Abstract
Rationale: Nontuberculous mycobacterial (NTM) infection is a growing problem in the United States and remains underrecognized in the developing world. The management of NTM infections is further complicated by several factors, including the need to use high systemic doses of toxic agents, the length of therapy, and the development of drug resistance.
Objectives: We have evaluated the use of monocyte-derived dendritic cells (DCs) as a delivery vehicle for a luminescent derivative of amikacin prepared by conjugation to fluorescein isothiocyanate (FITC) (amikacin-FITC) into granulomas formed in the tissues of mice infected with Mycobacterium avium.
Methods: Amikacin-FITC was prepared and quantitative fluorescence was used to track the intracellular uptake of this modified antibiotic. The antibiotic activity of amikacin-FITC was also determined to be comparable to unmodified amikacin against M. avium. Amikacin-FITC–loaded DCs were first primed with M. avium, and then the cells were injected into the tail vein of infected mice. After 24 hours, the mice were sacrificed and the tissues were analyzed under fluorescence microscope.
Measurements and Main Results: We found that we were able to deliver amikacin into granulomas in a mouse model of disseminated mycobacterial infection. No increase in levels of monocyte chemoattractant protein-1 and its CCR2 as markers of inflammation were found when DCs were treated with amikacin-FITC.
Conclusions: DC–based drug delivery may be an adjunct and useful method of delivering high local concentrations of antibiotics into mycobacterial granulomas.
Keywords: dendritic cells, amikacin, granuloma formation, mycobacteria
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Infection with nontuberculous mycobacteria is a major public health problem worldwide. An efficient treatment without attendant systemic side effects is needed to avoid relapse of the disease and an increase in the resistance rate of the organism. The use of dendritic cells as a vehicle for drug delivery is a new approach for the treatment of mycobacterial infection that provides site-directed therapy with minimal systemic absorption and hence fewer side effects.
What This Study Adds to the Field
We demonstrate an organism-directed delivery of amikacin-loaded dendritic cells into the granulomas of Mycobacterium avium–infected mice without evidence of systemic presence of amikacin.
Dendritic cells (DCs) contribute to generating Mycobacterium avium–specific CD4+ and CD8+ T-cell responses (1). They have been described as the most potent antigen-presenting cells (2, 3), showing clear superiority in inducing primary immune responses as compared with other antigen-presenting cell types (4, 5). They are among the first cells to encounter a pathogen (6) and have the capacity to internalize and process its antigens before migration to granulomas (7).
It is recognized that Mycobacterium avium complex (MAC) causes pulmonary disease in humans (8). MAC is described as an opportunistic pathogen (9–12) and is an environmentally ubiquitous organism. Interaction with airway epithelial cells and alveolar macrophages are likely key events in the pathogenesis of MAC, which causes chronic airways infection and granulomatous inflammation leading to bronchiectasis (9, 13)
A major issue in the management of mycobacterial infection is the difficulty in clearance of the organisms from tissues, where they may remain trapped in granulomas (14–16).
Amikacin is an aminoglycoside antibiotic that has bactericidal activity against several mycobacteria. Bakker-Woudenberg and colleagues have reported the use of amikacin as an efficient drug to kill MAC in a mouse model of MAC infection (17). However, the use of the drug is limited by the need to achieve a balance between high levels to achieve a bactericidal effect and the association of high levels with ototoxicity and nephrotoxicity.
In the present study, the application of DCs loaded with luminescent fluorescein-isocyanate (FITC)–modified derivative of amikacin (amikacin-FITC) is investigated as a potential therapeutic route for treating MAC. By using the ability of DCs to target and infiltrate granulomas and active M. avium populations while carrying concentrated intracellular drug cargos, localized therapy (15) without systemic toxicity may be possible. This would allow for a reduction of treatment duration and as a result perhaps improve patient compliance (16).
The objectives of this study were to determine first the capacity and the mechanisms by which monocyte-derived DCs uptake amikacin-FITC, and second, whether these DCs loaded with amikacin-FITC and primed by mycobacteria recognize and migrate to the site of infection in an M. avium–infected mouse. We show the presence of amikacin-FITC inside DCs and inside the granulomas in mouse tissue in a model of disseminated MAC infection. Additionally, we demonstrate measured killing of mycobacteria coincubated with amikacin-FITC–loaded DCs. Importantly, the carrier cells (DCs) did not release proinflammatory chemokines, such as monocyte chemoattractant protein-1 (MCP-1/CCL2) and its receptor CCR2 (18, 19), when loaded with amikacin.
The results obtained in this study demonstrate for the first time a cell-based method of antibiotic delivery into a specific site of infection with MAC.
METHODS
Detailed methodology is provided in the online data supplement.
Cell Culture
Monocytes were isolated from mouse spleen by density gradient centrifugations (Ficoll-Paque; Sigma Aldrich, St. Louis, MO) and CD11b magnetic cell selection (MACS Pre-Separation Filter; Miltenyi Biotec, Bergisch Gladbach, Germany).
Mouse monocytes and RAW 264.7 (TIB-71) mouse monocyte-macrophage cell line were transformed into DCs (20) and labeled with anti-mouse CIRE (CD-SIGN, CD209) (eBioscience, San Diego, CA).
Preparation of Amikacin-FITC
Amikacin (Sigma Aldrich) was reacted with FITC (Sigma Aldrich) in pH 9.0 buffer, extracted, and dissolved in phosphate-buffered saline (PBS) before application. (Additional details provided in the online data supplement).
