SUMMARY
The cytolytic activity of natural killer (NK) cells is regulated by inhibitory receptors that detect the absence of self molecules on target cells. Structural studies of missing self recognition have focused on NK receptors that bind MHC. However, NK cells also possess inhibitory receptors specific for non-MHC ligands, notably cadherins, which are down-regulated in metastatic tumors. We determined the structure of killer cell lectin-like receptor G1 (KLRG1) in complex with E-cadherin. KLRG1 mediates missing self recognition by binding to a highly conserved site on classical cadherins, enabling it to monitor expression of several cadherins (E-, N- and R-) on target cells. This site overlaps the site responsible for cell–cell adhesion, but is distinct from the integrin αEβ7 binding site. We propose that E-cadherin may co-engage KLRG1 and αEβ7, and that KLRG1 overcomes its exceptionally weak affinity for cadherins through multipoint attachment to target cells, resulting in inhibitory signaling.
INTRODUCTION
Natural killer (NK) cells are an ssential component of innate immunity against tumors and virally infected cells (Yokoyama and Plougastel, 2003; Lanier, 2005; Bryceson and Long, 2008). The cytolytic activity of NK cells is triggered by the ligation of various activating receptors, each of which recognizes a different molecule on the surface of target cells. Activating receptors include NKG2D and DNAM-1, which recognize MICA/B and CD155, respectively. The activation threshold of NK cells is set by the integration of inhibitory signals that counteract the activation signals. Inhibitory receptors recognize molecules that are expressed on normal cells as a means of protecting them from lysis by NK cells. Classically, inhibitory receptors recognize MHC class I (MHC-I) molecules and include members of the Ly49 and PIR receptor families in rodents, and of the KIR and LILR families in human (Deng and Mariuzza, 2006). Down-regulation of surface MHC-I molecules, which is often a hallmark of infectious or tumorigenic processes, results in loss of inhibition of NK cell activation. In this way, cells with abnormal MHC-I expression become targets of NK lytic activity (‘missing self’ recognition) (Yokoyama and Plougastel, 2003; Lanier, 2005; Bryceson and Long, 2008).
Although NK cell function is clearly regulated by MHC-I molecules on target cells, recent studies have revealed that NK cells also possess inhibitory receptors specific for non-MHC ligands (Bryceson and Long, 2008). By mediating NK cell inhibition through MHC-independent signaling, these receptors broaden the missing self hypothesis to comprise molecules besides MHC. Thus, the NKRP-1 family of C-type lectin-like NK receptors includes inhibitory receptors that bind to lectin-like ligands: Clrb molecules in mice (Iizuka et al., 2003; Carlyle et al., 2004) and the related LLT1 molecule in humans (Aldemir et al., 2005; Rosen et al., 2005). Whereas Clrb, like MHC-I, is broadly expressed on normal hematopoietic cells, it is frequently down-regulated on tumor cells (Carlyle et al., 2004). The immunoglobulin (Ig)-like inhibitory receptor LAIR-1 recognizes collagen as its biological ligand, showing that extracellular matrix proteins can also inhibit NK cell function (Lebbink et al., 2006).
Killer cell lectin-like receptor G1 (KLRG1) is a C-type lectin-like inhibitory receptor that contains an immune receptor tyrosine-based inhibitory motif (ITIM) motif in its cytoplasmic region (Guthman et al., 1995; Hanke et al., 1998). In humans, KLRG1 is expressed by NK cells (50–80%) and by CD4+ (~20%) and CD8+ (~40%) αβ T cells that exhibit an effector or effector–memory phenotype (Voehringer et al., 2001). In mice, KLRG1 is expressed by ~30% of NK cells and ~10% of αβ T cells. Infection with viruses or parasites leads to substantial increases in KLRG1 expression (Voehringer et al., 2001; Robbins et al., 2003; Thimme et al., 2005; Ibegbu et al., 2005). The inhibitory function of KLRG1, its predominant expression on effector cells, and its induction during infections, together suggest that KLGR1 raises the activation threshold of NK and T cells, thereby attenuating effector responses and preventing autoreactivity (Bryceson and Long, 2008; Colonna, 2006).
