Abstract
Objective
Patients with spontaneous intracerebral hemorrhage (ICH) presenting within 24 hours of symptom onset are known to be increased risk of hematoma expansion which is closely correlated with morbidity and mortality. We investigated whether tiny enhancing foci ('Spot sign') on axial view of 3-dimensional computed tomography angiography (3D-CTA) source images can predict subsequent hematoma expansion in spontaneous ICH.
Methods
During a 2-year period (March 2007-March 2009), we prospectively evaluated 3D-CTA of 110 patients with spontaneous ICH. Based on source images of 3D-CTA, patients were classified according to presence or absence of 'Spot sign'; 'Spot sign' (+) group, 'Spot sign' (-) group. Radiological factors and clinical outcomes were compared between two groups.
Results
Hematoma expansion occurred in 16 patients (15%). Mean Glasgow Coma Scale (GCS) score of patients with hematoma expansion was significantly different compared to score of patients without hematoma expansion (5 vs. 9, p < 0.001). Nineteen patients (16%) of 110 ICH patients demonstrated 'spot sign' on 3D-CTA. Among the 'spot sign' (+) group, 53% of patients developed hematoma expansion. Conversely 7% of patients without 'spot sign' demonstrated the hematoma expansion (p < 0.001). Initial volume and location of hematoma were significantly not associated with hematoma expansion except shape of hematoma.
Conclusion
Our study showed that patients with hematoma expansion of spontaneous ICH had significant clinical deterioration. And the fact that 'spot sign' (+) group have higher risk of hematoma expansion suggests the presence of 'spot sign' on source images of 3D-CTA can give a clue to predict hematoma expansion in spontaneous ICH.
Keywords: Intracerebral hemorrhage, Computed tomography angiography, Hematoma expansion, Prognosis
INTRODUCTION
Spontaneous intracerebral hemorrhage (ICH) is defined as spontaneous, nontraumatic bleeding into the parenchyma of the brain18,22,32). It is responsible for 10-15% of stroke cases in the US populations and up to 20-30% in Asian group15,23,31,32). And, the outcome of ICH is significantly worse than with that of ischemic stroke, with up to 50% mortality at 30 days2,3,5,6,24). Recent reports confirmed that morbidity and mortality in spontaneous ICH are correlated with hematoma expansion1,2,6,7,13,14,24). In order to treat acute ICH effectively and properly, it is important to identify and predict which patients would develop hematoma expansion.
Hypertension, diabetes mellitus, liver disease, amyloid angiopathy, and coagulopathy have been regarded as risk factors of hematoma expansion2,7,8,13,17,24,26,28,30). Other known clinical risk factors for hematoma expansion include both antecedent warfarin use and ultra-early presentation7). Unfortunately, in most patients with ICH there are no established markers for identifying patients with risk of expansion. Thus, accurate and reliable predictors of hematoma expansion are needed for better outcome.
Computed tomography angiography (CTA) is a rapid, noninvasive investigation for patients with ICH and has been proven useful for identifying potentially treatable entities such as aneurysms and other vascular lesions2,11,16,19,25,31). Various analyses and investigations to validate its usefulness have also been reported recently11,16,19,31). In this study, we investigated the incidence of 'spot sign' at spontaneous ICH and analyze the correlation between a 'spot sign' and hematoma expansion.
MATERIALS AND METHODS
Patients
Between March 1st 2007 and March 31st 2009, we prospectively studied patients with spontaneous ICH visited at our institution within 24 hours of symptom onset. These consecutive patients with spontaneous ICH who underwent a standard CT protocol were enrolled in our database. Patients were excluded if ICH was secondary to head trauma, ischemic stroke with hemorrhagic transformation, tumor, vascular malformation, or cerebral aneurysm. All patients were evaluated with brain computed tomography (CT) scans and 3D-CTAs within 24 hours of symptom onset. Also, all patients were evaluated with at least one more brain CT scan within 48 hours after their 3D-CTA.
According to the study design, 110 patients were included and 69 patients (63%) were male. The median age was 62 years (range, 33 to 88 years). Clinical data were collected by 2 neurosurgeons during admission period.
