Abstract
Parkinsonism is a primary neurotoxic manifestation of organophosphate pesticide intoxication. We report here the case of a 67-year-old man who developed acute parkinsonism with tremors and rigidity following exposure to fenitrothion, an organophosphate pesticide. His parkinsonism disappeared, and 2 months later he was able to walk alone without antiparkinsonian drug treatment. To identify particular lesions in the brain, Z score images were obtained from SPECT (single photon emission computed tomography) scans of the patient during the acute poisoning and a follow-up scan performed 2 months later. We indicate that reversible parkinsonism correlated with putaminal hyperperfusion as observed in the Z score images obtained during the acute event; this condition resolved later in concert with resolution of the clinical parkinsonism. We believe that the SPECT scan Z score images in this study are an important find in elucidating parkinsonism manifestations due to organophosphate poisoning.
BACKGROUND
Organophosphate pesticides are widely used not only in developing countries but also in the developed world, including Japan. Sahin et al reported that, among cases of poisoning in Turkey, organophosphate poisoning is responsible for 15% of all occurances.1 Organophosphates suppress the action of the cholinesterase that catalyses the hydrolysis of the neurotransmitter acetylcholine, and as a result, victims develop the symptoms of acute cholinergic crises. In the acute phases of organophosphate poisoning, symptoms include muscarinic actions such as miosis, hypersalivation, and sudoresis and nicotinic actions such as muscle weakness and fasciculation. After the initial remission of these acute cholinergic crises, there is an intermediate phase of neuropathy and muscle weakness (intermediate syndrome).2 Delayed polyneuropathies emerge later, and finally a phase involving the appearance of neuropsychiatric manifestations is possible. Following acute exposure, and in some cases following low dose chronic exposure, several neurobehavioral changes have been observed and together are termed as “chronic organophosphate induced neuropsychiatric disorder (COPIND).”3,4
Only 0.5% of organophosphate poisoning patients develop neurotoxic manifestations in the form of extrapyramidal syndromes such as parkinsonism.5 After an acute cholinergic crisis, patients may develop parkinsonism symptoms including rigidity, tremors, bradykinesia, and masked face. Eighty-one per cent of patients with extrapyramidal syndrome developed the symptoms within 2 weeks of their poisoning.6 Seventy-seven per cent of patients with extrapyramidal symptoms recovered, and 71% of the patients recovered without antiparkinsonian drug treatments.6 The duration of extrapyramidal symptoms ranges from 7 days to 2 months.6 Intermediate syndrome was not observed in all poisoning patients with extrapyramidal syndrome.5
The patient in this case study developed parkinsonism due to acute organophosphate poisoning. Previous reports have indicated that certain extrapyramidal symptoms, including parkinsonism, are subject to the possibility of spontaneous remission. The mechanism of their pathogenesis, however, remains unclear.7,8 To ascertain the effects of organophosphate pesticides on the central nervous system, SPECT (single photon emission computed tomography) studies were employed to identify the mechanisms of parkinsonism caused by organophosphate poisoning.
CASE PRESENTATION
In an attempted suicide, a 67-year-old man poisoned himself with about 200 ml of 50% fenitrothion, an organophosphorus pesticide. His symptoms were deep coma (Glasgow Coma Scale E1V1M1), hypotension (80/59 mm Hg), tachycardia (120/min), miosis (pupils 2 mm of size), hypersalivation, and sudoresis. The patient was administered activated charcoal with a cathartic and was mechanically ventilated. In addition, he was continuously infused with atropine sulfate with PAM (2-pyridine aldoxime methiodide). His plasma cholinesterase value decreased remarkably to 7 U/l (reference 170–420 U/l), and his blood fenitrothion value was abnormally high at 4260 ng/ml (reference <1 ng/ml). His consciousness and respiratory status improved, and mechanical ventilation was discontinued on the 4th day. The patient’s consciousness deteriorated again on the 5th day, however, and he developed respiratory muscle paralysis. We diagnosed this as intermediate syndrome accompanied by the typical characteristics of organophosphate poisoning in which symptoms improve and then worsen. From that time onward, a resting tremor similar to pill-rolling and cogwheel rigidity developed in the left dominant bilateral upper and lower limbs. The neurological examination revealed masked face, resting and postural tremors, and marked cogwheel rigidity manifesting as a rigid posture and fixed flexion of the elbows. The diagnosis of left dominant bilateral parkinsonism was established 7 days after ingestion of the organophosphate insecticide.
