Abstract
We herein report a case of a 33-year-old man in remission from Hodgkin lymphoma, who presented with reduced potency and hair loss. Initial endocrine tests revealed autoimmune hypothyroidism. An MRI of his pituitary gland at onset revealed hyperplasia. He tolerated replacement endocrine therapy with good response, but with no improvement in his alopecia universalis. A repeat MRI, 6 months after his initial endocrine manipulation, showed resolution of his pituitary hyperplasia.
Background
Introduction
Polyglandular autoimmune syndrome (PAS) comprises a wide spectrum of autoimmune conditions of the endocrine glands that results in reduced hormone production. Glandular abnormalities of the endocrine system are prone to occurrence in clusters, with up to 25% of patients with evidence of glandular hypofunction having evidence of other endocrinopathies. The concept of this syndrome is not new, and was first described in 1853 by Thomas Addison, when he elucidated details on patients with adrenocortical insufficiency that also appeared to have pernicious anaemia; but the classification and clarification was established in 1980 by Neufeld and Blizzard.1
We herein report the case of a 33-year-old Caucasian man of Irish extraction who presented with features and investigations that fulfil the criteria for PAS.
Case presentation
Case history
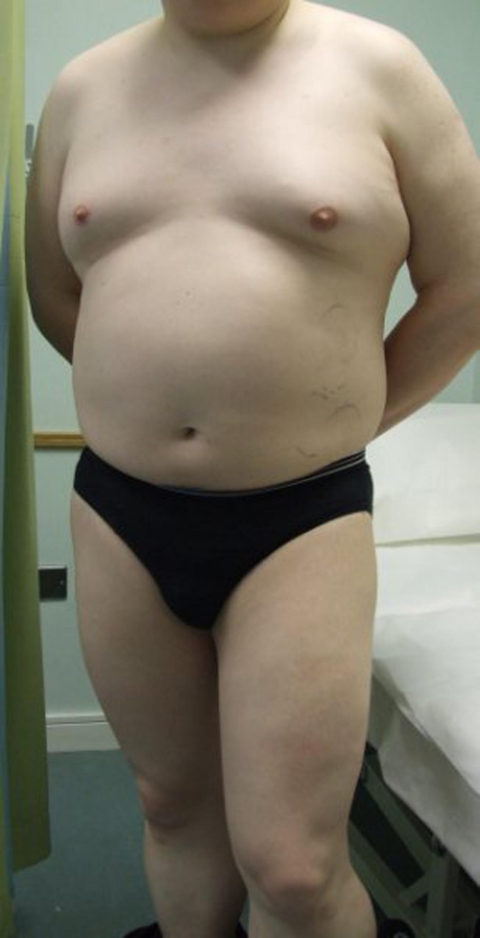
The patient, a 33-year-old Caucasian computer engineer with a history of Hodgkin lymphoma – stage IA nodular sclerosing subtype, and had undergone six cycles of chemotherapy with ABVD (adriamycin, bleomycin, vincristine and dacarbazine) and was in the 3rd year of his follow-up after a complete response to his primary therapy. January 2009, RMcG was reviewed routinely and revealed that he had been feeling mildly fatigued with reduced libido and significant hair-loss from all over his body (figure 1). Clinical examination was normal with no evidence to suggest recurrence of his malignancy, although note was made of his alopecia universalis. Examination of his external genitalia was normal in appearance. No enlargement of his thyroid was noted. Full neurological examination was within normal limits. His initial and subsequent blood tests were reviewed and clinical imaging is below.
Figure 1.
Clinical images of patient at presentation.
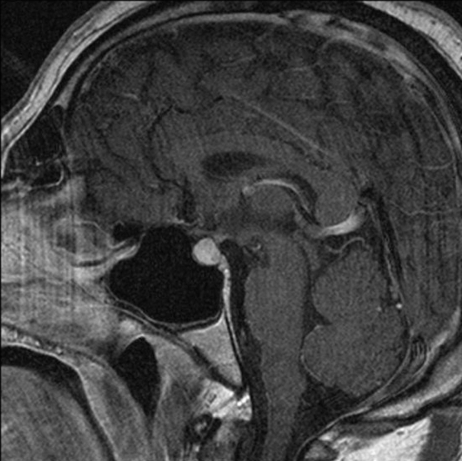
He also underwent MRI of his brain following his preliminary results, and this revealed a symmetrically enlarged pituitary which measured 14 mm × 10 mm, with suprasellar extension close to the optic chiasm, which had uniform enhancement postgandolinium. There was no evidence of extension laterally, and no other intracranial abnormalities (see figure 2A).
