Abstract
Objective
This study was designed to validate the cell trafficking efficiency of the in vivo bioluminescence image (BLI) study in the setting of transplantation of the luciferase expressing bone marrow-derived mesenchymal stem cells (BMSC), which were delivered at each different time after transient middle cerebral artery occlusion (MCAO) in a mouse model.
Methods
Transplanting donor BMSC were prepared by primary cell culture from transgenic mouse expressing luciferase (LUC). Transient focal infarcts were induced in 4-6-week-old male nude mice. The experiment mice were divided into five groups by the time of MSC transplantation : 1) sham-operation group, 2) 2-h group, 3) 1-day group, 4) 3-day group, and 5) 1- week group. BLI for detection of spatial distribution of transplanted MSC was performed by detecting emitted photons. Migration of the transplanted cells to the infarcted area was confirmed by histological examinations. Differences between groups were evaluated by paired t-test.
Results
A focal spot of bioluminescence was observed at the injection site on the next day after transplantation by signal intensity of bioluminescence. After 4 weeks, the mean signal intensities of 2-h, 1-day, 3-day, and 1-week group were 2.6×107 ± 7.4×106, 6.1×106 ± 1.2×106, 1.7×106 ± 4.4×105, and 8.9×106 ± 9.5×105, respectively. The 2-h group showed significantly higher signal intensity (p < 0.01). The engrafted BMSC showed around the infarct border zones on immunohistochemical examination. The counts of LUC-positive cells revealed the highest number in the 2-h group, in agreement with the results of BLI experiments (p < 0.01).
Conclusion
In this study, the results suggested that the transplanted BMSC migrated to the infarct border zone in BLI study and the higher signal intensity of LUC-positive cells seen in 2 hrs after MSC transplantation in MCAO mouse model. In addition, noninvasive imaging in real time is an ideal method for tracking stem cell transplantation. This method can be widely applied to various research fields of cell transplantation therapy.
Keywords: Stroke, Bone marrow-derived mesenchymal stem cells, Bioluminescence image
INTRODUCTION
Although there have been vast advances in the prevention and treatment of cerebrovascular diseases, stroke remains the main cause of serious adult disability and mortality. Bone marrow-derived mesenchymal stem cells (BMSC) transplantation has been employed in animal models of stroke5,12,13,15,19,23) as well as in human stroke patients2). Those studies have shown improvement of survival and functional outcome with BMSC therapy. However, there is uncertainty about the mechanism by which cell transplantation might improve stroke deficits. Transplanted cells would be expected replace cells that are damaged by ischemia and take over the function of these cellular elements21,26). It is also possible that transplanted cells secrete trophic factors that help to maintain marginally surviving cells or otherwise enhance the local environment sufficiently to improve function. Transplantation might also conceivably produce a host reaction that could include sprouting of new axons and synapse formation26).
The ability of stem cells to migrate to the sites of cerebral pathology was demonstrated in the model of intracranial gliomas1). Stroke is another pathologic event that causes neuronal and glial cell death by abrupt cessation of blood flow. So, the similar pathologic condition can be resulted in the area of insult. The migration of neural progenitor cells (NPC) to sites of cerebral ischemic infarct followed by neural and astrocytic differentiation has been demonstrated9). The migration and differentiation of transplanted cells in the brain is principally based on histological analyses. These techniques do not permit dynamic evaluation of the proliferation, migration, and fate of transplanted cells, because only one time point of datum can be obtained from each experimental animal. Non-invasive cellular imaging allows the real-time tracking of grafted cells as well as the monitoring of their migration. Bioluminescence imaging (BLI) using the firefly luciferase gene permits repeated and non-invasive monitoring of the survival and fate of transplanted cells8). BLI is also crucial to clarifying the factors needed for successful engraftment of donor cells and assessing the safety of their transplantation.
It remains unclear whether engrafted stem cells could generate different effects if cells are transplanted at different time points after stroke. Promising data have been accumulating regarding to the effectiveness of this neurorestorative approach. In rat models for stroke, injected MSC on 1 day (acute) or 7 days (subacute) after stroke induced neurological and functional recovery and the beneficial effects persisted for at least 4 months6,14).