Amikacin-FITC Uptake
RAW 264.7 cells were incubated in serum-free medium with different doses of amikacin-FITC for 6 hours at 4 and 37°C. The cells were pelleted and the fluorescence was measured with a spectrophotometer at 485 and 528 nm. Cell viability was measured with Trypan blue dye exclusion assay.
Quantitative Polymerase Chain Reaction Analysis
Total RNA from cultured cells was purified by a commercial kit (RNeasy Mini Kit; Qiagen Science, Valencia, CA). The quantitative analysis of MCP-1 and CCR-2 genes was assessed as described (21, 22). Supernatants were also tested for MCP-1 using ELISA (R&D Systems, Minneapolis, MN).
Efficacy of Amikacin-FITC in Bacterial Growth
Mycobacterium avium subspecies avium Chester was purchased from ATCC-15769 (Manassas, VA). The bacteria were incubated with different doses of both free amikacin and amikacin-FITC for 30 minutes on a rotary shaker at room temperature (RT) and then plated in triplicate in Middlebrook and Cohn 7H10 agar plates (BD Bioscience, San Jose, CA) for 14 days at 37°C in 95% O2 and 5% CO2. After this incubation period the colonies were counted.
A mouse-virulent strain of Staphylococcus aureus was purchased from ATCC and was used to expedite the identification of the minimum dose of the antibiotic necessary to kill or inhibit the growth of the bacteria.
Mycobacterium Avium Mouse Model
C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida.
Mice were inoculated intranasally with 1 × 106 M. avium in 30 μl of PBS once per week for 3 weeks. Alternatively, control mice were inoculated with an equal volume of PBS.
Amikacin-FITC Treatment
DCs were pretreated for 24 hours with 50 and 100 mg/L of amikacin-FITC and primed with M. avium for 1 hour at 37°C to activate the bacteria antigen to target the drug in the site of infection. Then the cells were harvested by scraper and centrifuged at 1,500 rpm for 5 minutes. Approximately 1.3 × 106 cells were resuspended in 200 μl of PBS and injected in M. avium–infected mice via tail vein.
Bronchoalveolar Lavage Fluid, Serum Collection and Tissue Harvest
The mice were killed 24 hours after injection of DCs with isoflurane (Forane; Webster Veterinary, Devens, MA). Bronchoalveolar lavage (BAL) fluid was collected by injecting 1,000 μl Hanks' balanced salt solution (Cellgro; Mediatech, Herndon, VA), into the lungs via tracheal cannulation. The tissues were harvested and stored in optimal cutting temperature compound for frozen section analysis. Tissue sections were stained using hematoxylin and eosin (H&E) staining.
RESULTS
Monocyte-Derived DCs Efficiently Uptake the Amikacin-FITC
We transformed monocytes derived from the mouse spleen and a mouse monocyte/macrophages cell line into immature DCs by adding granulocyte-macrophage colony-stimulating factor and IL-4 cytokines to complete RPMI medium in separate experiments. After 5 to 7 days, both cell types tested positive for the CD209 marker, confirming the formation of immature DCs (Figures 1E and 1F). We plated 1 × 106 cells per well in 2-well chamber slides and incubated with amikacin-FITC for 24 hours at 37°C. Cells were fixed with 4% paraformaldehyde and the nuclei were stained with DAPI. Cells were observed under fluorescence microscopy. As shown in Figures 1A–1D, the green signal was localized inside most of the cells analyzed, indicating an efficient uptake of the amikacin-FITC by the cells. We did not find any signal outside the cells when both culture types were analyzed for a period of 48 hours after the initial loading experiment in the absence of cell lysis. This suggests that leaching from living cells is minimal.
Figure 1.
Detection of amikacin–fluorescein isothiocyanate (FITC) inside the cells. Dendritic cells (DCs) were treated with amikacin-FITC and cells were then observed under the fluorescence microscope; the pictures indicate the merge image with fluorescent filter and differential interference contrast (DIC) bright field. (A, B) CD11b monocyte DCs incubated with amikacin-FITC for 24 hours. The blue signal indicates the nuclei of the cells stained with DAPI and green signal due to the presence of amikacin conjugated with FITC dye. (A) 200× magnification; (B) 400× magnification. (C, D) RAW 264.7 cell line incubated with amikacin-FITC for 24 hours. The nuclei of the cells were stained with DAPI. Amikacin conjugated with FITC dye was visualized by green. (C) 100× magnification; (D) 100× magnification and merge with DIC bright field. (E, F) RAW 264.7 cells transformed into DCs in vitro with granulocyte-macrophage colony-stimulating factor and IL-4 cytokines for 5 to 7 days. Cells were marked with CD209 coated with FITC dye and observed under the fluorescence microscope. Blue indicates the nuclei stained with DAPI. Green localizes the CD209 mouse marker in the cells. (E) 200× magnification; (F) 200× magnification and merge with DIC bright field.