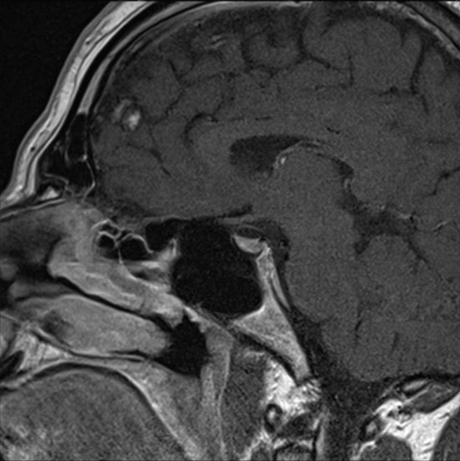
Figure 2.
Sagital T1 postgandolinium MRIs performed (A) January 2009 and (B) June 2009.
Investigations
Treatment
The patient was started on replacement therapy initially with thyroxine and then testosterone. He was reviewed regularly and underwent repeated endocrine testing, until his thyroid and gonadal function returned to normal limits (see table 1). His clinical symptomatology has abated, although his hair loss remains. He underwent repeat MRI, 6 months later. This showed reduction in the size of his pituitary gland with return to its normal conture and shape, with no other intracerebral abnormality was identified or other focus of abnormal enhancement (see figure 2B).
Table 1.
Summary of initial blood tests performed
| Blood tests (fasting morning samples) | Results Prehormonal treatment | Normal range | Results Posthormonal treatment |
|---|---|---|---|
| FBC | All normal | All normal | |
| U+Es | All normal | All normal | |
| Random plasma glucose | 5.0 mmol/l | 4.1–6.6 mmol/l | Not repeated |
| TSH | 85.10 mU/l | 0.15–3.2 mU/l | 0.95 mU/l |
| Repeat | 81.0 mU/l | ||
| Free thyroxine (free T4) | 12.1 pmol/l | 10.3–24.5 pmol/l | 23.7 pmol/l |
| Repeat | 11.3 pmol/l | ||
| Total thyroxine (total T4) | 56 nmol/l | 58–161 nmol/l | Not repeated |
| Repeat | 58 nmol/l | ||
| Prolactin | 211 mU/l | 30–500 mU/l | 204 mU/l |
| Testosterone | 9.3 nmol/l | 9.9–52.4 nmol/l | 14.9 nmol/l |
| SHBG | Not performed | 13–17 nmol/l | 10.8 nmol/l |
| LH | 1.9 U/l | 0.5–10.0 U/l | 1.4 U/l |
| FSH | 4.9 U/l | 0.8–9.0 U/l | 3.1 U/l |
| TPO | 288 IU/l | Not repeated | |
| TG | 652 IU/l | Not repeated | |
| Antinuclear antibody | Negative | Not repeated | |
| Short synthacen test | Not repeated | ||
| Serum cortisol presynacthen level | 287 nmol/l | ||
| Serum cortisol 30-min postsynacthen level | 858 nmol/l |
FBC, full blood count; FSH, follicle stimulating hormone; LH, luteinising hormone; SHBG, sex hormone binding globulin; TG, antithyroglobulin; TPO, antithyroid peroxidise; TSH, thyroid stimulating hormone; U+Es, urea and electrolytes.