The present study was designed to validate the cell trafficking efficiency for time differently delivered intracerebral transplantation BMSC in transient middle cerebral artery occlusion (MCAO) mouse model. An in vivo BLI optical imaging technique was used to detect the photons emitted by the transplanted MSC at different time point after transplantation. Histological distributions of the transplanted BMSC were also evaluated via luciferase immunohistochemical staining methods.
MATERIALS AND METHODS
Isolation and culture of BMSC
Six weeks old male FVB/N-Tg (β-Actin-luc)-Xen transgenic mice (Xenogen Biosciences, Cranbury, NJ, USA) that express luciferase were used for BMSC preparation. After the femurs were dissected away from attached muscle and soft tissue, both ends were cut. The marrow was extruded by inserting a 21-gauge needle into the shaft of the bones flushing it with 2 mL of Dulbecco's modified Eagle's (DMEM; Nissui Co., Tokyo, Japan) containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/mL penicillin G, From 10 to 15×106 whole marrow cells were placed in a 150 cm2 tissue culture flask coated with collagen I (Becton Dickinson Labware, Oxford, UK), in DMEM/10% FBS. After 48 h, the nonadherent cells were removed by replacing the medium, and the adherent cells were continuously subcultured as BMSC. The culture medium was replaced two times per week. The second or third passages were used for the following experiments.
In vitro assay
In order to confirm luciferase activity in MSC, serial twofold dilutions of 2×105 cells of cultured BMSC were prepared in a 96 well plate with 100 µL of PBS to determine 50% decreasing of the maximum peak of luminescent activity. 293T cells were placed in another row of the 96-well plate as a control. The cells were stimulated with adding d-luciferin (150 µg/mL) before measuring the luminescent activity by Wallac 1450 MicroBeta Jet Luminometer (Perkin-Elmer, Wellesley, MA, USA). For in vitro imaging, BMSC were diluted two fold serially from 2×105 cells into medium in a row of black, clear bottom 96-well plates (Grenier, Germany). D-Luciferin (150 µg/mL) was added to each well and incubated at room temperature for 5 min prior to imaging by the IVIS Imaging System (Caliper, Hopkinton, MA, USA) comprised of a highly sensitive CCD camera mounted in a light-tight camera box. The maximum peak of luminescent activity was observed at 15-20 min after stimulation. Images and intensities of bioluminescent signals were acquired and analyzed using Living Image Software (Caliper, Hopkinton, MA, USA).
Experimental groups
The four mice were randomly assigned to each of four groups that were divided by the time of MSC transplantation after MCAO : sham-operation group, 2-h group, 1-day group, 3-day group, and 1-week group. For in vivo imaging of cells, mice were anesthetized with ketamine/xylazine mixture (7.0 and 0.4 mg per 100 g of body weight, respectively) intra-peritoneally with 50 mg/kg D-luciferin at 5-10 min before imaging. Regions of Interest (ROIs) were drawn, using Living Image software. Imaging was performed at 5, 10, and 15 min after intraperitoneal luciferin injection until obtaining maximal signal intensity at each time points. In order to control for background photon emission, obtained data were subjected to average background subtraction, using data from control animals that were only injected with identical doses of luciferin. Photon radiance on the surface of an experimental animal was expressed as photons/sec/cm2/sr. Images shown in figures are compound pictures generated by Living Image software.
Animals and ischemic model
All experiments and procedures were approved by the local animal care committee and were in line with the Canadian Council of Animal Care guidelines. The in vivo experiments were carried out on 4-6-week-old male nude mice (Charles River, MA, USA) weighing 25 to 30 g. The mice had free access to food and water before the start of the experiment. Sixteen mice underwent focal ischemia using the intraluminal filament method and five were prepared as sham-operated.