Clinical parameters
All subjects and their family members were interviewed for clinical data including history of hypertension, diabetes mellitus, coronary artery disease, and medications. Patient's data were classified by the factors of sex, age, smoking or alcohol history, and other factors. Laboratory studies including serum glucose, platelet count, and normalized ratio of the prothrombin time were taken at the time of admission. The first documented systolic blood pressure (SBP) and diastolic blood pressure (DBP) were taken as admission SBP and DBP. Admission Glasgow Coma Scale (GCS) score was measured immediately after arrival at emergency room. Time of symptom onset was defined as the last time the patient was known to be symptom free.
Brain CT protocol
First, non-enhanced brain CT scan, as an initial baseline study, was performed using a helical CT scanner (Somatom Sensation 64; Siemens Medical Solutions USA, Inc. Malvern, Pennsylvania, USA). This was followed by helical scanning during the administration of 100 mL of nonionic contrast agent (Ultravist®, Bayer Healthcare Pharmaceuticals, Inc., Nordrhein-Westfalen, Germany) at 3 to 5 mL/second with a 25 to 40 second prep delay using standard scan parameters of 120 kVp and 200 mAs. Section thickness was 5 mm for non-enhanced scans and 3D-CTA. Also, 48 hours after the initial brain CT scan, follow-up brain CT scan was performed.
Hematoma expansion
Volume of ICH was determined from baseline brain CT scans with ABC/2 method20). Hematoma expansion within 48 hours was defined by an increase in volume of > 30% or > 6 mL from baseline brain CT scan by the criteria of Wada et al.31). Volumes of ICH were compared between the brain CT performed at the time of 3D-CTA and the last brain CT performed up to 48 hours after 3D-CTA. Images were electronically transferred in DICOM (digital imaging and communication in medicine) format to a workstation for analysis (Marosis Maroview software®; Marotech, Inc. Seoul, Korea). Location of hematoma was classified as basal ganglia, thalamus, lobar, cerebellum, pons and multiple types. Shape of hematoma was classified as round, ovoid and irregular. Clinical deterioration was defined by aggravation of consciousness disturbance or neurologic deficits including motor deficits and cranial nerve palsy.
Spot sign
All studies were prospectively evaluated for the presence or the absence of 'spot sign' on 3D-CTA31). 'Spot sign' was defined as 1 or more 1- to 2-mm sized foci of enhancement within hematoma on axial view of 3D-CTA source images (Fig. 1). An ovoid or round shape of foci was also included as 'spot sign'. The location of 'spot sign' was inspected as a center of hematoma or peripheries of hematoma. We excluded the foci which was located outside the hematoma. By the definition of 'spot sign', foci of enhancement within hematoma on axial view of 3D-CTA source images were divided as 'spot sign' (+) group and 'spot sign' (-) group. Clinical and radiological factors and outcomes were compared between 'spot sign' (+) group and 'spot sign' (-) group (Fig. 2).
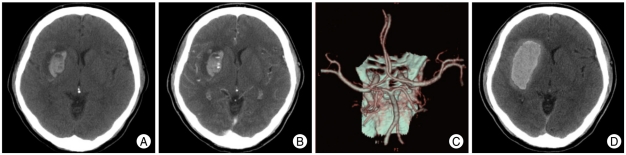
Fig. 1.
Images obtained from a 58-year-old male with a history of hypertension who presented with stupor state and left side weakness. A : Non-contrast brain computed tomography (CT) scan demonstrates spontaneous intracerebral hemorrhage (ICH) with mild surrounding edema in right basal ganglia. The patient's initial Glasgow Coma Scale (GCS) score was 9. B : Axial view in the source image of 3-dimensional computed tomography (3D-CTA) reveals that a small focus of enhancement is seen and consistent with the 'spot sign'. C : 3D-CTA shows no evidence of cerebral aneurysms or vascular malformations. D : Hematoma expansion is demonstrated on follow-up brain CT scans and the patient's GCS score was lowered to 6.
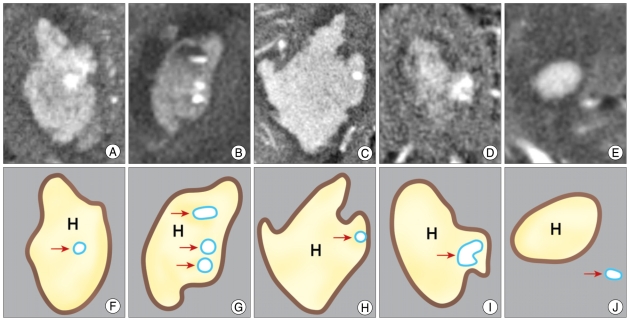
Fig. 2.