Discussion
PAS form a diverse cluster of autoimmune conditions and are uncommon endocrinopathies that are typified by the occurrence of at least two glandular autoimmune mediated diseases.2 Two major subtypes of PAS, types I and II are described, and can be distinguished based on their age of presentation, characteristic patterns of disease combinations and different modes of inheritance.3 The co-existence of adrenal failure with either autoimmune thyroid disease and/or type I diabetes mellitus is defined as Schmidt's or Carpenter's syndrome. A third subtype has been described in adults which contrary to types I and II, does not involve the adrenal cortex. PAS type III has been further classified into the following categories:
-
▶
PAS IIIa – autoimmune thyroiditis with immune-mediated diabetes mellitus
-
▶
PAS IIIb – autoimmune thyroiditis with pernicious anaemia
-
▶
PAS IIIc – autoimmune thyroiditis with vitiligo and/or alopecia and/or other organ-specific autoimmune disease
Three major factors have been implicated in the aetiology of PAS III; these include autoimmunity, environmental factors and genetic factors. Autoimmune disease affecting a solitary endocrine gland may be followed by the impairment of other glands, resulting in reduced function of multiple endocrine glands. In 1956, Roitt et al noted the development of circulating precipitating auto-antibodies to thyroglobulin in patients with Hashimoto's thyroiditis.4 The identification of circulating organ-specific auto-antibodies served as the earliest and strongest support for the auto-immune pathogenesis of polyglandular failure syndromes. Histological examination of the affected endocrine glands has demonstrated similar results, that is, mononuclear infiltrate compromised mainly of lymphocytes, macrophages, natural killer cell and plasma cells. The most significant feature is the sparing of the adjacent non-target tissues.
It is believed, that environmental influences on autoimmunity might play a role in the development of polyglandular autoimmunity. Viral infection is thought to potentially augment the ongoing immune response and precipitate multiple gland failure, although no human epidemiological studies have been able to demonstrate a triggering pathway.5 PAS III, as well as PAS II, has been associated with human leukocyte antigen (HLA) class II gene with apparently distinctive HLA alleles for each. The underlying non-HLA genes of PAS III remain to be further defined genetically. PAS III is often observed in individuals in the same family, suggesting that its inheritiance may be autosomal dominant with incomplete penetrance.5
The international prevalence of PAS III is unknown, and the natural history of this syndrome is determined by the individual components of the syndrome. It typically affects middle-aged females, but can affect persons of any age and of either gender.
The MRI findings seen for this patient were unexpected, and after an exhaustive review within the literature, we failed to find any cases presenting with the exact triad of symptoms or signs (alopecia universalis, autoimmune thyroiditis and pituitary hyperplasia), we widened our search, looking at cases with abnormal pituitary imaging associated with endocrinopathies. Differential diagnoses with such MRI findings, were considered and the most common were, pituitary adenomas, lymphocytic hypophysitis (LYH) and pituitary hyperplasia.6 Given the clinical and radiological details of this case, the pituitary enlargement appears unlikely to be related to a pituitary adenoma or LYH, given the pituitary stalk appears uninvolved within this case, although there is uniform enlargement and enhancement of the pituitary in keeping with what is published in the literature.7 These images were reviewed in the multidisciplinary setting, and it was consensus opinion that the pituitary enlargement could not be attributed to LYH, especially in the absence of tissue biopsy.
LYH is an uncommon autoimmune condition that affects the pituitary gland.8 It is characterised by infiltration of the pituitary gland by lymphocytes, plasma cells and macrophages and as such its function may be impaired; the symptoms appreciated depend on the part of the gland that is affected.
Also review of the literature, suggests that patients with LYH are predominantly female with ratios varying from 5:1 to 8:1.9 10 The mean age at presentation was 34.5 years for females and 44.7 years for males.7 LYH is normally experienced within pregnancy, usually the 1st trimester.9 These features differed in this case, as our patient is male and was 33 years at the time of presentation. However, LYH has been associated with a series of endocrine autoimmune diseases, including Hashimoto's thyroiditis, diabetes mellitus, parathyroiditis, Grave's disease, Addison's disease and PAS types I and IIIa.7 Our patient has PAS IIIc, which does not appear to have been associated with LYH.
Pituitary hyperplasia (enlargement) in patients with long-standing hypothyroidism is well documented within the literature.11 12 CT and MRI features of these conditions and subsequent regression with the introduction of thyroid hormone therapy have been previously described.13 14 The postulated pathway for the development of the MRI findings is related to the thyroid gland secreting low levels of thyroid hormones, thus resulting in increased serum levels of thyrotropin-releasing hormone and can lead to eventual hyperplasia of thyrotropin-producing cells and in subsequent enlargement of the pituitary gland.15 All of the patients involved in these published reports mentioned above had undergone either thyroidectomies or thyroid ablation with radio-active iodine, our case was different in the fact that he had not undergone either of these procedures, but he did have evidence of an autoimmune thyroiditis, which had a therapeutic regression with the introduction of thyroid hormone replacement therapy, which he remains maintained on.