Transient focal infarcts were microsurgically induced using the previously described method of intraluminal suture by Longa et al17). After a ventral midline incision in the neck, the right common and external carotid artery were exposed and isolated under an operating microscope (Leica Wild M3C), two branches of the external carotid artery (ECA), the occipital artery and superior thyroid artery, were isolated and then cut between double ligatures (6-0 silk suture). The right ECA was dissected further distally and double ligated as distally as possible. A 5-0 silk suture was tied loosely around the proximal ECA. The right internal carotid artery (ICA) was isolated and carefully separated from the adjacent vagus verve. Two micro vessel clips were placed across the CCA and ICA on either side of the origin of the ECA. The pterigopalatine artery from the proximal portion of the internal carotid artery was electrocoagulated and cut to minimize collateral circulation effect after ICA occlusion. A length (10-12 mm, determined according to the animal' weight) of 7-0 silicon-coated 7-0 nylon monofilament suture occluder was prepared and was immersed in saline containing 50 U/mL heparin before use. The monofilament suture was introduced through an arteriotomy between the distal ligature of the ECA and the cervical carotid bifurcation and was advanced a short distance into the ECA lumen. The silk suture around the ECA was tightened around the intraluminal nylon suture to prevent bleeding, and then the micro vessel clips were removed. The ECA was cut between the two distal ligatures and the resulting stump was bent in the same direction as the ICA. The monofilament nylon suture was then gently advanced from the ECA to the ICA lumen. The position of the suture within the ICA lumen could be seen as it reached the base of the skull. When introducing the suture 12 mm from the cervical carotid bifurcation, obvious resistance was felt and a slight curvature was observed, which indicated the initiation of MCAO. After the exclusion of hemorrhage, the surgical wound was closed with a 4-0 silk suture. After 1 h of MCAO, the animals were reanesthetized and reperfusion was achieved by withdrawing the nylon suture. The sham-operated group received the same procedure without inserting filament in the MCA.
Measurement of cerebral blood flow
For all animals, transcranial measurements of cerebral blood flow (CBF) were made by laser-Doppler flow metry (LDF) (Periflux System 5000; Perimed, Stockholm, Sweden). A 0.5-mm diameter microfiber laser-Doppler probe (Probe 418; Primed) was attached to the skull with cyanoacrylate glue, 3 mm lateral and 1 mm anterior to bregma, to monitor the regional cerebral blood flow (rCBF) in the cortex of right MCA territory before the MCAO procedure. The data were continuously stored in a computer and analyzed using Perimed data acquisition and analysis system (Perimed AB, Järfälla, Sweden). Regional CBF was expressed as a percentage of preischemic baseline values. MCAO animals were only included if occlusion caused a decrease in rCBF to 30% of the original blood flow.
2, 3, 5-triphenyltetrazolium chloride staining
Mice were deeply anesthetized with an injection of pentobarbital (70 mg/kg IP) and subjected to intracardiac perfusion with 50 mL of PBS delivered by hand injection. The head was then removed with a small surgical forcep and the brain carefully dissected out en bloc. After a brief (< 1 min) period of cooling on a bed of ice, the brain was sliced coronally at 1-mm intervals. Individual slices were freed from dura mater and vascular tissue and soaked for 10 minutes in a solution of 2% TTC in 0.1 M PBS (pH adjusted to 7.4), warmed to 37℃ in a water bath. Gentle stirring of the slices ensured even exposure of the surfaces to staining. Excess TTC was then drained, and slices were refrigerated in 10% formalin.
BMSC transplantation procedures
The luciferase expressing BMSCs were transplanted into the contralateral striatum of the mice subjected to transient MCA occlusion at different time point after the insult. MSC transplantation for the sham-operated mice was performed just after the sham operation. The animals were anesthetized on the above mentioned condition. All procedures were performed under aseptic condition on a clean bench (VWP-1000; Nihon Ika, Osaka, Japan). The animals were fixed to a stereotactic apparatus (Model DKI-900; David Kopf Instrument, Tujunga, CA, USA) and the cranium was exposed through midline skin incision. BMSCs were intracerebrally transplanted by inserting a 26-gauge needle with a Hamilton syringe into the right striatum (anteroposterior = 0 mm; lateral to midline = 2.0 mm; depth = 3.5 mm) from bregma, based on the atlas given by Paxinos et al.22), after making a small burr hole using a dental drill. One million of MSC in 4-µL fluid volumes were transplanted into each animal over a 5-min period using an automatic microinjection pump system (Model KDS-310; Muromachi Kikai, Tokyo, Japan). No immunosuppressive drugs were used in any animals.