Recently, the biological ligand for KLRG1 was identified as E-cadherin, a classical cadherin (Ito et al., 2006; Gründemann et al., 2006; Tessmer et al., 2007). Cadherins comprise a family of transmembrane proteins that mediate Ca2+-dependent cell–cell adhesion by homotypic interactions (Pokutta and Weis, 2007; Gumbiner, 2005). They contribute to tissue morphogenesis during embryonic development and to the maintenance of tissue architecture. E-cadherin, whose extracellular region comprises five Ig-like domains (EC1–EC5), is localized at the basolateral membrane of epithelial cells where it establishes tight binding between neighboring cells in adherens junctions (Gumbiner, 2005). Besides E-cadherin, KLRG1 recognizes N- and R-cadherins (Ito et al., 2006), which are present in analogous structures in other cell types.
The binding of E-cadherin to KLRG1 prevents lysis of E-cadherin-expressing target cells by KLRG1+ NK cells (Ito et al., 2006). Moreover, KLRG1–cadherin interactions inhibit antigen-induced proliferation of CD8+ T cells and their acquisition of effector functions (Gründemann et al., 2006; Tessmer et al., 2007). These results indicate that KLRG1 may prevent cytotoxic cells from damaging tissues expressing E-, N-, or R-cadherins (Colonna, 2006; Bryceson and Long, 2008). In addition, they suggest a role for KLRG1 in tumor immunosurveillance analogous to missing self recognition by inhibitory NK receptors that bind MHC-I (Ly49s and KIRs) (Colonna, 2006; Schwartzkopff et al., 2007). The malignancy of epithelial tumors, such as breast cancers, is often associated with down-regulation of E-cadherin, which renders tumor cells invasive and metastatic (Takeichi, 1993; Cowin et al., 2005; Jeanes et al., 2008). The KLRG1–E-cadherin system may serve to detect potentially metastatic epithelial tumors with abnormal cadherin expression (Colonna, 2006; Bryceson and Long, 2008).
So far, structural studies of missing self recognition have focused on NK receptors that recognize MHC-I molecules (Deng and Mariuzza, 2006). Crystal structures of Ly49–MHC-I complexes showed that Ly49s engage MHC-I at a site beneath the peptide-binding platform formed by the heavy chain α1α2 and α3 domains and β2-microglobulin (Dam et al., 2003; Held and Mariuzza, 2008). By contrast, KIRs contact MHC-I through the α1 and α2 helices and the bound peptide in an orientation resembling the docking mode of T cell receptors onto MHC-I (Boyington et al., 2000; Fan et al., 2001). We recently determined the structure of the Ig-like NK receptor 2B4 (CD244) bound to CD48 that represents a multifunctional receptor–ligand system mediating both activating and inhibitory effects (Velikovsky et al., 2007). To understand the basis for MHC-independent missing self recognition by an exclusively inhibitory NK receptor that recognizes a ligand whose down-regulation is often associated with tumor progression, we determined the structures of human and mouse KLRG1 bound to human E-cadherin and of mouse KLRG1 in unbound form. The KLRG1–E-cadherin complexes revealed the nature and location of the KLRG1 binding site on E-cadherin relative to the sites that mediate E-cadherin interactions with itself and with the heterotypic ligand αEβ7 integrin. Collectively, these interactions contribute to regulating the complex interplay between cytotoxic lymphocytes and epithelial cells that occurs under inflammatory and neoplastic conditions.