Inclusive and exclusive illustrations of 'spot sign' on source images of 3-dimensional computed tomography (3D-CTA). Source images of '3D-CTA' are shown in A, B, C, D, E and corresponding schematic illustrations are shown in F, G, H, I, J. When 'spot sign' is located at the center of hematoma regardless of number (A, B), the cases included in 'spot sign' (+) group. Even though 'spot sign' (arrow) is at the periphery or within the boundary of hematoma (C), it is included in 'spot sign' (+) group. When the shape is round or ovoid (D), it is included in 'spot sign' (+) group. But the enhancing focus (E) is located out of hematoma, the focus was excluded. H : hematoma
Data analysis
A total of 110 patients were grouped by presence of 'spot sign'. The factors and outcomes of clinical, radiological values were analyzed by using the SPSS/PC statistical program (version 12.0 for windows; SPSS, Inc). Results were given as mean ± standard deviation values. Mean of age, initial GCS score, initial volume of hematoma, systolic blood pressure were calculated in each groups. Two-tailed Student's t-tests were used to calculate p values of these parameters. Factors including sex, smoking, alcohol abuse and history of diabetes mellitus and hypertension were recorded according to the groups. Chi-square tests were used to calculate p values of these parameters. Radiological and clinical outcomes between 'spot sign' (+) group and 'spot sign' (-) group were also given as mean ± standard deviation values. Mortality rate was calculated by results of survival of patients of each group in 3 months.
RESULTS
Incidence of hematoma expansion
According to follow-up brain CT scans, hematoma expansion in spontaneous ICH occurred in 16 patients (14.5%).
Clinical outcome according to presence of hematoma expansion
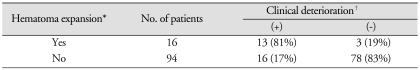
After hematoma expansion, mean GCS scores of patients with hematoma expansion were significantly different compared with the scores with the patients of non-hematoma expansion (GCS score 5 vs. 9, p <0.001). Clinical deterioration was not seen in 3 patients (19%) of hematoma expansion group (Table 1). In these patients, mass effects were not observed from the CT scans after hematoma expansion due to cerebral atrophy. Clinical deterioration occurred in 16 patients (17%) of non-hematoma expansion group. Eleven of them were diagnosed as hydrocephalus at the time of clinical deterioration. Five patients were associated with cerebral edema when clinical deterioration was observed.
Table 1.
Number of patients with clinical deterioration
*Hematoma expansion was defined by an increase in volume of > 33% or > 6 mL from baseline within 48 hours of computed tomography angiography. †Clinical deterioration means an aggravation of consciousness disturbance or neurological deficits during 48 hours from the onset of hemorrhage, which was judged from medical records.
Two group comparison according to presence or absence of 'spot sign'
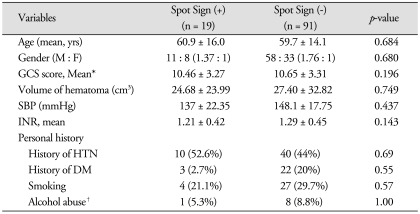
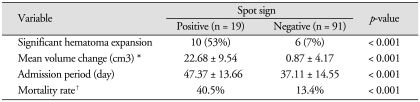
Nineteen (17%) patients of spontaneous ICH demonstrated "spot sign" (Table 2). Initial mean GCS score was not significantly different between 'spot sign' (+) group and 'spot sign' (-) group. The baseline characteristics including mean age, gender, history of hypertension or diabetes mellitus, SBP, INR, and history of anticoagulants, smoking, alcohol abuse of the groups with and without 'spot sign' were not different statistically. Initial volume of hematoma of 'spot sign' (+) group (24.68 ± 23.99 cm3) was lesser than the volume of 'spot sign' (-) group (27.40 ± 32.82 cm3). Initial volume of hematoma was not associated with hematoma expansion.
Table 2.