Amikacin-FITC Internalizes Through the Cell Membrane
To study the mechanism by which the amikacin-FITC is transported inside the cells, experiments were performed at 4°C, at which temperature energetic active uptake processes, such as endocytosis/pinocytosis, are inhibited and passive mechanisms, such as membrane diffusion, are dominant, as well as at 37°C, a temperature at which the cells are metabolically active and both passive and active transport processes would coexist. We incubated RAW 264.7 cells with different doses of amikacin-FITC at 4 and 37°C for 3 and 6 hours. After the incubation time, the cells were collected and fluorescence levels were measured spectrophotometrically. We observed a clear dose-dependent increase of fluorescence in the amikacin-FITC treated cells as compared with the untreated control (Figure 2). We did not find any significant differences between the two temperatures, 4 and 37°C, when we compared the fluorescence profile. Our results indicated that amikacin-FITC is a cell membrane–permeable antibiotic and further that its transport is not significantly influenced from 4 to 37°C, enabling efficient passive uptake over a wide range of conditions. Moreover, the viability of the culture was not appreciably altered by the different temperatures assayed or by the different doses of amikacin-FITC (Figure 3).
Figure 2.
Levels of amikacin–fluorescein isothiocyanate (FITC) inside the RAW 264.7 cells. When the culture was confluent, cells were treated with 50 and 100 mg/L doses of amikacin-FITC and incubated for 6 hours at 4 and 37°C. The cells were collected and the fluorescent levels were measured spectrophotometrically. There is a dose-related increase in the fluorescence level for both temperatures assayed. The results are expressed as the mean of three different experiments.
Figure 3.
Cell viability in RAW 264.7 cells incubated with amikacin–fluorescein isothiocyanate (FITC). Cells were treated with 50 and 100 mg/L doses of amikacin-FITC and incubated at 4 and 37°C for 6 hours. Then cells were pelleted and counted by Trypan blue dye exclusion. There were no significant differences between the control and the treated cell for any of the temperatures assayed. The results indicate the percentage of live cells and are expressed as the mean of three different experiments.
Amikacin-FITC Does Not Enhance MCP-1 Cytokine Gene Expression or CCR-2 Protein Production in RAW 264.7 Cells
To ensure there was not a significant inflammatory effect of amikacin-FITC on the RAW 264.7 cells, they were incubated with different doses of amikacin-FITC at 4 and 37°C for 3 and 6 hours. The supernatants of the cells were then collected and the levels of cytokine inflammatory marker MCP-1 were measured. We found no difference in MCP-1 levels between the treated and control cells (Figure 4). Furthermore, the low temperature (4°C) also did not alter the cytokine level profile when we compared control and treated samples. It is noted that the cells incubated at 4°C showed lower levels of MCP-1 secreted in the supernatants for all the doses assayed, as compared with the levels measured in the culture at 37°C. This is probably due to the lower metabolic rate the cells have when they are incubated at 4°C.
Figure 4.
Cytokine production of RAW 264.7 cells incubated with different doses of amikacin–fluorescein isothiocyanate (FITC). Cultured cells were treated with different doses of amikacin-FITC (range from 25 to 50 mg/L) at 4 and 37°C. The supernatants were collected after 6 hours of incubation time and the monocyte chemoattractant protein (MCP)-1 levels were measured by ELISA. The results indicate no significant differences between the treatment and the control sample. The cells incubated at 4°C show less production of MCP-1 compared with the cells incubated at 37°C.
Once we measured the cytokine levels produced by the RAW 264.7 cells treated with amikacin-FITC, we went on to analyze the expression of both MCP-1 and CCR-2 in the same samples with reverse transcriptase–polymerase chain reaction (PCR). As shown in Figures 5A and 5B, we found that cells treated with amikacin-FITC did not express higher levels of MCP-1 or CCR-2 as compared with the untreated sample. The results obtained for MCP-1 mRNA were in concordance with the previous results we obtained with the ELISA.
Figure 5.
Gene expression of monocyte chemoattractant protein (MCP)-1 and CCR-2 proteins in RAW 264.7 cells. Cells were incubated with 50 and 100 mg/L of amikacin–fluorescein isothiocyanate (FITC) and were exposed to 4 and 37°C for 6 hours. The expressions of the MCP-1 and CCR-2 genes were analyzed by real-time polymerase chain reaction. The results were related to the expression of the RNA16S as a housekeeping gene. There were no significant differences between the treatment and the untreated sample for both MCP-1 and CCR-2. The different temperatures assayed did not alter the gene expression profile.
M. avium and S. aureus Are Susceptible to Amikacin-FITC
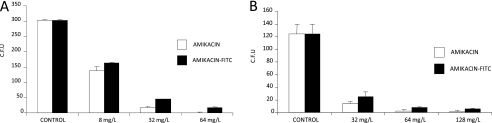
To assess the efficacy of amikacin as an antibiotic when it was conjugated with FITC, we studied the minimum dose of free amikacin and amikacin-FITC needed to reduce the growth of both S. aureus and M. avium bacteria in vitro. We incubated the bacteria with different doses of both free amikacin and amikacin-FITC, and then counted the colonies formed and compared them with an untreated control. We observed no significant difference in the decrease in the cfu for both types of amikacin. For M. avium a dose of 64 mg/L of the antibiotic provoked a decrease of CFU from 300 counted in control to 1 with the free amikacin and 16 for the amikacin-FITC (Figure 6A). When S. aureus colonies were analyzed for 64 mg/L, it resulted in a decrease from 124 cfu in control plates to 2.5 in plates treated with free amikacin, and 7.6 in the treatment with amikacin-FITC (Figure 6B). These results confirmed that FITC dye did not substantially alter the capacity of the amikacin to kill the bacteria.
Figure 6.