RESULTS
Interaction of KLRG1 with Cadherins
We used surface plasmon resonance (SPR) to demonstrate specific binding of KLRG1 to E-cadherin. To permit directional coupling to streptavidin-coated biosensor surfaces, peptide tags containing biotinylation sequences were added to the N-termini of human or mouse KLRG1. Affinities were measured under equilibrium binding conditions by flowing one- or two-domain constructs (EC1 or EC1–EC2) of human or mouse E-cadherin over surfaces immobilized with human or mouse KLRG1. SPR sensograms for the interaction of the N-terminal Ig-like domain of human E-cadherin (hEC1) with human KLRG1 (hKLRG1) and mouse KLRG1 (mKLRG1), which is characterized by very fast on and off rates, are shown in Figure 1. Equilibrium binding constants (KDs) are presented in Table 1. In all cases, KDs in range of 100–200 µM were obtained, irrespective of whether EC1 or EC1–EC2 was used, and of the presence or absence of Ca2+, which is required for homotypic cadherin interactions (Pokutta and Weis, 2007). These surprisingly low affinities are 10–100-fold weaker than for any previously characterized NK cell receptor–ligand pair, including Ly49–MHC-I, KIR–MHC-I NKG2D–MICA and 2B4–CD48 (Boyington et al., 2000; Deng et al., 2008; Lengyel et al., 2007; Velikovsky et al., 2007).
Figure 1. Equilibrium Binding of Human E-Cadherin to Human and Mouse KLRG1.
SPR sensograms for the interaction of hEC1, at concentrations of 33 µM (blue), 67 µM (magenta), 135 µM (brown) and 270 µM (cyan), with immobilized hKLRG1 (450 RU) (A) and of hEC1, at concentrations of 19 µM (blue), 37 µM (magenta), 75 µM (brown) and 150 µM (cyan), with immobilized mKLRG1 (430 RU) (B) after correction for nonspecific binding. Insets show Scatchard plots of the equilibrium binding data; Req is the corrected equilibrium response at a given concentration C. KD = 200 µM for hKLRG1 and 130 µM for mKLRG1.
Table 1.
Dissociation Constants (M) of KLRG1–Cadherin Interactions
| hKLRG1 | mKLRG1 | |
|---|---|---|
| hEC1 | 2.0 ×10−4 | 1.3 ×10−4 |
| hEC1–EC2 | 1.0 ×10−4 | 8.7 ×10−5 |
| mEC1 | 1.6 ×10−4 | |
| mEC1–EC2 | 1.2 ×10−4 | |
| mNC1–NC2 | 1.3 ×10−4 | |
| hPC1–PC5 | NB |
Equilibrium dissociation constants (KDs) were derived from Scatchard analysis of SPR data (Figure 1). mNC1–NC2, domains 1 and 2 of mouse N-cadherin. hPC1–hPC5, P-cadherin-Fc chimera. NB, no binding.
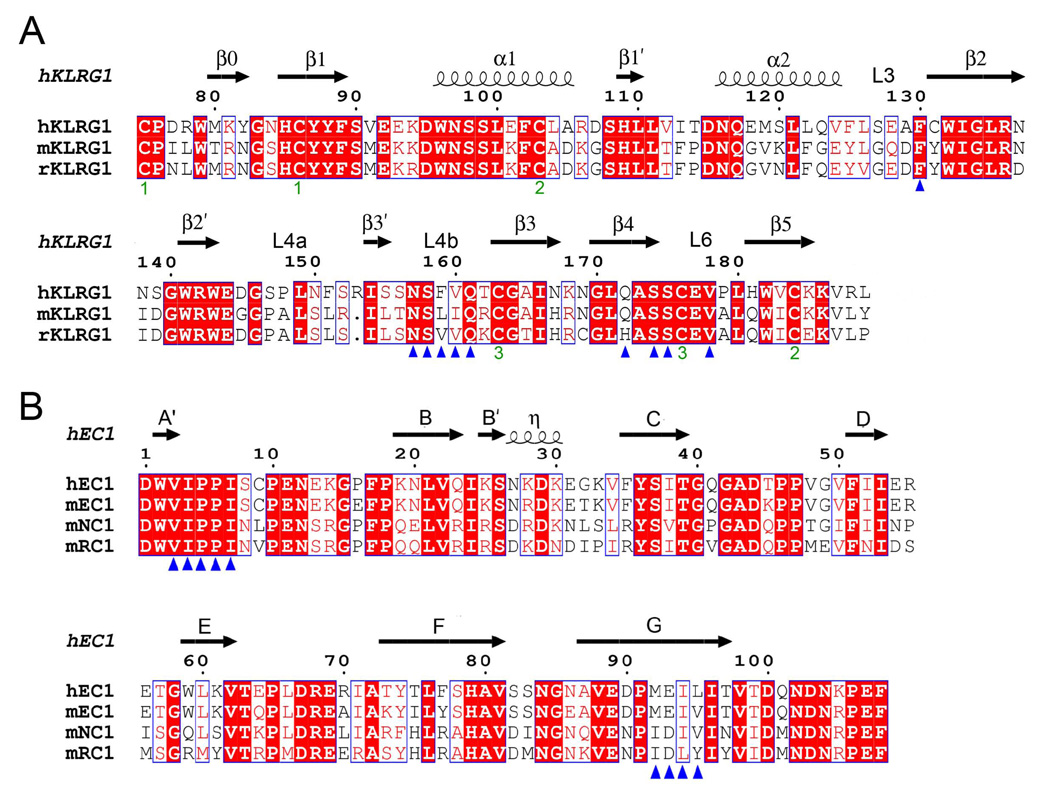