Clinical factors of patients with or without Spot Sign
*Initial Glasgow Coma Scale score and systolic blood pressure were collected by medical records, †Alcohol abuse was defined by history of medical treatment or objective symptoms (delirium, hallucinations, insomnia, etc.). GCS : Glasgow Coma Scale, SBP : systolic blood pressure, INR: international normalized ratio
Two group comparison according to presence or absence of 'spot sign'
In each group, 53% of patients with 'spot sign' and 7% of patients without 'spot sign' demonstrated the hematoma expansion (p <0.001). Mean admission period was 47.37 ± 13.66 days in 'spot sign' (+) group and 37.11 ± 14.55 days in 'spot sign' (-) group, respectively (p <0.001) (Table 3). The mortality rate during 3 months of admission period was 40.5% and 13.4% in each group, respectively (p <0.001).
Table 3.
Radiological and clinical outcomes in intracerebral hemorrhage patients with and without spot sign
*Mean volume change was calculated by the volume differences on brain computed tomography scan between initial images and follow-up images, †Mortality rate was estimated during 3 months of admission period
Shape and location of ICH
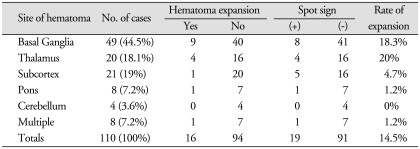
Basal ganglia (44.5%) was the most common site of spontaneous ICH and cerebellum (3.6%) was the least one (Table 4). The location of hematoma did not show significant association with hematoma expansion. Hematomas on basal ganglia on initial brain CT scan were present in 49 patients and the rate of expansion was 18.3%. Rate of expansion was highest at thalamus (20%) and expansion of hematoma at cerebellum was not seen.
Table 4.
The incidence of hematoma expansion among locations of intracerebral hemorrhage
Irregular shape of hematoma was present in 19 patients in spontaneous ICH on initial presentation. Eleven patients (70%) with hematoma expansion and 8 patients (9%) without hematoma expansion showed irregular shape of hematoma on initial brain CT scans, respectively. Irregular shape of hematoma was showed significant association with hematoma expansion (p <0.001).
DISCUSSION
Spontaneous ICH is responsible for 20% to 30% of all strokes, and mortality exceeds 50%. The incidence doubles with each decade of life above 45 years29). It can cause severe, permanent neurologic deficits and complications with just a single attack. In addition, hematoma expansion is the main cause of mortality and morbidity of spontaneous ICH after hospitalization6,14,18). Hematoma expansion can persist for up to 6 hours postictus25). Several authors have reported that the bleeding can continue even later during the treatment course in some patients with spontaneous ICH1,15). Continued bleeding may lead to expansion of an existing hematoma, resulting in progressive neurological deterioration1,4,5,15).
In our study, 16 of 110 patients showed hematoma expansion. Thirteen patients (81%) of them experienced clinical deterioration including disturbance of consciousness and motor deficits. But, in 94 patients without hematoma expansion, clinical deterioration was occurred only in 16 patients (17%). Thus, this study results confirmed, as speculated, that hematoma expansion is a crucial risk factor of clinical outcome in spontaneous ICH.
The risk factors for hematoma expansion in ICH have been investigated by many researchers previously. On several studies, blood pressure had been regarded as the most important risk factor for acute stage hematoma expansion in spontaneous ICH4,13,17,18,24,26). Kazuhiro et al.26) reported that elevated blood pressure increases the risk of hematoma expansion and that an effort to lower systolic blood pressure below 150 mmHg may prevent this risk. Kazui et al.13) reported that although it remained controversial whether antihypertensive drugs should be used in the acute phase of intracerebral hemorrhage, poorly controlled diabetics with high systolic blood pressure (> 200 mmHg) on admission also were at high risk of hematoma enlargement. In our study, mean systolic blood pressure were relatively higher than normal range of systolic blood pressure. But, we could not find any significant differences in systolic blood pressure during admission period between those with hematoma expansion and those without hematoma expansion. Thus, systolic blood pressure was not a definite predictive risk factor of hematoma expansion in our study.