Tissue processing and immunohistochemical staining
The anesthetized animals were killed by decapitation. The brains were rapidly removed and placed in 4% paraformaldehyde at 4℃ for 48 h. Coronal sections (100 µm of thickness) were cut on a vibratome. Sections were mounted on poly-L-lysine-coated slides. After blocking, sections were incubated with anti-luciferase antibody (Cortex Biochem, San Leandro, CA, USA) diluted (1 : 100) in PBS/0.1% BSA for 1 h at 37℃ and then, fluorescein-conjugated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA, USA) was added, followed by incubation with the ABC reagent (Vector Laboratories). Specimens were viewed with a fluorescence microscope and a confocal microscope (Ultraview 2; Perkin Elmer, Boston, MA, USA).
Statistical analysis
All data are expressed as mean ± SD. Difference between groups were evaluated by paired t-test using SPSS 15.0 statistical software. Data were considered statistically significant at a value of p < 0.05.
RESULTS
In vitro luciferase activity in BMSCs after primary culture
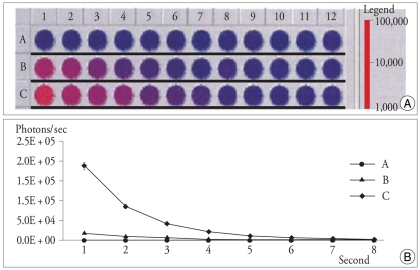
After 24 h of the culture of cells from the bone marrow of a mouse femur, heterogeneous mixture of morphologically distinct adherent cells, including fibroblastoid and hematopoietic cell types, were observed. After 1 week of the culture, populations consisting of spindle-shape fibroblastoid cells were predominantly adhered to the plastic plate. After the second cell passage, the MSC became homogeneous in appearance as elongated or spindle-shaped cells. These cells had a high replicative potential and persisted in the subsequent cultures without morphological change (data not shown). In order to confirm the expression of luciferase from the cultured BMSC, evaluation of the luciferase activity was performed with luminometry (Fig. 1).
Fig. 1.
Two-fold serial dilutions were used to determine the luciferase activity by luminometry : Luminometer image of 96 well plate showing luciferase expression tests on progressive dilutions from 105 (row B), 106 (row C) of BMSC, and 106 (row A) of 293 T cells as a control. Each wells are containing 150 µL of PBS and 150 µg/g of luciferin was added followed by incubation period of 15 min at room temperature and measured with a second/well integration time (A). Plotting of luciferase activity examined by luminometer discloses two-fold decrease of photons counts per second in different initial cell numbers of BMSC (B : 105 cells, C : 106 cells). 293 T cells (A) show no detectable luminometric photon signal (B).
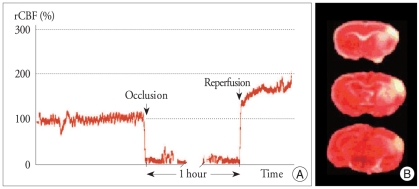
Induction of focal cerebral ischemia using laser-doppler
Cortical blood flow measured using a laser-doppler flowmetry (LDF) was decreased after MCA occlusion by at least 70% in all animals. After insertion of the intraluminal filament, the mean rCBF was decreased to less than 15% of the preocclusion value (Fig. 2A). The decreased rCBF value was sustained relatively constant during the intraischemic period. Immediately after reperfusion, the LDF reading was returned to baseline or more than the baseline preocclusion value. LDF measurements were recorded for 60 min after reperfusion. There was no difference statistically in the rCBF during MCAO (mean 10.1%, p < 0.5). One mouse died in each group from subarachanoid hemorrhage, for an overall mortality of 16.7%. Brain infarct area in the cerebral cortical and striatal areas over a series of sections were determined by 2, 3, 5-triphenyltetrazolium chloride monohydrate (TTC) staining (Fig. 2B).
Fig. 2.
Infarction of right MCA territory in the reperfusion ischemia models. Percent rCBF compared with preischemic baseline evaluated (A). Sudden drop of CBF below the baseline level was regarded as a successful MCAO. Once reperfusion was induced after 1 h of MCAO, the CBF returned to same or higher than preocclusion level. Mouse brain slices were stained by TTC solution after ischemia. Representative sections show the infarction area, which is evident as white tissue due to lack of staining (B).