Effect of amikacin–fluorescein isothiocyanate (FITC) and free amikacin on the growth of (A) Mycobacterium avium and (B) Staphylococcus aureus. M. avium and S. aureus bacteria were treated with different doses of both free amikacin and amikacin conjugated with FITC dye. After 30 minutes of treatment, the bacteria cultures were grown in the specific agar plates and incubated at 37°C. After the incubation time, formed colonies were counted. (A) M. avium was incubated at 37°C for 14 days after the treatment. (B) S. aureus colonies were counting after 30 minutes' treatment with amikacin and amikacin-FITC. Plates incubated overnight at 37°C. The results show the mean of cfu from three plates of each dosage. There was the same profile in the decrease of colonies when amikacin-FITC was compared with free amikacin for both M. avium and S. aureus.
To determine if a therapeutic concentration is achieved inside of the DCs, the average amount of amikacin-FITC was estimated inside of the cells by fluorescence. For a 24-hour exposure of DCs to 50 mg/L amikacin-FITC the resulting intracellular concentration of amikacin-FITC was estimated to be in the range of 15 to 29 mg/ml, which is well above the therapeutic concentration identified above. This intracellular amikacin-FITC concentration is also above the mean lethal dose (LD50) reported for mice for a commercial amikacin sulfate product (Amikacin Sulfate Injection, USP; Teva Parenteral Medicines, Irvine, CA), wherein the LD50 for an intravenous injection in a mouse is disclosed at 181 mg/kg for a mouse, which would roughly provide a serum level of 1 to 3 mg/ml.
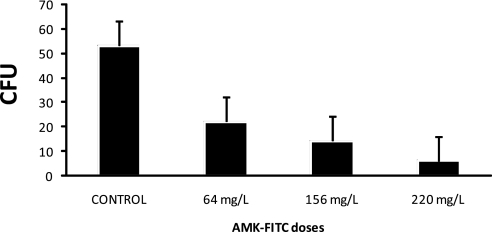
To demonstrate that the monocyte-transformed DCs loaded with amikacin-FITC are capable of killing M. avium, we infected populations of the amikacin-FITC–bearing cells with M. avium for 24 hours. After the incubation period, the cells were lysed and the lysate was serially diluted and plated onto 7H10 agar plates. Plates were incubated at 37°C for 14 days and the number of colonies quantified. A dose-related decrease in the cfu when M. avium was treated with DCs loaded with amikacin-FITC was found as compared with the control (Figure 7).
Figure 7.
Inhibition of Mycobacterium avium growth inside the RAW 264.7 dendritic cell (DC)-loaded amikacin–fluorescein isothiocyanate (FITC). RAW 264.7 DCs were incubated with different doses of amikacin-FITC for 24 hours and cells were infected with M. avium at a 50:1 ratio. After 24 hours, cells were lysed and serial dilutions were plated in agar and incubated at 37°C. The number of colonies was quantified after 14 days.
Furthermore, PCR measurements were used to ensure that the M. avium death occurs within the amikacin-FITC–loaded DCs using specific primers to detect M. avium (Figure 8). Because PCR is performed immediately after the DC/M. avium incubation endpoint is achieved it provides a better indication of M. avium death inside of the DCs. Colony-counting experiments, on the contrary, reflect the impact of cell death inside the DCs as well as from amikacin-FITC being released during DC lysis that occurs before colony growth/counting experiments.
Figure 8.
Electrophoresis of polymerase chain reaction (PCR) products amplified with mycobacteria-specific primers. After PCR using chromosomal DNA as the template and specific primers, DNAs were separated in 2% agarose gel electrophoresis followed by ethidium bromide staining. Observation of a 435-bp PCR indicates the presence of mycobacteria. M, DNA size marker 100 bp (Bio-Rad, Hercules, CA); lane 1, RAW 264.7 cells without Mycobacterium avium; lane 2, RAW 264.7 cells incubated with M. avium for 24 hours (4× stock); lane 3, RAW 264.7 cells incubated with M. avium for 24 hours (2× stock); lane 4, RAW 264.7 cells loaded with amikacin–fluorescein isothiocyanate (FITC) (8 mg/L) and incubated with M. avium for 24 hours; lane 5, RAW 264.7 cells loaded with amikacin-FITC (64 mg/L) and incubated with M. avium for 24 hours; lane 6, H2O.
Interestingly in both cases, we do not observe a considerable change in M. avium death or inhibition with the intracellular amikacin-FITC concentration. This appears to suggest that the killing rate of M. avium is dependent on and limited by the kinetics of internalization into the loaded DCs rather than amikacin-FITC concentration-dependent phenomena; however, a more detailed analysis is necessary to confirm this.
Colocalization of Amikacin-FITC and M. avium Inside the DCs
After incubating M. avium with amikacin-FITC–loaded DCs for 24 hours, the cells were fixed then stained with DAPI and auramine-rhodamine (for labeling M. avium). After washing the samples with PBS, the samples were analyzed under a fluorescence microscope. A positive signal for auramine-rhodamine and therefore M. avium inside the DCs was observed along with the amikacin-FITC signal demonstrating the colocalization of the antibiotic inside the same cells (Figure 9).
Figure 9.
Colocalization of Mycobacterium avium and amikacin–fluorescein isothiocyanate (FITC) inside dendritic cells (DCs). Imaging of DCs stained with DAPI, amikacin-FITC visualized with FITC filter in green and M. avium stained with auramine-rhodamine and visualized with Texas-red filter in red. 400× magnification.
