Abstract
Objective
This prospective study evaluated the use of continuous sedation using propofol and remifentanil when carpal tunnel release (CTR) was performed under local anesthesia.
Methods
We sedated 60 patients undergoing CTR using local anesthesia with remifentanil at loading and continuous doses of 0.5 µg kg-1 and 0.05 µg kg-1min-1, respectively, and propofol, using a target controlled infusion (TCI) pump set to a target of 2 µg mL-1 (group A), or with the same drug doses except that the continuous remifentanil dose was 0.07 µg kg-1min-1 (group B) or 0.1 µg kg-1min-1 (group C).
Results
In group B, the levels of pain when local anesthetics were administered (p = 0.001), intraoperative pain (p < 0.001) and anxiety (p = 0.001) were significantly lower than those of group A. Furthermore, the incidence of adverse events, including desaturation (p < 0.001) and vomiting (p = 0.043), was significantly lower in group B than in group C.
Conclusion
Continuous sedation using an appropriate dose of remifentanil and propofol can be used as safe, efficacious ambulatory anesthesia in cases of CTR under local anesthesia, performed using only 2 mL of local anesthetic, with a high degree of patient satisfaction.
Keywords: Carpal tunnel syndrome, Propofol, Remifentanil
INTRODUCTION
Carpal tunnel release (CTR) can be performed under general, regional, intravenous regional, or local anesthesia. Among these techniques, local anesthesia is commonly used for median nerve release11,15). Local anesthesia has many advantages, such as reduced cost and a shortened hospital day, and less complications from general or regional anesthesia, making it suitable for outpatient surgery. However, anxiety, pain at the injection site, and intraoperative pain, including that caused by tourniquet use, can result in patient discomfort and dissatisfaction. Anatomical distortion due to infiltration at the site of incision is also a major limitation of this technique18). Consequently, we examined modifications of the anesthetic technique in an attempt to make the procedure more comfortable for the patient and surgeon, and planned sedation that involved the use of only 2 mL of lidocaine subcutaneous infiltration, and outpatient surgery.
We sedated patients who underwent CTR under local anesthesia before infiltrating local anesthetic and during surgery with a continuous remifentanil and propofol infusion. We report the application, safety, complications, and patient satisfaction with continuous sedation as outpatient anesthesia for CTR and the optimal dose of remifentanil in combination with propofol.
MATERIALS AND METHODS
Following approval from our institutional ethics committee and after obtaining written informed consent, 60 adult patients (ASA I or II) undergoing CTR under local anesthesia between May 2009 and March 2010 were included in this prospective randomized study. Exclusion criteria were age < 20 or > 65 years, weight > 100 kg, oral opening class IV Mallampati classification, history of chronic sedative use, history of alcohol or drug abuse, and known or suspected psychiatric disturbance. No sedative premedication was used. Patients were allocated randomly to one of three groups, randomized to receive a remifentanil injection at loading and continuous doses of 0.5 µg kg-1 and 0.05 µg kg-1/min-1, respectively, with propofol, using a target controlled infusion (TCI) pump set to a target of 2 µg mL-1 (group A), or injection of remifentanil at a loading and continuous dose of 0.5 µg kg-1 and 0.07 µg kg-1 min-1 with the same propofol dose (group B), or injection of remifentanil at loading and continuous doses of 0.5 µg kg-1 and 0.1 µg kg-1min -1 with the same dose of propofol (group C) for sedation.
Sedation
After arrival in the operating room, noninvasive arterial pressure (NIBP), heart rate (HR), pulse oximetry (SpO2), and electrocardiography (ECG) were monitored. All patients were given O2 at a rate of 5 L min-1 using a face mask. After an initial monitoring of group A, patients received a remifentanil injection at a loading dose of 0.5 µg kg-1 and a continuous dose of 0.05 µg kg-1 min-1. The sedation level was assessed using the Observer's Assessment of Alertness/Sedation (OAA/S) scale, modified by reversing the order of the scores (with a composite score of 1 = awake and alert to 5 = unresponsive)3). Five minutes after the remifentanil injection, propofol was infused using a TCI pump set to a target of 2 µg mL-1. After completing administration of the loading and continuous doses of the study drugs, an operator blinded to the experimental groups draped the operation site and injected local anesthetic along the site of the incision using 2 mL, and then performed the CTR. The operation was performed using a tourniquet. In groups B and C, we used the same method of sedation as in group A, except that the continuous doses of remifentanil were 0.07 and 0.1 µg kg-1 min-1, respectively.