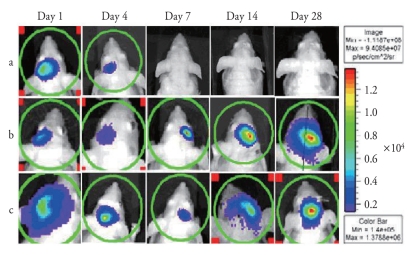
In vivo bioluminescence imaging of BMSC migration to ischemic area
Cultured BMSC (1×106) were stereotactically transplanted into the sham and infarction model mice, and cell distribution and signal intensity were assessed in living animals using BLI. The use of BLI for detection of spatial distribution of transplanted BMSCs was validated by detecting emitted photons. A focal spot of bioluminescence was observed at the injection site on the next day after transplantation, crossing the midline, and finally at the site of infarct in a spatio-temporal sequential patterns (Fig. 3). Sequential evaluation of grafted cell viability was possible with the BLI system since the luciferin-luciferase reaction depends on ATP and only living cells release photons. Drastic reductions in signal intensity within the first 7 days after transplantation were observed at the injection site. Unlike the infarction animals, there was no signal from the contralateral side of injection in the control group.
Fig. 3.
Transplanted BMSCs are initially located in the injected site (left hemisphere). Gradual disappearance of photon emission during 7 days after injection on the injection site is noted. No migration is observed in control group (a). Experimental mice injected of cells 2 h (b) and 1 week (c) after MCAO show signals in infarction area (right hemisphere) after crossing the midline and subsequent increasing intensity over time.
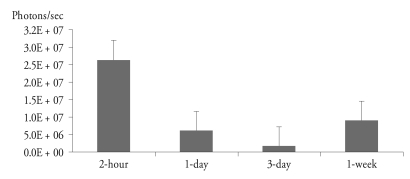
Quantitative analysis
The mean peak BLI signal intensities of one day after transplantation in control and experimental groups were 3.7 ×106 ± 1.6×105 and 3.7×106 ± 1.3×105 photons per sec, respectively, and there was no significant difference between two groups. After 7 days of transplantation, BLI signal intensities of the control group decreased to 1.3×103 ± 0.5×103 photons per second, which was almost the same number of back ground signal intensity. However, BLI signal intensities of groups with MCAO injury persisted for 4 weeks. At 4 weeks after transplantation of BMSC, BLI signal intensity of the 2-h group was significantly higher than those of other experimental groups (Fig. 4).
Fig. 4.
BLI signal of 4 weeks after transplantation of BMSC in different groups of injection time in MCAO mice. The mean signal intensity of 2-h, 1-day, 3-day, and 1-week group was 2.6×107 ± 7.4×106, 6.1×106 ± 1.2×105, 1.6×105 ± 4.4×104, and 8.9×106 ± 9.5×105, respectively. The 2-h group shows the highest signal intensity comparing to other experimental groups (p < 0.01). The signal of the 1-week group is higher than that of 1-day group without statistically significant.
The number of BMSCs engrafted in the infarcted area
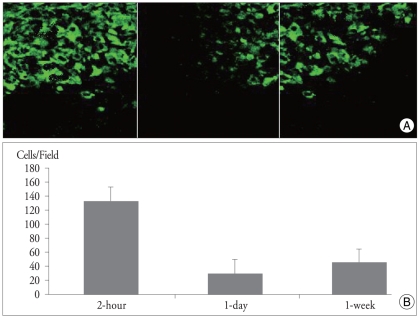
Immunohistochemical staining obtained 28 days after injection showed many BMSC clustering around the site of infarct (Fig. 5). In consistent with the BLI results, numbers of luciferase-positive cells were significantly higher in 2-h group than those of other groups. In control animals without infarct, a scanty number of cells were scattered around the injection site, suggesting transplanted cell death.
Fig. 5.
Immunohistochemical staining examination performed 4 weeks after transplantation showing the BMSC (green cells) engrafted around the infarct border zone (black area) in different experimental groups. Original magnification ×20. (A). Analysis of positive cell counts demonstrate that the 2-h group shows the highest number of cells among the experimental groups (p < 0.01) (B).