Monocyte-derived DCs Target Granulomas in M. avium–infected Mice
We conjugated the amikacin with FITC dye to track the antibiotic under fluorescence microscopy once taken up by the cells. We first successfully tested the efficacy of the DCs to uptake the amikacin-FITC. We also succeeded in demonstrating that amikacin-FITC did not alter the cell viability and did not initiate an inflammatory response in the form of release of the cytokine MCP-1 and the expression of the CCR2 receptor in the treated cells as compared with the control. Subsequently, we used the same type of monocyte DCs, loaded with amikacin-FITC, as a vehicle for drug delivery.
Our group has developed a mouse model of disseminated NTM infection by infecting mice with M. avium using the intranasal route. Six months later, the mice developed typical granulomas at the sites of infection, characteristic of mycobacteria infection. We injected amikacin-FITC–loaded DCs, previously primed in vitro with M. avium, into the mice via the tail vein. Twenty-four hours later, we killed the mice and harvested the tissues for frozen sections. The H&E staining confirmed granuloma formation in the lungs, spleens, and livers of the infected mouse (Figure 10). We analyzed the sections under fluorescent microscopy. As represented in Figure 10, the lungs, spleens, and livers from the infected mice showed a clear signal of the FITC dye, indicating the presence of amikacin-FITC inside the granulomas. We collected the BAL to measure the levels of amikacin-FITC to determine if the DCs loaded with the antibiotic traveled from site of injection to the infected lungs. As expected, several BAL cells observed under the fluorescence microscope had enhanced green fluorescence indicating the presence of amikacin-FITC inside the infected lungs. We measured the levels of amikacin in both serum and BAL samples. As shown in Figure 11, amikacin was detected in BAL but not in serum samples from infected mice that developed granulomas in the tissues. A total of 0.5 μg/ml of amikacin-FITC was found in the lung BAL of infected mice, which suggests that minimum inhibitory concentration (MIC) is achieved not only inside the DCs but also in the lungs 24 hours after administration. The MIC of amikacin for M. avium is reported to be 1 μg/ml (23). Because 1 ml of Hanks' balanced salt solution was used for the BAL and the amount of lung lining fluid for a mouse is much less than 1 ml (more than twofold dilution), it is anticipated that we have achieved several times the MIC in the lung lining fluid after 24 hours. Because we have not observed significant amikacin-FITC leaching from the cells in the absence of cell lysis, we anticipate that the BAL concentrations were due to carrier cells that died at the site of infection. We injected roughly 1.3 × 106 cells that collectively contained 75 μg of amikacin-FITC; hence, the death of 1% of the injected cells at the site of infection could have led to the measured BAL concentrations. However, this may be an underestimation because the released amikacin-FITC may subsequently be uptaken by other cells that are proximal or downstream.
Figure 10.
Presence of granuloma formation in the tissue of Mycobacterium avium–infected mice and detection of amikacin–fluorescein isothiocyanate (FITC) in the same sections. Imaging of mouse tissue staining with hematoxylin and eosin (H&E), and fluorescence for DAPI and amikacin-FITC. Lung: (A, B) H&E-stained sections; (C, D) fluorescence staining. The blue signal indicates the nuclei of the cells stained with DAPI and green signal the amikacin conjugated with FITC dye. Spleen: (A, B) H&E-stained sections; (C, D) fluorescence staining. The nuclei of the cells stained with DAPI and amikacin conjugated with FITC dye. Liver: (A, B) H&E-stained sections; (C, D) fluorescence staining. The nuclei of the cells stained with DAPI and amikacin conjugated with FITC dye. Kidney: (A, B) H&E-stained sections; (C, D) fluorescence staining. The nuclei of the cells stained with DAPI and amikacin conjugated with FITC dye. Heart: (A, B) H&E-stained sections; (C, D) fluorescence staining. The nuclei of the cells stained with DAPI and amikacin conjugated with FITC dye.
Figure 11.
Levels of amikacin in bronchoalveolar lavage (BAL) and serum samples in infected and noninfected mice. Mice were infected with Mycobacterium avium for 6 months and injected with dendritic cells (DCs) loaded with amikacin–fluorescein isothiocyanate (FITC). Twenty-four hours later the mice were killed and serum and BAL were collected. Levels of amikacin were measured in both serum and BAL samples. We repeated the same protocol injecting DCs with amikacin-FITC in noninfected mice. Serum and BAL samples were analyzed for amikacin. Amikacin levels were detected in BAL but not in serum only in the group of mice infected with M. avium; in this group the presence of granulomas was confirmed by hematoxylin and eosin staining.
We injected the same DCs loaded with amikacin-FITC into a noninfected mouse via tail vein as a control. We harvested the lungs, spleen, liver, kidney, and heart 24 hours later following the same procedure we followed above. When we analyzed the samples under the fluorescence microscope, we did not observe any FITC signal in the lung tissues (data not shown). Furthermore, we found no detectable levels of amikacin in the BAL and serum from noninfected mice, with no granulomas in the tissues.
DISCUSSION
MAC infects immunocompromised hosts as well as those with underlying lung abnormalities (24–30). Furthermore, NTM causes primary infections in patients with no history of respiratory illness (31–35), and what is of greater concern is the increasing frequency of infection in recent years (30, 31, 35, 36) in these patients. Therefore, it is important to find an efficient treatment that succeeds in the cure of mycobacterial infections, with shorter periods of treatment and lower systemic drug dosage. High-dose, targeted drug delivery at the site of infection was our goal in this study. By achieving this, patient compliance could be improved and antimicrobial drug resistance could be avoided.