The ability of mKLRG1 to bind both mouse EC1 (mEC1; KD = 160 µM) and hEC1 (130 µM) is consistent with the finding that human E-cadherin is a xenogenic ligand for mKLRG1 (Table 1) (Ito et al., 2006). Since hKLRG1 exhibits similar affinities for hEC1 and hEC1–EC2 (200 µM and 100 µM, respectively), as does mKLRG1 for mEC1 and mEC1–EC2 (160 µM and 120 µM, respectively), KLRG1 mainly recognizes the N-terminal Ig-like domain of E-cadherin. We also found that the affinity of mKLRG1 for N-cadherin, another classical cadherin identified as a KLRG1 ligand (Ito et al., 2006; Tessmer et al., 2007), is effectively indistinguishable from that for E-cadherin (Table 1). No binding was detected to P-cadherin, which is not a KLRG1 ligand (Ito et al., 2006). Together, these results suggest that KLRG1 binds to a site that is structurally conserved in several classical cadherins, as well as in cadherins from different species.
Structure of KLRG1
KLRG1 is a type II transmembrane glycoprotein composed of a C-type lectin-like domain (CTLD) connected by a stalk region of 19 residues to a transmembrane segment and cytoplasmic domain (Guthman et al., 1995; Hanke et al., 1998). On the cell surface, KLRG1 exists both as a monomer and as a disulfide-linked homodimer with interchain disulfides in the stalk region (Corral et al., 2000). We determined the crystal structure of the CTLD of mKLRG1 to 1.8 Å resolution, as well as the structure of mKLRG1 bound to hEC1 to 2.0 Å resolution (Table 2). We also determined the structure of the CTLD of hKLRG1 in complex with hEC1 to 1.7 Å resolution.
Table 2.
Data Collection and Refinement Statistics
| hKLRG1–hEC1 | mKLRG1–hEC1 | mKLRG1 | |
|---|---|---|---|
| Data collection statistics | |||
| Space group | P21 | P212121 | C2 |
| Unit cell (Å, °) | a = 54.1, b = 83.4, c = 56.5, γ = 106.1° | a = 53.8, b = 90.5, c = 107.6 | a = 59.1, b = 56.9, c = 68.1, γ = 97.2° |
| Resolution (Å) | 30–1.8 | 30–2.0 | 30–1.7 |
| Observations | 41,8170 | 52,0356 | 78,240 |
| Unique reflections | 44,610 | 36,121 | 24,208 |
| Completeness (%)a | 99.6 (100) | 100 (100) | 97.7 (94.7) |
| Mean I/σ(I)a | 65.3 (6.8) | 46.9 (6.2) | 10.0 (2.8) |
| Rsym (%)a,b | 5.8 (38.8) | 5.1 (44.1) | 5.7 (34.3) |
| Resolution range (Å) | 30–1.8 | 30–2.0 | 30–1.8 |
| Refinement statistics | |||
| Rwork (%)c | 21.4 | 21.3 | 22.3 |
| Rfree (%)c | 24.6 | 25.1 | 25.7 |
| Protein atoms | 3,336 | 3,385 | 1,860 |
| Ca2+ | 3 | ||
| Water molecules | 309 | 250 | 169 |
| Average B values (Å2) | |||
| Protein main chain | 27.0 | 27.9 | 21.0 |
| Protein side chain | 30.1 | 35.2 | 25.0 |
| Ca2+ | 28.9 | ||
| Water molecules | 34.2 | 34.3 | 31.9 |
| R.m.s. deviations from ideality | |||
| Bond lengths (Å) | 0.0093 | 0.0098 | 0.0050 |
| Bond angles (°) | 1.56 | 1.54 | 1.25 |
| Ramachandran plot statistics | |||
| Most favored (%) | 90.3 | 92.2 | 91.9 |
| Additionally allowed (%) | 9.2 | 7.0 | 7.1 |
| Generously allowed (%) | 0.6 | 0.3 | 1.0 |
| Disallowed | 0 | 0 | 0 |
Values in parentheses are statistics for the highest resolution shells.