Learning points.
-
▶
The patient has clinical, biochemical and radiological features of PAS IIIc syndrome.
-
▶
He has responded favourably to replacement therapy and is undergoing regular surveillance for further target gland dysfunction.
-
▶
There is not much information is available on the occurrence of such features noted within this patient, or association with Hodgkin lymphoma, hence our reporting this case, especially given the radiological findings seen.
-
▶
This case report hopefully will augment the information in the established literature.
-
▶
As far as we are aware, this combination of clinical and radiological features have not been previously described within the literature, and it is therefore hoped that others may be more aware of this triad, that is, alopecia universalis, autoimmune thyroiditis (PAS IIIc) and pituitary hyperplasia.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Neufeld M, Blizzard Rm. Polyglandular autoimmune disease. In: Pinchera A, Doniach D, Fenzi DF, Barchieri L, eds. Autoimmune aspects of endocrine disorders. UK: Academic Press, 1980:357–65 [Google Scholar]
- 2.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. NEJM 2004:350;2068–79 [DOI] [PubMed] [Google Scholar]
- 3.Jenkins RC, Weetman V. Disease associated with autoimmune thyroid disease. Thyroid 2002:12;977–88 [DOI] [PubMed] [Google Scholar]
- 4.Doniach D, Hudson RV, Roitt IM. Human auto-immune thyroiditis: clinical studies. Br Med J 1960;1:365–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aung K, Salmon M. Polyglandular autoimmune syndrome, type III. http://www.emedicine.medscape.com/article/124398 (Accessed August, 2009)
- 6.Thodou E, Asa SL, Kontogeorgos G, et al. Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab 1995:80;2302–11 [DOI] [PubMed] [Google Scholar]
- 7.Bellastella A, Bizzarro A, Coronella C, et al. Lympocytic hypophysitis: a rare or underestimated disease? Eur J Endocrinol 2003:149;363–76 [DOI] [PubMed] [Google Scholar]
- 8.Sato N, Sze G, Endo K. Hypophysitis: endocrinologic and dynamic MR findings. Am J Neuroradiol 1998:19;439–44 [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K, Takao T, Makino S. Lymphocytic adenohypophysitis and lymphocytic infundibuloneurohypophysitis. Endocr J 1997:44;1–10 [DOI] [PubMed] [Google Scholar]
- 10.Duran MM, Santonja C, Pavon, de Paz I, et al. Lymphocytic hypophysitis: report of an unusual case of a rare disorder. J Endocrinol Invest 2001:24;190–3 [DOI] [PubMed] [Google Scholar]
- 11.Bigos ST, Ridgway EC, Kourides IA, et al. Spectrum of pituitary alterations with mild and severe thyroid impairment. J Clin Endocrinol Metab 1978;46:317–25 [DOI] [PubMed] [Google Scholar]
- 12.Scheithauer BW, Kovacs K, Randall RV, et al. Pituitary gland in hypothyroidism. Histologic and immunocytologic study. Arch Pathol Lab Med 1985;109:499–504 [PubMed] [Google Scholar]
- 13.Pita JC, Jr, Shafey S, Pina R. Diminution of large pituitary tumor after replacement therapy for primary hypothyroidism. Neurology 1979;29:1169–72 [DOI] [PubMed] [Google Scholar]
- 14.Sarlis NJ, Brucker-Davis F, Doppman JL, et al. MRI-demonstrable regression of a pituitary mass in a case of primary hypothyroidism after a week of acute thyroid hormone therapy. J Clin Endocrinol Metab 1997;82:808–11 [DOI] [PubMed] [Google Scholar]
- 15.Koller KJ, Wolff RS, Warden MK, et al. Thyroid hormones regulate levels of thyrotropin-releasing-hormone mRNA in the paraventricular nucleus. Proc Natl Acad Sci USA 1987;84:7329–33 [DOI] [PMC free article] [PubMed] [Google Scholar]