We have developed an M. avium–infected mouse model to study the characteristic of disseminated infections. Six months after the inoculation of the mycobacteria, the mice present with features of the disease, such as the formation of the typical granulomas in the lungs, spleen, and liver. In this study, we used M. avium–infected mice to test the efficacy of DCs as a vehicle for drug delivery.
The use of DCs for carrying amikacin in vivo takes advantage of their “memory” properties to target the drug delivery into specific sites. The use of DCs as a vehicle for drug delivery is novel because of their ability to recognize pathogens and present them to T cells. These cells are used in cancer therapy and vaccination with specifically primed DCs that can induce immunological as well as clinical responses in selected patients with advanced cancer (37–39). The same principle can be applied to infectious diseases and the unique qualities of DCs can be mined to provide targeted therapy.
Other groups have demonstrated the efficacy of liposomes as drug carriers for the treatment of mycobacterial infections (40, 41). The encapsulation of aminoglycoside antibiotics in liposomes has been described (42). The advantage of using liposomes for the encapsulation of amikacin is to enhance the intracellular concentration at safe serum levels to avoid toxic side effects of this antibiotic (43). However, certain chemicals or therapeutic agents that show success in cell culture fail to produce the same effect in the human body because of the limitations in targeting the designated area; as a result, higher doses are given to patients, potentially resulting in more intense side effects (44).
To bypass the difficulties associated with immune system recognition of artificial carriers, physical clearance, and inefficiencies of receptor-mediated targeting paradigms, our group has suggested the use of the patient's own DCs to transport the drug. These cells are not recognized by the host as foreign cells, so they are innocuous to the immune system, thus avoiding an immune response. Furthermore, living cells have an innate ability to actively migrate to sites via chemo- and mechanotaxis.
To enhance the targeting capacity of DCs, we primed the DCs in vitro with the M. avium used to infect the mice. Thus, once injected into the mice they easily target the site of infection. In this study, our group has demonstrated the efficacy of monocyte-derived DCs to carry the antibiotic amikacin into the granulomas formed by M. avium infection. For this purpose, we transformed RAW 264.7 mouse monocyte cells into DCs by culturing with granulocyte-macrophage colony-stimulating factor and IL-4 cytokines, and then treated the cells with amikacin labeled with FITC dye in vitro. After the cells took up the amikacin-FITC, we injected them into an M. avium–infected mouse. Figure 10 shows the presence of amikacin-FITC in the lungs, spleen, and liver. Moreover, we did not find any positive signal in the noninfected mice treated with the same DCs loaded with amikacin-FITC. Mice treated with unlabeled DCs also did not exhibit any discernable green fluorescence. The presence of amikacin levels only in the BAL from infected mice and the negative values of the antibiotic in the serum of the same animals suggest an efficient delivery and retention of amikacin in the infected tissues (Figure 11).
The amikacin-FITC is easy to track using fluorescence microscopy once it is taken up by DCs. We evaluated the amikacin-loaded cells for in vitro toxicity. As shown in Figure 3, there was no significant difference in cell viability when they were treated with amikacin-FITC at any of the doses assayed as compared with the control. Furthermore, when we measured the level of a key cytokine and its receptor, we did not find any alteration in the profile of MCP-1 and CCR-2 as compared with the control (Figures 4 and 5), even at a high doses, indicating that amikacin itself does not provoke an inflammatory response. We also analyzed the efficacy of amikacin when conjugated with FITC dye as compared with free amikacin against both S. aureus and M. avium bacteria. The results showed no change in the efficacy of the antibiotic in its ability to decrease the growth of the organisms, including S. aureus and M. avium. We found the same bactericidal profile when we compared amikacin-FITC with free amikacin. Taken together, these data confirm that amikacin-FITC had the same antibiotic properties as free amikacin. Moreover, there was no apparent impact on the viability of the amikacin-FITC–loaded DCs.
To study the mechanism by which this drug enters the cells, we treated the mouse monocyte cell line with different doses of amikacin-FITC at two different temperatures. At 37°C, cells are metabolically active, so the drug could be taken up either by a passive mechanism through the phospholipid bilayer or by an active system such as a specific transporter, pinocytosis, or endocytotic pathway. However, at 4°C cells are in a quiescent state, so although they are alive they are not metabolically active. By measuring the fluorescence in the cells treated with amikacin-FITC we estimated the amount of the drug inside the cells. When we measured the fluorescence in the cells incubated with amikacin-FITC for 6 hours at 4 and 37°C, we did not find any significant difference between the two temperatures assayed (Figure 2). Furthermore, we found the same profile in the gene expression of MCP-1 and CCR-2 cytokines analyzed from cells at both the temperatures (Figure 5). These data not only indicate that there is no effect in the cells incubated at low temperature (4°C) but also suggest that the drug uses a passive mechanism to enter the cell. The main advantage of the passive transport through the cell membrane is that this compound might avoid the interaction with some of the receptors that could alter the behavior of DCs. This indicates that there is no apparent interaction between the amikacin-FITC and the cell membrane that initiates any kind of inflammatory or signaling pathways that can potentially interfere with cell-based drug delivery.