Operative procedure
We resected the transverse carpal ligament using a mini-open skin incision under tourniquet control. An approximately 2-cm skin incision was made and the transverse carpal ligament was identified after dissecting the subcutaneous soft tissue. Then, the skin was retracted proximally and distally, and the overlying hypertrophied transverse carpal ligament was resected. After resecting the carpal ligament, we confirmed complete resection of the ligament and decompression of the median nerve.
Outcome measures of sedation
The sedation level was assessed by different investigators during the operation. An investigator graded the patient sedation status using an OAA/S scale score. At 2 h after surgery, the patients were questioned by anesthesiologists in the recovery room. Pain when local anesthetic was applied and during the operation, including tourniquet pain, was assessed using a visual analog scale (VAS) : 0 = no pain to 10 = severe pain. Anxiety was assessed using a VAS : 0 = no anxiety to 10 = most anxiety. Sedation satisfaction was also assessed using a VAS : 0 = extremely dissatisfied to 10 = extremely satisfied. Adverse events, including postoperative headache, nausea, and vomiting, were checked.
Statistical analyses
We used a nonparametric test for the statistical analysis because of the small sample size. Statistical analyses were performed using SPSS software (ver. 12; SPSS, Chicago, IL, USA), and p values < 0.05 were deemed to indicate statistical significance. Ratio data were analyzed using the Kruskal-Wallis test, and nominal data were analyzed using the Pearson chi-squared test. Mean VAS scores for pain at the local anesthetic injection site, pain during the operation, anxiety, headache, satisfaction, and OAA/S scale scores were compared between groups using the Kruskal-Wallis test. The incidence of anxiety, headache, nausea, vomiting, and hypoxemia, was compared between groups using the Pearson chi-squared test.
RESULTS
Patient demographics
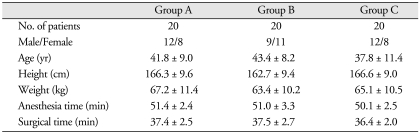
The three groups were similar with respect to age, gender, height, weight, and duration of anesthesia and surgery (Table 1).
Table 1.
Patient demographics and clinical profiles
Data are the means ± SDs. No significant differences were observed among the three groups
Pain on administration of local anesthetics and during the operation
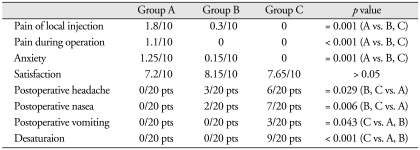
In group A, nine patients had injection pain and their mean VAS score was 3.89/10, whereas seven patients had operation pain and their mean VAS score was 3.14/10. As a result, the overall pain score for group A was 1.8/10 at the injection site and 1.1/10 during the operation. By contrast, in group B, three patients felt pain only at the injection site, and their mean pain score was 2/10, whereas the overall group B score was 0.3/10. None of group-C patients experienced pain. The mean injection pain scores for group A were significantly higher than those of groups B and C (p = 0.001). Additionally, the mean operation pain scores for group A were significantly higher than those of groups B and C (p < 0.001) (Table 2).
Table 2.
Results from three different groups
Data are shown as means ± SD. A means Group A, B means Group B, C means Group C, vs. means versus, pts means patients
Anxiety during the operation
In group A, nine patients felt anxiety, and their mean VAS anxiety score was 2.78/10. As a result, the overall anxiety score for group A was 1.25/10. By contrast, in group B, only one patient felt anxious, and her anxiety score was 3/10, whereas the overall group B score was 0.15/10. Furthermore, none of group-C patients felt anxiety. The incidence of anxiety in group A was significantly higher than that of groups B and C (p = 0.001), and the mean anxiety score for group A was significantly higher than that of groups B and C (p = 0.001) (Table 2).