Rsym = Σ|Ij – <I>|/ΣIj, where Ij is the intensity of an individual reflection and <I> is the average intensity of that reflection.
Rwork = Σ‖Fo| – |Fc‖/Σ|Fo|, where Fc is the calculated structure factor. Rfree is as for Rwork but calculated for a randomly selected 5.0% of reflections not included in the refinement.
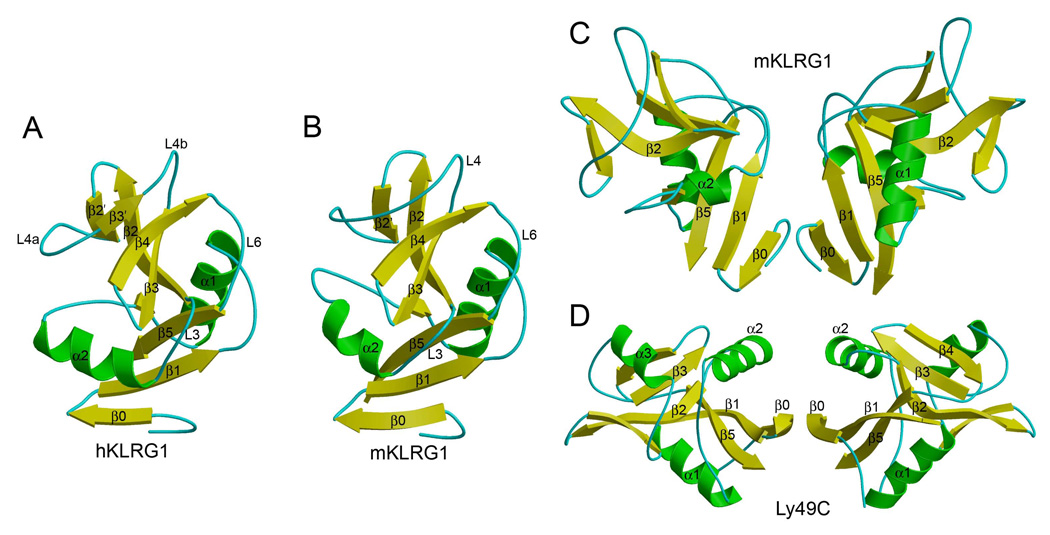
As expected from sequence analysis, KLRG1 adopts a fold characteristic of other CTLDs, such as Ly49 and NKG2D, comprising two α-helices (α1 and α2) and two anti-parallel β-sheets (Figures 2A and 2B). The two β-sheets are formed by β-strands β0, β1, β5 and β1’, and β-strands β2’, β2, β4 and β3’, respectively. There are three intrachain disulfide bonds in the KLRG1 CTLD, Cys75–Cys86, Cys103–Cys184 and Cys163–Cys176, of which Cys103–Cys184 is invariant in all CTLDs and Cys163–Cys176 is unique to KLRG1. The structures of human and mouse KLRG1 are very similar, with a root mean squared (r.m.s.) difference of 1.58 Å for 113 α-carbon atoms for a comparison of hKLRG1 in the hKLRG1–hEC1 complex with mKLRG1 in the mKLRG1–hEC1 complex. The main structural difference between the human and mouse receptors resides in helix α2, which is shorter in mKLRG1 and oriented slightly differently with respect to the β-sheets. In addition, the long loop (L4; residues 144–162) that connects β-strands β2’ and β3 in mKLRG1 (Figure 2B) is interrupted by a short β-stand (β3’) in hKLRG1 (Figure 2A). Nevertheless, the portion of the L4 loop that interacts with E-cadherin displays similar conformations in human and mouse KLRG1 (see below).