Once we tested the antibiotic modified with the FITC dye in the in vitro system, we injected the DCs loaded with the same drug into the bloodstream of M. avium–infected mice. After 24 hours, the tissues were harvested and frozen sections obtained. The lungs, spleen, liver, kidneys, and hearts of the mice were examined under fluorescence microcopy. H&E staining confirmed the granuloma formation provoked by the M. avium bacteria in the lungs, liver, and spleen (Figure 10). We also observed a clear signal of the FITC dye in the same tissues, indicating the presence of DCs loaded with amikacin-FITC at the sites of infections. This colocalization results in increased killing rate of M. avium (Figure 8).
The DCs act to effectively target sites of M. avium infection and as drug concentrators. By loading the DCs with amikacin-FITC, localized intracellular concentrations of the drug have been estimated at levels above the MIC and the LD50 for a commercial amikacin sulfate formulation in mice. However, at the same time, the total amikacin-FITC if administered in the free form would not be expected to provide a lethal dose (estimated levels at ∼15–29 mg/ml) in the mice as administered in this research. Hence, the current therapeutic intervention appears to enhance the therapeutic index of intravenous amikacin-based treatments by providing the advantage of highly targeted and concentrated antibiotic delivery to sites of infection with reduced concern for system toxicity.
In conclusion, we demonstrated that DCs can be used as a vehicle for delivery of drugs to specific sites of infection. This finding is important in mycobacterial infection because it allowed us to deliver amikacin directly into the granulomas at potent concentrations. The delivery of drugs into granulomas is known to be extremely difficult. The experiments were set to evaluate if drug delivery was feasible and we did directly measure drug levels in the granulomas. The implications of this work may extend beyond MAC to tuberculosis and other diseases wherein local delivery of drugs is necessary to achieve high levels and to avoid systemic toxicity. This work opens up a new avenue for the treatment of mycobacterial infections. However, further studies are needed to support the use of DCs as a drug delivery tool for the treatment of human infectious disease. Future experiments are needed to demonstrate killing in vivo with an effective MIC at the site of action, with low plasma drug concentrations, and without detrimental side effects. Additional research will be required to identify the optimal dosage and treatment time points to kill the MAC inside the mice.
Supplementary Material
Acknowledgments
The authors thank Barbara Locke, C.V.T. L.A.T.G., for her excellent technical assistance.
Supported by National Institutes of Health grants RO1 AI080349 and R21 AA 014796 (V.B.A.). Partial support was also received from the Center for Nano-Bio Sensors at the University of Florida.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200912-1888OC on October 1, 2010
Author Disclosure: A.M.-W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.B. received grant support from the National Institutes of Health (more than $100,001). D.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.F.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B. received grant support from the National Institutes of Health (more than $100,001). V.B.A. received lecture fees from GlaxoSmithKline ($50,001–$100,000).
References
- 1.Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med 1986;163:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 1991;9:271–296. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–252. [DOI] [PubMed] [Google Scholar]
- 4.Mohagheghpour N, Gammon D, Kawamura LM, van Vollenhoven A, Benike CJ, Engleman EG. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in 19-kDa lipoprotein. J Immunol 1998;161:2400–2406. [PubMed] [Google Scholar]
- 5.Sprent J, Schaefer M. Antigen-presenting cells for unprimed T cells. Immunol Today 1989;10:17–23. [DOI] [PubMed] [Google Scholar]
- 6.Holt PG, Schon-Hegrad MA. Localization of T cells, macrophages and dendritic cells in rat respiratory tract tissue: implications for immune function studies. Immunology 1987;62:349. [PMC free article] [PubMed] [Google Scholar]
- 7.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med 1996;183:1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton AM, Chadwick MV, Nicholson AG, Dewar A, Feldman C, Wilson R. Investigation of mycobacterial colonisation and invasion of the respiratory mucosa. Thorax 2003;58:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras MA, Cheung OT, Sanders DE, Goldstein RS. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis 1988;137:149–152. [DOI] [PubMed] [Google Scholar]
- 10.Hjelte L, Petrini B, Kallenius G, Strandvik B. Prospective study of mycobacterial infections in patients with cystic fibrosis. Thorax 1990;45:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson R, Abdallah S. Pulmonary disease caused by nontuberculous mycobacteria in immunocompetent patients. In: Wilson R, editor. Tuberculosis. The European Respiratory Monograph. Volume 4. Sheffield: European Respiratory Society; 1997. pp. 247–272.