In the hyperacute stage of spontaneous ICH, reported risk factors associated with hematoma expansion include liver dysfunction, excessive alcohol consumption, anticoagulant therapy, thrombocytopenia8,17,24,30). Yukihiko et al.8) reported that liver dysfunction was an important factor influencing hematoma volume and concluded that patients with liver dysfunction and coagulation abnormalities should be closely observed for at least 6 hours after onset in preparation for emergent surgery, since the risk of hematoma expansion in these circumstances is high. Akikazu et al.30) hypothesized that an impaired coagulation system facilitates hematoma expansion in spontaneous ICH. Plasma levels of both fibrinopeptide A and thrombin-antithrombin complex were statistically analyzed between hematoma expansion group and non-expansion group. Their results showed that coagulation system seemed to be highly activated depending on the hemorrhage volume within 3 hours after ictus and the hematoma expansion seemed to be occurred when thrombin generation was not sufficient after bleeding. They reported that plasma levels of the coagulation markers on admission could be useful predictors of the possible hematoma expansion which may lead to a poor outcome. However, Kim et al.18) reported that liver dysfunction and platelet count were not associated with hematoma expansion. Lim et al.24) studied about risk factors such as age, sex, blood pressure, history of diabetes mellitus, liver disease and hypertension. In our study, patient's previous history of liver disease and alcohol abuse were not statistically different between the hematoma expansion group and group without hematoma expansion. Furthermore, the laboratory results such as aspartate aminotransferase (AST)/alanine aminotransferase (ALT) and other liver function markers were not related with international normalized ratio (INR), prothrombin time (PT)/activated Partial thromboplastin time (aPTT) level nor hematoma expansion significantly. Although our study did not reveal the relationship between liver function markers, coagulation markers and hematoma expansion in spontaneous ICH patients, the risk of hematoma expansion in patients of liver disease and coagulopathy is unclear.
Initial GCS score was also concerned as one of the predictive risk factors of hematoma expansion6,10,11,13,19,24). Our findings of initial GCS score between patients with hematoma expansion and without hematoma expansion did not show as an indicator of hematoma expansion. Initial GCS score has known to be associated with patients' clinical outcome regardless of hematoma expansion rather than risk factor of hematoma expansion3,27,32).
It has been hypothesized by many that certain shape of the hematoma, volume of hematoma and location of hematoma on initial CT scans are associated with presence of hematoma expansion. Yukihiko et al.8) studied that ICH patients with irregularly shaped large hematomas should be closely observed because of high risk of hematoma expansion. But, other studies reported that these factors do not influence hematoma expansion statistically. Several studies showed that the volume or location of hematoma did not lead to hematoma expansion frequently5,6,8,12,16,17,32). Also, initial volume and location of hematoma did not correlate with hematoma expansion in our study. The shape of hematoma may be related with small vessel injury or coagulopathies and liver disease12). In our study, the shape of hematoma was considered somehow meaningful as a risk factor of hematoma expansion. In particular, patients with irregular shape of hematomas tended to have hematoma expansion more frequently. It can be explained that irregularly shaped hematomas may be resulted by actively bleeding from multiple arterioles. But, these results are not statistically significant and large-scaled studies are needed.
Recently, many reports concluded that extravasation on cerebral angiography is a useful marker of determining and identifying active bleeding21). Goldstein et al.11) reported that contrast extravasations was independently associated with continued bleeding on spontaneous ICH and this effect was independent of time to presentation. Becker et al.2) showed that contrast extravasations into hematoma was associated with increased fatality and the risk of contrast extravasations was increased with hypertension, depressed consciousness, enlarged hematoma.
When cerebral angiography is not available in emergency circumstances, structural causes of hemorrhage can not be detected on routine CT scanning, including bleeding from a cerebral aneurysm or a vascular malformation. Recently, 3D-CTA is regarded as a highly sensitive modality for determining the source of hemorrhage11,25). As 3D-CTA is being developed rapidly especially in resolution qualities, the source images of 3D-CTA could give us many information about small vessel injuries and anormalies as well as cerebral aneurysm like lesions1,8,16). Thus, we included 3D-CTA as a routine modality in spontaneous ICH.