Figure 2. Structure of KLRG1.
(A) Ribbon diagram of human KLRG1, as observed in the hKLRG1–hEC1 complex. Secondary structure elements are labeled. α-helices are colored in green, β-stands in yellow, and loops in cyan.
(B) Structure of mouse KLRG1 in unbound form.
(C) Mouse KLRG1 homodimer, as observed in the mKLRG1–hEC1 complex. This dimer was not observed in structures of mKLRG1 alone or in the hKLRG1–hEC1 complex.
(D) Structure of the Ly49C homodimer (Protein Data Bank accession code 3C8J). Secondary structure elements are colored as in (A).
All three crystals (mKLRG1, mKLRG1–hEC1 and hKLRG1–hEC1) contain two KLRG1 molecules or two KLRG1–EC1 complexes per asymmetric unit (Table 2). However, the KLRG1 monomers form three totally different dimers in the three crystals. In one case, that of the mKLRG1–hEC1 complex, the overall mode of KLRG1 dimerization resembles that of the Ly49C or NKG2D homodimers (Dam et al., 2003; Li et al., 2001), in which the N-termini of the CTLDs point in the same direction (i.e., towards the NK cell membrane) and appear in close enough proximity to allow the stalks to form interchain disulfides (Figures 2C and 2D). Therefore, the KLRG1 dimer observed in the mKLRG1–hEC1 structure could represent a biologically relevant form of the receptor.
The total solvent-accessible surface area buried in the KLRG1 dimer interface is 1272 Å2, comparable to that in the Ly49C dimer (1598 Å2) but less than in NKG2D (2228 Å2). The interface shows a relatively low degree of shape complementarity, based on a calculated shape correlation statistic (Sc) (Lawrence and Colman, 1993) of 0.60 (Sc = 1.0 for interfaces with geometrically perfect fits), which is significantly less than the Sc values for Ly49C (0.80) and NKG2D (0.69). In addition, there are no main-chain–main-chain hydrogen bonds linking the β0 strands in the KLRG1 interface, whereas several such bonds are present in Ly49s and NKG2D. These factors may explain the behavior of KLRG1 CTLD (mouse or human) as a monomer in size exclusion chromatography (not shown), and why the putative dimer was only observed in one of the three crystal structures presented here. By contrast, Ly49 and NKG2D CTLDs form stable dimers in solution and in the crystal (Dam et al., 2003; Deng et al., 2008; Li et al., 2001). Weak association between KLRG1 CTLDs may also explain the existence of KLRG1 as a mixture of monomers and dimers on the cell surface (Ortega Soto and Pecht, 1988; Corral et al., 2000; Rosshart et al., 2008), whereas Ly49s and NKG2D are exclusively dimeric (Yokoyama and Plougastel, 2003; Lanier, 2005). Nevertheless, interchain disulfides within the stalk region of KLRG1 may drive association of the monomers through the interface observed in the mKLRG1–hEC1 structure (Figure 2C).