The patient had not been taking any medications, such as psychotropic drugs, known to cause or exacerbate parkinsonism and cholinergic properties. He has no personal or familial history of parkinsonism.
INVESTIGATIONS
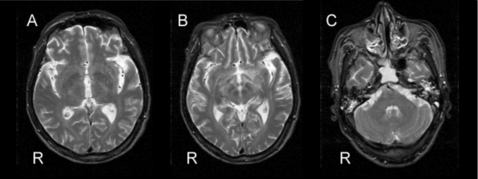
Magnetic resonance imaging (MRI) of the brain from the 16th day did not reveal lesions on the mesencephalon, basal ganglia, or cerebellum (fig 1A–C).
Figure 1.
Magnetic resonance imaging from 16 days after ingestion of organophosphate insecticide failed to reveal lesions on the mesencephalon, basal ganglia, or cerebellum. T2-weighted axial images (A–C).
A statistical analysis of the tomographic brain images obtained via N-isopropyl-p[123I] iodoamphetamine single photon emission computed tomography (123I-IMP SPECT) was performed. The spatial distribution of abnormal cerebral blood flow (CBF) was evaluated using Neurological Statistical Image Analysis Software,9 and the value of the Z score was calculated. A positive Z score represented an increase in the patient’s regional CBF relative to the control mean. The 23 control subjects (mean (SD) age 72.0 (4.1) years) were cognitively normal.
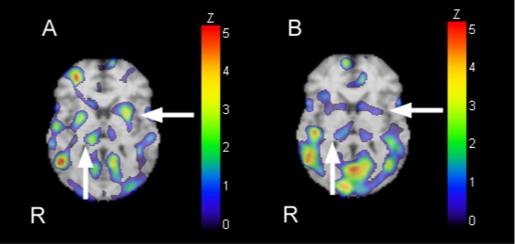
A 123I-IMP SPECT image from the 19th day showed an increased Z score in the bilateral putamen hyperperfusion (fig 2A).
Figure 2.
Hyperperfusion was shown in the putamen and thalamus by an examination of the 123I-IMP SPECT Z score increasing (arrow) 19 days after ingestion of organophosphate insecticide (A). The Z scores of the putamen were >2.0, which is a significant value. As shown by the 123I-IMP SPECT Z-score increasing (arrow) 2 months after ingestion of the organophosphate insecticide (B), the patient lost all signs of parkinsonism and he was able to walk alone. Perfusion deviation improved, and the Z scores of the putamen were <1.4, which is not a significant value. Other areas of hyperperfusion are also shown in the frontal, temporal, and occipital lobes in panel A or B.
DIFFERENTIAL DIAGNOSIS
Idiopathic Parkinson disease
Parkinsonian syndrome
Vascular parkinsonism.
TREATMENT
We performed no treatment for parkinsonism due to organophosphate pesticide intoxication.
OUTCOME AND FOLLOW-UP
On the 24th day, the patient’s consciousness normalised, and he had neurologic manifestations such as persecutory delusions and anxiety. His left dominant bilateral parkinsonism (tremor, rigidity) showed improvement but remained on the 30th day. Through natural causes, his parkinsonism disappeared, and 2 months later he was able to walk alone and lost all signs of parkinsonism. Additionally, as shown by the SPECT image of the increasing Z score (fig 2B), the perfusion deviation improved. Delayed polyneuropathies showed no significant influence. His plasma cholinesterase value improved to 111 U/l by the 60th day.
DISCUSSION
Organophosphate poisoning induces parkinsonism through the effect of cholinesterase inhibition. Davis et al reported the first such case in 1978.10 The mechanisms of extrapyramidal parkinsonism, however, remain unclear. Senanayake and Sanmuganathan proposed that organophosphates might exert selective access to the basal ganglia and impair the balance between dopamine and acetylcholine therein and in the substantia nigra.11 According to conventional theory, this imbalance should cause parkinsonism. Hsieh et al proposed that organophosphate pesticides impair the function of acetylcholinesterase in the modulation of the nigrostriatal dopaminergic system, which is dependent on the hydrolysing of acetylcholine.5
On the other hand, to our knowledge, this case is the first report on hyperperfusion of the putamen utilising brain SPECT imaging in the investigation of parkinsonism due to organophosphate poisoning. We believe that the perfusion abnormality in the putamen revealed by SPECT imaging contributes to the manifestation of parkinsonism in victims of organophosphate poisoning. The correlation is in fact shown by follow-up SPECT images that, with the resolution of parkinsonism, reveal bilateral perfusion improvement in the putamen. Hyperperfusion of the putamen correlates clinically with dysfunction in the affected basal ganglia. It is difficult to explain why hyperperfusion of the putamen occurs with organophosphate poisoning. As for hemiconvulsions followed by Todd’ paralysis, the mechanism underlying hyperperfusion is thought to be impairment of cerebrovascular autoregulation in the dysfunctional region.12,13 Considering that the MRI revealed no basal ganglia lesions,14 cerebral hyperperfusion might imply the reversible dysfunction of the putamen due to organophosphate poisoning. In a report on fluorine-18 entitled fluorodeoxyglucose-positron emission tomography (PET) for a case with extrapyramidal symptoms due to organophosphate poisoning, hypermetabolism of the putamen was shown.15 On the other hand, Hsieh et al reported a case with a normal PET study.5 We believe that hyperactive neurons in the putamen might be constitutively inhibitory.