DISCUSSION
The main findings of this study are; 1) transplanted BMSC migrated to the site of infarction in mouse MCAO model, 2) BLI was useful to confirm the accurate and successful transplantation by demonstrating the intensity of luminescence, 3) BLI and histological findings were well correlated, and 4) the optimal time regarding to transplanted BMSC migration was observed in 2-h group.
The transplanted cells have been known to migrate to the injured parenchymal area5,13,15,18). However, the factors that induce or promote their migration remain largely undefined. Ischemic brain tissue extract selectively induces chemotaxis of mesenchymal progenitors, and the expression of chemotactic factors such as monocyte chemoattractant protein-1 (MCP-1) is increased in the ischemic brain18). MCP-1 increased after the onset of cerebral infarction and induced the migration of BMSC in a microchemotaxis chamber18). Adhesion molecules such as intercellular adhesion molecule, vascular adhesion molecule-1, and E-selectin are also highly expressed on the endothelial cells in the ischemic lesions27). Focal cerebral ischemia elicits an inflammatory response characterized by the infiltration and accumulation of leukocytes, as well the secretion of inflammatory mediators7). The interaction of locally produced stromal cell-derived factor-1α (SDF-1α) by reactive astrocytes in the ischemic penumbra and its cellular receptor CXCR4 expressed on the BMSC membrane plays an important role in the migration of transplanted cells, suggesting that it might be a potential approach to modulate the expression of the two molecules in order to enhance the therapeutic effects using BMSC24,25,28). The interaction of these molecules may promote the targeting of transplanted bone marrow cells to the ischemic lesions.
BLI is useful for tracking the cells and vectors that express the reporter genes. Signal intensities of BLI have been shown to correlate with cell viability in a number of models because the luminescence is produced by enzymatic reaction in living cells8). In this study, the migration of transplanted BMSC expressing luciferase gene could be longitudinally monitored in living animals. As shown in previous reports8,9), histological findings were in concordance with BLI elicited by the migrated BMSC. The cells implanted in control animals without infarction showed no migratory behavior. Instead, cell death occurred at the site of injection, which was confirmed by absence of bioluminescence signal. However, reporter gene expressing cells or vectors are always required to perform BLI studies. In addition, BLI has relatively low spatial resolution compared with modalities such as MRI10,29). Nonetheless, noninvasive monitoring using BLI provides an accurate, practical, and cost-effective method to assess the efficiency of cell transplantation therapy.
Although BMSC administration is a promising strategy to augment recovery from stroke, the optimal time to transplant cells is still unknown. Most studies for MSC transplantation in transient focal ischemia, injection of BMSC are performed at 1 day after stroke. In those studies, improvement of functional outcome obtained by various mechanisms including the production of trophic factors by the BMSC5), reduction of apoptosis, and enhancement of cellular proliferation in the subventricular zone and promotion of bFGF expression in the ischemic boundary area4). Some studies reported 1 week time interval between the transplantation and MCAO4,14). Release of excitotoxic neurotransmitters, free radicals, and proinflammatory mediators in the acute setting could threaten cells introduced into the peri-infarct region16). The longest time window tested for stroke cell therapy in animal models was 1 month after focal ischemia, in which NT2 cells were implanted directly into ischemic striatum3). In a study of injection of male BMSCs at 1 month after stroke into older female rats, significant improvement of neurologic functional recovery obtained in relation with the induction of neurogenesis and reduction of glial scar formation24).
In agreement with our study, previous reports showed that mesenchymal stromal cell therapy resulted in the significant improvement of functional outcome and the decrease of infarction volume when cells were administered 2 h after stroke30). This therapeutic benefit may reflect increased production of growth factors, including neurotrophins adjusted to the needs of the compromised tissue with an array of reducing host cells' apoptosis in the ischemic boundary zone, including neurons, and promoting functional recovery of the remaining neurons6,22). In a report of intravenous administration of autologous bone marrow cells transplantation 3-72 h after MCAO in the rat model, benefits were confirmed by both histological and behavior analyses11). They concluded that the earlier the transplantation was performed after the ischemic insult the greater the beneficial outcome.