Structure of E-Cadherin
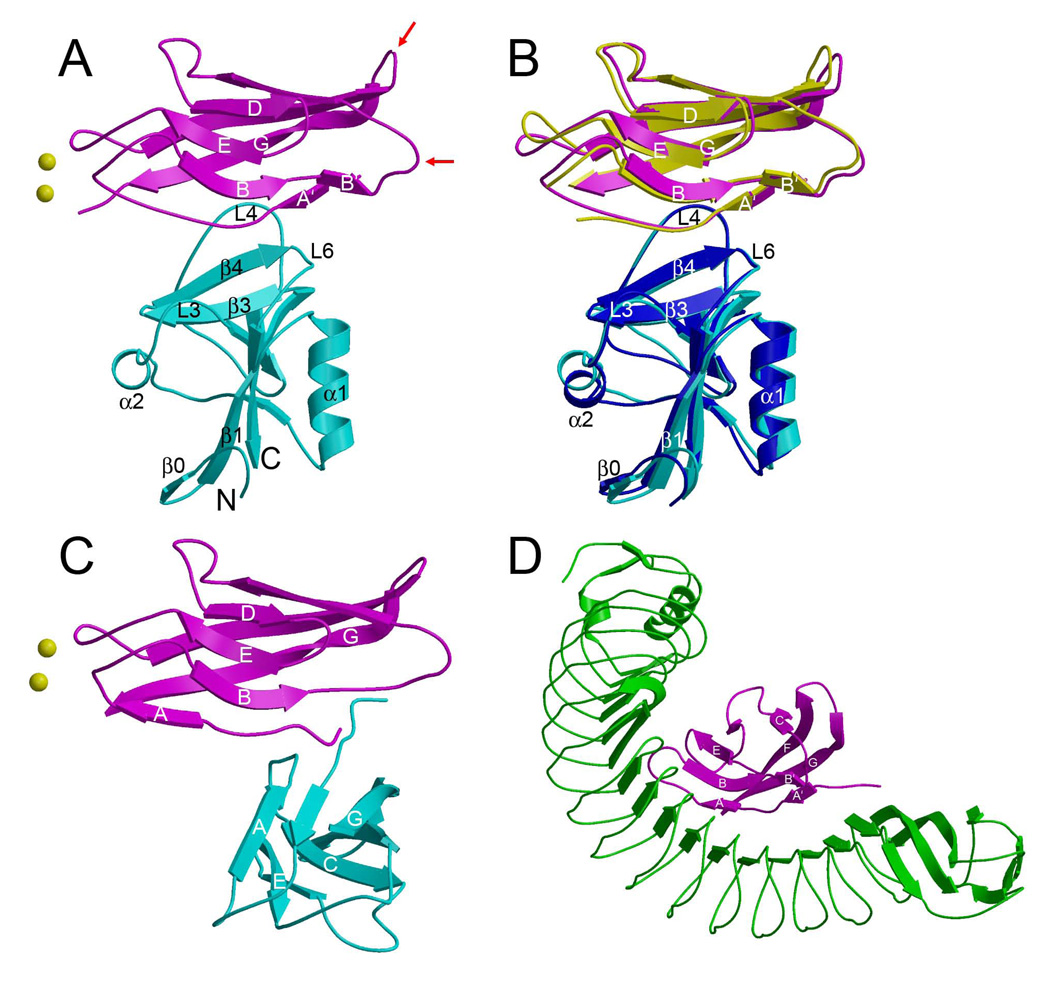
The EC1 domain of E-cadherin, as bound to KLRG1, consists of eight β-strands assembled into two anti-parallel β-sheets of strands designated A’BB’ED and CFG, closely related to the Ig fold (Figure 3A). The KLRG1 binding site on EC1 partially overlaps the site that mediates homotypic contacts between E-cadherin molecules on opposing cells (trans interactions) that are crucial for cell–cell adhesion (Nagar et al., 1996; Pokutta and Weis, 2007; Patel et al., 2003) (Figure 3C). Remarkably, it also overlaps the site recognized by the invasion protein internalin from the bacterium Listeria (Schubert et al., 2002), even though internalin, a leucine-rich repeat protein, is completely unrelated to KLRG1 (Figure 3D). In both KLRG1–EC1 complexes, the side chain of EC1 residue Trp2 fills a hydrophobic pocket in the body of the EC1 domain, as observed in one crystal form of mEC1 (Pertz et al., 1999) and in hEC1 bound to internalin (Schubert et al., 2002) (Figure 4A). By contrast, all other cadherin structures show a strand exchange between neighboring EC1 domains, placing Trp2 into the corresponding pocket of the second molecule to produce a strand-exchanged dimer that mediates cell adhesion (Nagar et al., 1996; Patel et al., 2003; Pokutta et al., 2007; Parisini et al., 2007). Hence, KLRG1 binding is incompatible with cadherin-mediated cell adhesion via the strand exchange mechanism.