In the absence of an underlying aneurysm or aneurysm-like lesions on 3D-CTA, the peripheral enhancement on source images of 3D-CTA supports the assertion that these foci represent active hemorrhage from secondarily damaged or torn perforations31). As a result, recent focus of radiologic markers in spontaneous ICH represents the enhancing foci. Recent studies concluded that 'spot sign' is regarded as tiny, enhancing foci within hematomas on 3D-CTA source images31). We defined 'spot sign' as 1 or more 1- to 2-mm sized foci of enhancement within the hematoma on axial section of 3D-CTA source images. These signs are with clear contrast extravasations in CTA. As 3D-CTA is used conventionally in most stroke centers, various data of 'spot sign' on source images of 3D-CTA can be analyzed in many ways. Because 'spot sign' is considered as small vessel injuries, it was hoped that hematoma expansion could be predicted by the presence of 'spot sign'. We therefore assessed the effectiveness of 'spot sign' in estimation of hematoma expansion on the acute stage of spontaneous ICH.
As our results of analysis between 'spot sign' (+) group and 'spot sign' (-) group, 'spot sign' itself seemed to raise the risk of hematoma expansion. And, clinical outcomes of 'spot sign' (+) group were significantly different with that of 'spot sign' (-) group. Mean admission period of 'spot sign' (+) group was 47.37 days, whereas that of 'spot sign' (-) group was 37.11 days. Mortality rate between two groups were also statistically different (40.5%, 13.4%, respectively). As seen in relationship of 'spot sign' and hematoma expansion, 'spot sign' could be a reliable radiologic predictor of clinical deterioration and poor outcomes in spontaneous ICH. Also, using source images of 3D-CTA in analysis, results of our study could be useful in clinical management based on its feasibility and affordability, especially in time course of treatment.
There are some limitations of our study to confirm 'spot sign' as a universal radiologic marker. Even though many radiologists defined 'spot sign' precisely, it is not absolutely objective but rather subjective. The authors tried to define 'spot sign' strictly in many points such as location, shape, number of this sign. Further study is required to define more precisely in objective way. Further improvement in resolution of 3D-CTA will be useful in this regard. Also, pathological characteristics of hematoma between 'spot sign' (+) group and 'spot sign' (-) group should be investigated in further studies. Our study data consisted of only 110 patients with spontaneous ICH, and patients of hematoma expansion and 'spot sign' was also relatively low in numbers.
CONCLUSION
The patients with hematoma expansion of spontaneous ICH showed significant clinical deterioration. Since 'spot sign' (+) group was associated with higher risk of hematoma expansion, its presence on axial sections of 3D-CTA source images at initial presentation can be considered as a one of useful predictors for possible subsequent hematoma expansion.
Acknowledgements
We thank Dr. Jang Se Youn and Dr. Huh Hoon in Seoul Medical Center for their faithful assistance to keep this enormous survey going.
References
- 1.Bae HG, Lee KS, Yun IG, Bae WK, Choi SK, Byun BJ, et al. Rapid expansion of hypertensive intracerebral hemorrhage. Neurosurgery. 1992;31:35–41. doi: 10.1227/00006123-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–2032. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30 day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 4.Broderick JP, Brott TG, Tomsick T, Barsan W, Spilker J. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–199. doi: 10.3171/jns.1990.72.2.0195. [DOI] [PubMed] [Google Scholar]
- 5.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Broderick J, Hennerci M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 7.Fujii Y, Takeuchi S, Saaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–1166. doi: 10.1161/01.str.29.6.1160. [DOI] [PubMed] [Google Scholar]
- 8.Fujii Y, Tanaka R, Takeuchi S, Koike T, Minakawa T, Sasaki O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J Neurosurg. 1994;80:51–57. doi: 10.3171/jns.1994.80.1.0051. [DOI] [PubMed] [Google Scholar]
- 9.Fujitsu K, Muramoto M, Ikeda Y, Inada Y, Kim I, Kuwbara T. Indications for surgical treatment of putaminal hemorrhage. Comparative study based on serial CT and time-course analysis. J Neurosurg. 1990;73:518–525. doi: 10.3171/jns.1990.73.4.0518. [DOI] [PubMed] [Google Scholar]