Depth of sedation
The level of sedation, as graded on the OAA/S scale, remained between 2 and 4 in the three groups, except for three patients in group C who sometimes had an OAA/S scale score of 5. A statistically significant difference was noted between group A and groups B and C. The group-A OAA/S scale score was significantly lower than that of groups B and C (p = 0.030).
Respiratory Evaluation
In group C, desaturation (< 90%) developed in nine patients (45%), and the SpO2 decreased significantly from 15 min after propofol injection. All patients in groups A and B maintained SpO2 levels above 97%. The incidence of hypoxemia in group C was significantly higher than that in groups A and B (p < 0.001) (Table 2).
Satisfaction with sedation
The average VAS satisfaction scores for groups A, B, and C were 7.2/10, 8.15/10, and 7.65/10, respectively. Patients in group B tended to have a higher satisfaction score than those in groups A and C, but the difference was not statistically significant (Table 2).
Adverse events
The incidence of adverse events was the highest in group C in which seven (35%) patients complained of nausea. Six (30%) of the seven patients had headache, and three (15%) patients of the seven vomited. In group B, three (15%) patients had a headache and two (10%) of the three patients complained of nausea. None of patients in group A experienced any adverse events. The incidence (p = 0.029) and mean score (p = 0.024) for headache in groups B and C were significantly higher than those of group A. The incidence (p = 0.006) and mean score (p = 0.005) for nausea in groups B and C were significantly higher than those of group A. The incidence of vomiting (p = 0.043) in group C was significantly higher than that in groups A and B (Table 2).
DISCUSSION
Anesthetic techniques used for CTR include general anesthesia, local anesthetic infiltration, intravenous regional anesthesia, and peripheral nerve block, either proximally at the brachial plexus or more distally at the peripheral nerves. The choice of anesthetic technique for CTR should ensure a bloodless operative field, without anatomical distortion, to enable clear identification of the transverse carpal ligament and median nerve, avoiding injury to the palmar cutaneous branch of the median nerve18).
Local anesthesia for CTR is the simplest, most cost-effective technique, providing rapid onset and prolonged anesthesia with no motor block19). Altissimi and Mancini1) described a technique of infiltrating 4-5 mL of 2% mepivacaine into the carpal tunnel in addition to infiltrating 3-4 mL of the same anesthetic into the subcutaneous tissue. They reported complete analgesia in most patients. However, they stressed the risk of median nerve injury. Furthermore, large volumes of local anesthetic can cause anatomical distortion. In this study, our sedation allowed local anesthetic infiltration along the site of the incision using only 2 mL, which resulted in no anatomical change, subcutaneous swelling, or median nerve injury.
To avoid median nerve injury, Gale5) suggested that local anesthetic be injected only into the subcutaneous tissues. Gibson6) reported slight discomfort on incising the flexor retinaculum in four of 98 patients and Baguneid et al.2) reported that 13% of patients had slight discomfort during the operation using similar techniques. Patil et al.14) compared the administration of local anesthetic into the carpal tunnel combined with subcutaneous infiltration (the modified Altissimi and Mancini technique) with only subcutaneous infiltration (the modified Gale technique). The mean pain scores on administration of the local anesthetic were 3.2 and 3.3, respectively, using a numerical rating scale (NRS) with the modified Gale and modified Altissimi and Mancini techniques. Of the 20 patients, six experienced intraoperative pain (NRS scores 8, 7, 5, 4, 2, and 2) with the modified Gale technique, whereas no patient experienced pain with the modified Altissimi and Mancini technique. Vossinakis20) identified three distinct causes of the discomfort associated with the administration of the local anesthetic before surgery.