LEARNING POINTS
Organophosphate poisoning induces the inhibition of anticholinesterase leading to excess of acetylcholine and acute cholinergic crises.
Parkinsonism can appear as a symptom of a common form of poisoning in the developing world.
Parkinsonism is a possible neurotoxic manifestation of chronic intoxication by organophosphates.
Parkinsonism following organophosphate poisoning is reversible without antiparkinsonian drug treatment.
SPECT images reveal that parkinsonism due to organophosphate pesticide intoxication may be associated with abnormalities in parts of the nigrostriatal system such as the basal ganglia.
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Sahin HA, Sahin I, Arabaci F. Sociodemographic factors in organophosphate poisonings: a prospective study. Hum Exp Toxicol 2003; 22: 349–53 [DOI] [PubMed] [Google Scholar]
- 2.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med 1987; 316: 761–3 [DOI] [PubMed] [Google Scholar]
- 3.Jamal GA. Neurological syndromes of organophosphorus compounds. Adverse Drug React Toxicol Rev 1997; 16: 133–70 [PubMed] [Google Scholar]
- 4.Jamal GA, Hansen S, Julu PO. Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology 2002; 181–182: 23–33 [DOI] [PubMed] [Google Scholar]
- 5.Hsieh BH, Deng JF, Ger J, et al. Acetylcholinesterase inhibition and the extrapyramidal syndrome: a review of the neurotoxicity of organophosphate. Neurotoxicology 2001; 22: 423–7 [DOI] [PubMed] [Google Scholar]
- 6.Shahar E, Bentur Y, Bar-Joseph G, et al. Extrapyramidal parkinsonism complicating acute organophosphate insecticide poisoning. Pediatr Neurol 2005; 33: 378–82 [DOI] [PubMed] [Google Scholar]
- 7.Mohit H, Bhatt MH, Elias MA, et al. Acute and reversible parkinsonism due to organophosphate intoxication: five cases. Nerurology 1999; 52: 1467–71 [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Andraws J. Extra-pyramidal parkinsonism complicating organophosphate insecticide poisoning. Eur J Paediatr Neurol 2001; 5: 261–4 [DOI] [PubMed] [Google Scholar]
- 9.Minoshima S, Frey KA, Koeppe RA, et al. A diagnostic approach in Alzheimer’ disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995; 36: 1238–48 [PubMed] [Google Scholar]
- 10.Davis KL, Yesavage JA, Berger PA. Single case study. Possible organophosphate-induced parkinsonism. J Nerv Ment Dis 1978; 166: 222–5 [DOI] [PubMed] [Google Scholar]
- 11.Senanayake N, Sanmuganathan PS. Extrapyramidal manifestations complicating organophosphorus insecticide poisoning. Hum Exp Toxicol 1995; 14: 600–4 [DOI] [PubMed] [Google Scholar]
- 12.Yarnell PR. Todd’ paralysis: a cerebrovascular phenomenon? Stroke 1975; 6: 301–3 [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Sejima H, Ozasa H, et al. Technetium-99m-HMPAO SPECT in patients with hemiconvulsions followed by Todd’ paralysis. Pediatr Radiol 1998; 28: 92–4 [DOI] [PubMed] [Google Scholar]
- 14.Teke E, Sungurtekin H, Sahiner T, et al. Organophosphate poisoning case with atypical clinical survey and magnetic resonance imaging findings. J Neurol Neurosurg Psychiatry 2004; 75: 936–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki M, Okada Y, Mii K. Clinical evaluation of central nervous system action in organophosphorous intoxication with positron emission CT. Jpn J Acute Med 1991; 15: 611–3 [Google Scholar]