- 12.Reddy VM. Mechanism of Mycobacterium avium complex pathogenesis. Front Biosci 1998;3:D525–D531. [DOI] [PubMed] [Google Scholar]
- 13.Lai YM, Mohammed KA, Nasreen N, Baumuratov A, Bellew BF, Antony VB. Induction of cell cycle arrest and apoptosis by BCG infection in cultured human bronchial airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L393–L401. [DOI] [PubMed] [Google Scholar]
- 14.de Steenwinkel JE, van Vianen W, Ten Kate MT, Verbrugh HA, van Agtmael MA, Schiffelers RM, Bakker-Woudenberg IA. Targeted drug delivery to enhance efficacy and shorten treatment duration in disseminated Mycobacterium avium infection in mice. J Antimicrob Chemother 2007;60:1064–1073. [DOI] [PubMed] [Google Scholar]
- 15.Khuller GK, Kapur M, Sharma S. Liposome technology for drug delivery against mycobacterial infections. Curr Pharm Des 2004;10:3263–3274. [DOI] [PubMed] [Google Scholar]
- 16.Pandey R, Khuller GK. Polymer based drug delivery systems for mycobacterial infections. Curr Drug Deliv 2004;1:195–201. [DOI] [PubMed] [Google Scholar]
- 17.Bakker-Woudenberg IA, van Vianen W, van Soolingen D, van Agtmael MA. Antimycobacterial agents differ with respect to their bacteriostatic versus bactericidal activities in relation to time exposure, mycobacterial growth phase, and their use in combination. Antimicrob Agents Chemother 2005;49:2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein 1 (MCP-1): An overview. J Interferon Cytokine Res 2009;29:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1). Immunol Today 1990;11:97–101. [DOI] [PubMed] [Google Scholar]
- 20.Pham W, Xie J, Gore JC. Tracking the migration of dendritic cells by in vivo optical imaging. Neoplasia 2007;9:1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasreen N, Mohammed KA, Mubarak KK, Baz MA, Akindipe OA, Fernandez-Bussy S, Antony VB. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-β1 in vitro. Am J Physiol Lung Cell Mol Physiol 2009;297:L115–L124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lean JM, Murphy C, Fuller K, Chambers TJ. CCL9/MIP-1γ and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem 2002;87:386–393. [DOI] [PubMed] [Google Scholar]
- 23.Horgen L, Jerome A, Rastogi N. Pulsed-Exposure and post-antibiotic leukocyte enhancement effects of amikacin, clarithromycin, clofazimine, and rifampin against intracellular Mycobacterium avium. Antimicrob Agents Chemother 1998;42:3006–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iseman MD. Medical management of pulmonary disease caused by Mycobacterium avium complex. Clin Chest Med 2002;23:633–641. [DOI] [PubMed] [Google Scholar]
- 25.Wallace RJ Jr, Cook JL, Glassroth J, Griffith DE, Olivier KN, Gordin F. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med 1997;156:S1–S25. [DOI] [PubMed] [Google Scholar]
- 26.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 27.Field SK, Cowie RL. Lung disease due to the more common nontuberculous mycobacteria. Chest 2006;129:1653–1672. [DOI] [PubMed] [Google Scholar]
- 28.Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci 2005;20:913–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yim JJ, Park YK, Lew WJ, Bai GH, Han SK, Shim YS. Mycobacterium kansaii pulmonary diseases in Korea. J Korean Med Sci 2005;20:957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iseman MD. Mycobacterium avium complex and the normal host: the other side of the coin. N Engl J Med 1989;321:896–898. [DOI] [PubMed] [Google Scholar]
- 31.Prince DC, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863–868. [DOI] [PubMed] [Google Scholar]
- 32.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: the Lady Windermere syndrome. Chest 1992;101:1605–1609. [DOI] [PubMed] [Google Scholar]
- 33.Epstein MD, Aranda CP, Bonk S, Hanna B, Rom WN. The significance of Mycobacterium avium complex cultivation in the sputum of patients with pulmonary tuberculosis. Chest 1997;111:142–147. [DOI] [PubMed] [Google Scholar]
- 34.Dhillon SS, Watanakunakorn C. Lady Windermere syndrome: middle lobe bronchiectasis and Mycobacterium avium complex infection due to voluntary cough suppression. Clin Infect Dis 2000;30:572–575. [DOI] [PubMed] [Google Scholar]
- 35.Yamazaki Y, Kubo K, Sekiguchi M, Honda T. Analysis of BAL fluid in M avium-intracellulare infection in individuals without predisposing lung disease. Eur Respir J 1998;11:1227–1231. [DOI] [PubMed] [Google Scholar]
- 36.Whiteside TL, Odoux C. Dendritic cell biology and cancer therapy. Cancer Immunol Immunother 2004;53:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thumann P, Moc I, Humrich J, Berger TG, Schultz ES, Schuler G, Jenne L. Antigen loading of dendritic cells with whole tumor cell preparations. J Immunol Methods 2003;277:1–16. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann J, Peters JH, Wischke C, Borchert HH, Lorenzen DR. Delivery of PGLA-microsphere encapsulated antigens to monocytes-derived dendritic cells prolongs their antigen presentation and promotes Th1-type cytokine production [abstract number U34]. Immunobiol 2003;208:270. [Google Scholar]
- 39.Pinto-Alphandary H, Andremont A, Couvreur P. Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents 2000;13:155–168. [DOI] [PubMed] [Google Scholar]
- 40.Bakker-Woudenberg IAJM. Liposomes in the treatment of infectious diseases. In: Puisieux F, Couvreur P, Delattre J, Devissaguet J-P, editors. Liposomes, new systems, and new trends in their application. Paris, France: Editions de Santé; 1995. pp. 373–396.
- 41.Schiffelers R, Storm G, Bakker-Woudenberg I. Liposome-encapsulated aminoglycosides in pre-clinical and clinical studies. J Antimicrob Chemother 2001;48:333–344. [DOI] [PubMed] [Google Scholar]
- 42.Leitzke S, Bucke W, Borner K, Müller R, Hahn H, Ehlers S. Rationale for and efficacy of prolonged-interval treatment using liposome-encapsulated amikacin in experimental Mycobacterium avium infection. Antimicrob Agents Chemother 1998;42:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawat M, Singh D, Saraf S, Saraf S. Nanocarriers: promising vehicle for bioactive drugs. Biol Pharm Bull 2006;29:1790–1798. [DOI] [PubMed] [Google Scholar]
- 44.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm 2008;5:487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.