In the present study, determination of an optimal time window for parenchymal administration of BMSC was pursued by injecting BMSC at four different time points after MCAO. Transplantation of BMSC at 2 h after MCAO showed a higher number of migration cells to the infarction area comparing to other time points. But, the difference in 1, 3 day group and 7 day group result was probably due to the acute inflammatory response in 1, 3 day group that caused decrease in transplantation of BMSC, where as 7 day group was with subacute inflammatory response. The acute inflammatory response may have diverse influence on cerebral tissue in stoke. Transplanted BMSC would have successful migration and integration in ischemic area after avoiding the harmful acute inflammatory reaction in acute phase. The underlying mechanisms of this observation are to be explored for establishment of the optimal time window for BMSC transplantation. And, specific gene transduction into BMSC can be performed for enhancing therapeutic benefits. These ex vivo BMSC can migrate and express exogenous gene products which interact with locally secreting molecules or various cells in the infarction area. Various cytokines secreted from BMSC or genomodified BMSC activate the proliferation and differentiation of endogenous neural stem and progenitor cells in the subventricular zone. Interestingly, hepatocyte growth factor (HGF) gene transferred to BMSC extended the therapeutic time window from superacute (2 h) to at least 24 h after ischemia occurred. Such combined therapy could improve the neurological recovery after stroke11).
Many of restorative events, such as angiogenesis, neurogenesis, and synaptic plasticity occur naturally after stroke, but they can also be amplified by multipotent stem cells to restore neurological function after a cerebral insult. An understanding of the dynamics of homing and engraftment of infused stem cells is crucial for the progression of stem cell therapeutics. Although 2 hours after infarction suggested as the best therapeutic time window here, another optimal time for BMSC delivery would be indicated depending on administration route of injection and proper combined cytokine genes transduced in stroke model. The mechanisms underlying the migratory characteristics of BMSC in the early phase remain to be further investigated. In addition to the migration, studies on the differentiation into glial or neuronal cells and functional recovery should be evaluated to offer a promise for human clinical treatment in future.
CONCLUSION
Noninvasive imaging provides feasibility and usefulness for tracking transplanted stem cell in real time fashion. The methods in the present study can be widely applied to various research fields of cell transplantation therapy. Combining this BLI method, basic stroke researches using stem cells would enable to perform a clinical application of MSC transplantation in the future.
Acknowledgements
One of the authors (Kyung-Sool Jang) is supported by a project grant from Clinical Research Laboratory and Institute of Catholic Integrative Medicine (ICIM) of Incheon St. Mary's Hospital.
References
- 1.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 3.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 6.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 7.Clark RK, Lee EV, White RF, Jonak ZL, Fenerstein GZ, Barone FC. Reperfusion following focal stroke hastens inflammation and resolution of ischemic injured tissue. Brain Res Bull. 1994;35:387–392. doi: 10.1016/0361-9230(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 8.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, et al. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol. 1997;66:523–531. doi: 10.1111/j.1751-1097.1997.tb03184.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004;35:952–957. doi: 10.1161/01.STR.0000120308.21946.5D. [DOI] [PubMed] [Google Scholar]
- 10.Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–414. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 11.Iihoshi S, Honmou O, Houkin K, Hashi K, Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, et al. Human marrow stromal cell therapy for stroke in rat : neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stoke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Lo EH, Dalkara T, Moskowitz MA. Mechanisms,challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 17.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. discussion 1203-1204. [PubMed] [Google Scholar]
- 19.Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- 20.Ourednik V, Ourednik J, Park KI, Snyder EY. Neural stem cells -- a versatile tool for cell replacement and gene therapy in the central nervous system. Clin Genet. 1999;56:267–278. doi: 10.1034/j.1399-0004.1999.560403.x. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 22.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 23.Rempe DA, Kent TA. Using bone marrow stromal cells for treat-ment of stroke. Neurology. 2002;59:486–487. doi: 10.1212/wnl.59.4.486. [DOI] [PubMed] [Google Scholar]
- 24.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, et al. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 25.Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 26.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, et al. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Zhang L, et al. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003;53:259–263. doi: 10.1002/ana.10467. [DOI] [PubMed] [Google Scholar]
- 30.Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, et al. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]