In our study, pain on administration of the local anesthetic was significantly low, although we infiltrated only 2 mL subcutaneously. The modified Gale technique and the original Gale technique injected 6 mL and 5-8 mL of 2% plain lidocaine, respectively. Furthermore, during the operation, groups B and C had no pain, including tourniquet pain. Tourniquet pain is another important point of anesthetic consideration. Several authors believe that some patients do not tolerate the conventional upper arm tourniquet when awake8,13). In our study, the patients who were sedated before local anesthetic infiltration tolerated both the tourniquet pain and infiltration pain well. Kwon et al.10) reported that endoscopic CTR under local anesthesia with upper arm tourniquet had excellent results compared with conventional surgery. We think that our sedation can be also used in this endoscopic CTR, and the patients may be more comfortable with the infiltration pain and tourniquet pain.
Sedation with sedative and analgesic agents has become popular for painful procedures and surgery under local anesthesia. Our sedation technique with propofol and remifentanil produced less pain and anxiety during the operation, while maintaining the advantages of local anesthesia and allowing outpatient surgery. The sedative propofol has a pharmacokinetic profile that is well-suited for continuous infusion because it has a rapid onset of action, short duration of effect, and minimal postanesthetic side effects16,21). Furthermore, the high clearance and favorable recovery profile of propofol offer advantages over other intravenous sedatives for ambulatory surgery17,22). Remifentanil is a short-acting, selective µ-opioid agonist with a rapid onset of effect and a rapid offset that is independent of the duration of infusion. The termination of its therapeutic effect is primarily dependent on metabolism by circulating plasma esterases, rather than redistribution4,7). Consequently, remifentanil has a pharmacokinetic profile well-suited for rapid recovery, even for more prolonged outpatient procedures requiring moderate-to-high levels of intraoperative analgesia. However, some studies have reported that patients receiving a continuous remifentanil infusion experience nausea, vomiting, headache, or pruritus.9,12).
In our study, all of the groups showed overall satisfaction with the sedation. Although there was not a statistically significant difference, the satisfaction scores of the three groups showed some tendencies that were due to the different continuous remifentanil doses. In group A, nine patients felt injection pain and seven felt operative pain. In group B, three patients felt pain only at the injection site, and their mean pain score was 2/10, whereas the overall group B score was 0.3/10. In group C, which received the highest continuous remifentanil dose, no patient felt pain. In terms of complications, however, the group-C patients had many adverse events; the most common was nausea (35%), followed by headache (30%) and vomiting (15%). Three patients in group B developed a headache and no group-A patient experienced such complications. Considering all of our results, we conclude that the appropriate continuous dose of remifentanil was 0.07 µg kg-1 min-1 with a loading dose of 0.5 µg kg-1 in combination with propofol given with a TCI pump set to a target of 2 µg mL-1.
CONCLUSION
Continuous sedation using an appropriate dose of remifentanil and propofol can be used to provide safe, efficacious ambulatory anesthesia for CTR under local anesthesia, with a high degree of patient satisfaction. Furthermore, with this sedation, local anesthesia can be performed using subcutaneous infiltration of only 2 mL, which do not cause anatomical distortion or median nerve injury.