- 10.Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasations on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 12.Hwnag SN, Ahn YH, Mok JH, Park K, Kim YB, Min BK, et al. An analysis of factors related to the shape of hypertensive intracerebral hematoma and its clinical significance. J Korean Neurosurg Soc. 1995;24:905–911. [Google Scholar]
- 13.Kazui S, Minematsu K, Yamamoto H, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–2375. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 14.Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27:1783–1787. doi: 10.1161/01.str.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 15.Kelley RE, Berger JR, Scheinberg P, Stokes N. Active bleeding in hypertensive intracerebral hemorrhage : computed tomography. Neurology. 1982;32:852–856. doi: 10.1212/wnl.32.8.852. [DOI] [PubMed] [Google Scholar]
- 16.Keum HJ, Whang K, Hu C, Kim HJ, Hong SK, Pyen JS, et al. The usefulness of contrast extravasation on CT angiography in spontaneous intracerebral hemorrhage. Korean J Cerebrovasc Surg. 2007;9:238–242. [Google Scholar]
- 17.Kim DH, Moon JG, Hwang YS, Kim HK, Yoo CS, Lee HD. Clinical analysis of the factors affecting the growth or rebleeding of spontaneous intracerebral hemorrhages. J Korean Neurosurg Soc. 1996;25:2411–2417. [Google Scholar]
- 18.Kim HY, Mok JH, Park HK, Lee KC, Park YS, Lee YB. The factors affecting enlargement of spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc. 2002;32:564–569. [Google Scholar]
- 19.Kim YW, Yoon WK, Kim SR, Kim SD, Park IS, Baik MW, et al. Is extravasation of radiographic contrast a predictor of hematoma enlargement in spontaneous supratentorial intracerebral hemorrhage? Korean J Cerebrovasc Surg. 2007;9:252–258. [Google Scholar]
- 20.Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 21.Kowada M, Yamaguchi K, Matsuoka S, Ito Z. Extravasation of angiographic contrast material in hypertensive intracerebral hemorrhage. J Neurosurg. 1972;36:471–473. doi: 10.3171/jns.1972.36.4.0471. [DOI] [PubMed] [Google Scholar]
- 22.Kwon Y, Kim CJ, Rhim SC, Kwun BD, Whang CJ. Comparative clinical analysis of stereotaxic surgery versus conservative treatment for spontaneous intracerebral hematoma. J Korean Neurosurg Soc. 1990;19:995–1000. [Google Scholar]
- 23.Leira R, Dávalos A, Silva Y, Gil-Peralta A, Tejada J, Garcia M, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associocted factors. Neurology. 2004;63:461–467. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 24.Lim JK, Hwang HS, Cho BM, Lee HK, Ahn SK, Oh SM, et al. Multivariate analysis of risk factors of hematoma expansion in spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:40–45. doi: 10.1016/j.surneu.2007.07.025. discussion 45. [DOI] [PubMed] [Google Scholar]
- 25.Murai Y, Takagi R, Ikeda Y, Yamamoto Y, Teramoto A. Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg. 1999;91:424–431. doi: 10.3171/jns.1999.91.3.0424. [DOI] [PubMed] [Google Scholar]
- 26.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage : relationship beween elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364–1367. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi AI, Safdar K, Weil EJ, Barch C, Bliwise DL, Colohan AR, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764–1767. doi: 10.1161/01.str.26.10.1764. [DOI] [PubMed] [Google Scholar]
- 28.Rosand J, Hylek EM, O'Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy : a genetic and pathologic study. Neurology. 2000;55:947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 29.Sacco RL, Wolf PA, Bharucha NE, Meeks SL, Kannel WB, Charette LJ, et al. Subarachnoid and intracerebral hemorrhage : Natural history, prognosis, and precursive factors in the Framingham study. Neurology. 1984;34:847–854. doi: 10.1212/wnl.34.7.847. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Urano T, Nagai N, Takada Y, Takada A. Progressive expansion of hypertensive intracerebral hemorrhage by coagulopathy. Am J Hematol. 1998;59:110–114. doi: 10.1002/(sici)1096-8652(199810)59:2<110::aid-ajh2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography "spot sign" predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 32.Wartenberg KE, Mayer SA. Reducing the risk of ICH enlargement. J Neurol Sci. 2007;261:99–107. doi: 10.1016/j.jns.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 33.Wolpert SM, Schatzki SC. Extravasation of contrast material in the intracerebral basal Ganglia. Radiology. 1972;102:83–85. doi: 10.1148/102.1.83. [DOI] [PubMed] [Google Scholar]