]
- 49.Shi W, Wang X, Shih DM, Laubach VE, Navab M, Lusis AJ. Paradoxical reduction of fatty streak formation in mice lacking endothelial nitric oxide synthase. Circulation 2002;105:2078–2082. [DOI] [PubMed] [Google Scholar]
- 50.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 2002;105:1656–1662. [DOI] [PubMed] [Google Scholar]
- 51.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 2003;111:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maier W, Cosentino F, Lutolf RB, Fleisch M, Seiler C, Hess OM, Meier B, Luscher TF. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J Cardiovasc Pharmacol 2000;35:173–178. [DOI] [PubMed] [Google Scholar]
- 53.Agewall S, Henareh L, Kublickiene K. Endothelial function in conduit and resistance arteries in men with coronary disease. Atherosclerosis 2006;184:130–136. [DOI] [PubMed] [Google Scholar]
- 54.Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens 2001;19:415–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