References
- 1.Altissimi M, Mancinic GB. Surgical release of the median nerve under local anesthesia for carpal tunnel syndrome. J Hand Surg Br. 1988;13:395–396. doi: 10.1016/0266-7681_88_90163-5. [DOI] [PubMed] [Google Scholar]
- 2.Baguneid MS, Sochart DH, Dunlop D, Kenny NW. Carpal tunnel decompression under local anaesthetic and tourniquet control. J Hand Surg Br. 1997;22:322–324. doi: 10.1016/s0266-7681(97)80394-4. [DOI] [PubMed] [Google Scholar]
- 3.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale : study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. [PubMed] [Google Scholar]
- 4.Egan TD, Lemmens HJ, Fiset P, Hermann DJ, Muir KT, Stanski DR, et al. The pharmacokinetics of the new short-acting opioid remifentanil (G187084B) in healthy adult male volunteers. Anesthesiology. 1993;79:881–892. doi: 10.1097/00000542-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gale DW. Surgical decompression of the carpal tunnel using infiltrative anaesthesia : description of technique. J R Coll Surg Edinb. 1991;36:341. [PubMed] [Google Scholar]
- 6.Gibson M. Outpatient carpal tunnel decompression without tourniquet : a simple local anaesthetic technique. Ann R Coll Surg Engl. 1990;72:408–409. [PMC free article] [PubMed] [Google Scholar]
- 7.Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid : remifentanil (G187084B) Anesth Analg. 1993;77:1031–1040. doi: 10.1213/00000539-199311000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Glynn A, Strunk S, Reidy D, Hynes DE. Carpal tunnel release using local anaesthetic and a forearm tourniquet. Ir Med J. 2005;98:144–145. [PubMed] [Google Scholar]
- 9.Gold MI, Watkins WD, Sung YF, Yarmush J, Chung F, Uy NT, et al. Remifentanil versus remifentanil/midazolam for ambulatory surgery during monitored anesthesia care. Anesthesiology. 1997;87:51–57. doi: 10.1097/00000542-199707000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Kwon YJ, Kim TS, Lim YJ, Rhee BA, Leem W, Kim GK. The Clinical analysis of patients with carpal tunnel syndrome underwent surgery : comparison between conventional and endoscopic surgery. J Korean Neurosurg Soc. 2000;29:372–378. [Google Scholar]
- 11.Litchman HM, Silver CM, Simon SO, Motamed M, Deutch SO. Carpal tunnel realease, efficacy as an ambulatory outpatient procedure under local anaesthesia. Orthop Rev. 1984;13:167–171. [Google Scholar]
- 12.Mingus ML, Monk TG, Gold MI, Jenkins W, Roland C Remifentail 3010 Study Group. Remifentanil versus propofol as adjuncts to regional anesthesia. J Clin Anesth. 1998;10:46–53. doi: 10.1016/s0952-8180(97)00220-1. [DOI] [PubMed] [Google Scholar]
- 13.Ng ES, Ting JR, Foo SL, Akram SA, Fadzlina AA, Alywiah JS, et al. The comparison of discomfort level between upper arm and forearm tourniquet. Med J Malaysia . 2006;61(Suppl B):23–26. [PubMed] [Google Scholar]
- 14.Patil S, Ramakrishnan M, Stothard J. Local anaesthesia for carpal tunnel decompression : a comparison of two techniques. J Hand Surg Br. 2006;31:683–686. doi: 10.1016/j.jhsb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Phalen GS. The carpal-tunnel syndrome. Seventeen years' experience in diagnosis and treatment of six hundred fifty-four hands. J Bone Joint Surg Am. 1966;48:211–228. [PubMed] [Google Scholar]
- 16.Sebel PS, Lowdon JD. Propofol : a new intravenous anesthetic. Anesthesiology. 1989;71:260–277. [PubMed] [Google Scholar]
- 17.Shafer SL. Advances in popofol pharmacokinetics and pharmacodynamics. J Clin Anesth. 1993;5:14S–21S. doi: 10.1016/0952-8180(93)90003-w. [DOI] [PubMed] [Google Scholar]
- 18.Sinha A, Chan V, Anastakis DJ. Anesthesia for carpal tunnel release. Can J Anesth. 2003;50:323–327. doi: 10.1007/BF03021026. [DOI] [PubMed] [Google Scholar]
- 19.Tomaino MM, Ulizio D, Vogt MT. Carpal tunnel release under intravenous regional or local infiltration anaesthesia. J Hand Surg Br. 2001;26:67–68. doi: 10.1054/jhsb.2000.0426. [DOI] [PubMed] [Google Scholar]
- 20.Vossinakis IC. Re : reduction in pain associated with open carpal tunnel decompression. J Hand Surg Br. 2001;26:503–504. doi: 10.1054/jhsb.2001.0640. [DOI] [PubMed] [Google Scholar]
- 21.White PF. Clinical pharmacology of intravenous induction drugs. Int Anesthesiol Clin. 1988;26:98–104. doi: 10.1097/00004311-198802620-00003. [DOI] [PubMed] [Google Scholar]
- 22.White PF. Propofol : pharmacokinetics and pharmacodynamics. Semin Anesth. 1988;1:4–20. [Google Scholar]