Figure 3. Structure of the KLRG1–EC1 Complex and Comparison with Other E-Cadherin Complexes.
(A) Structure of the mKLRG1–hEC1 complex. Mouse KLRG1 is cyan and hEC1 is purple. The N- and C-termini of KLRG1 are labeled. Bound Ca2+ ions are drawn as yellow spheres. Red arrows indicate the BC and FG loops of hEC1, which bind αEβ7 integrin (Taraszka et al., 2000).
(B) Superposition of the mKLRG1–hEC1 and hKLRG1–hEC1 complexes. In the hKLRG1–hEC1 complex, hKLRG1 is blue and hEC1 is yellow.
(C) Structure of the hEC1–hEC1 strand-swapped adhesion dimer (2O72). The orientation of the upper hEC1 monomer (purple) is similar to that of hEC1 in (A).
(D) Structure of the Listeria internalin–hEC1 complex (1O6S). The hEC1 domain (purple) bound to internalin (green) is oriented similarly to hEC1 in (A).
Figure 4. Comparison of E-Cadherins.
(A) Superposition of hEC1 obtained in complex with KLRG1 (purple), hEC1 obtained in complex with Listeria internalin (yellow) (1O6S), one monomer of a strand-exchanged hEC1 dimer (cyan) (2O72), and the monomeric form of mEC1 (green) (1FF5). In monomeric EC1, and in the KLRG1 EC1 and internalin KLRG1–EC1 complexes, Trp2 occupies an acceptor pocket in the EC1 domain. In the strand-exchanged EC1 dimer, Trp2 forms part of the adhesion arm that mediates dimerization.
(B) The superposed EC1 structures in (A) are oriented to highlight conformational differences in the N-terminal region of the domains.
As in the internalin–hEC1 complex (Schubert et al., 2002), β-strands A’ and B’ of hEC1 form a small anti-parallel β-sheet in the KLRG1–EC1 complexes (Figure 4B). This β-sheet is probably induced by ligand binding, since the segments corresponding to strands A’ and B’ in KLRG1-bound hEC1 exist as loops in both monomeric and strand-exchanged EC1 structures (Patel et al., 2003). In the KLRG1–EC1 complexes, EC1 residues Ile4–Ser8 are extended in a polyproline type II conformation, whereas they form part of β-strand A in all other E-cadherin structures, including hEC1 bound to internalin (Figure 4B). This unique conformation is most likely attributable to KLRG1 binding, since EC1 residues 4–8 contact the receptor (see below). By contrast, KLRG1 does not undergo any significant structural rearrangements upon engaging hEC1 (r.m.s. difference of 0.71 Å for 113 α-carbon atoms for free versus bound mKLGR1).
Overview of the KLRG1–E-Cadherin Complex
The structure of the hKLRG1–hEC1 complex closely resembles that of the mKLRG1–hEC1 complex (Figure 3B), with r.m.s. differences ranging from 1.46 Å to 1.60 Å for 196 α-carbon for comparisons of the two complex molecules in the asymmetric unit of each crystal. The only significant difference between the hKLRG1–hEC1 and mKLRG1–hEC1 structures is that residues 11–13 of the loop connecting β-strands A’ and B of hEC1 are disordered in hKLRG1–hEC1; however, this part of the loop does not contact KLRG1 (Figure 3B). By contrast, the entire A’B loop is well defined in the mKLRG1–hEC1 complex, where it appears to be stabilized by two bound Ca2+ ion (mKLRG1–hEC1, but not hKLRG1–hEC1, was crystallized in the presence of Ca2+). Although these Ca2+ sites have low occupancy (~0.5), they correspond to Ca2+-binding sites previously described for human E-cadherin (Figure 3C) (Parisini et al., 2007). Further analysis is based on the mKLRG1–hEC1 structure, unless stated otherwise.
In the complex, one KLRG1 CTLD binds one EC1 molecule (Figures 3A and S1A). In this respect, KLRG1 recognition of its non-MHC ligand is reminiscent of Ly49 recognition of MHC-I, in which each CTLD monomer contains an entire ligand-binding site (Figure S1B). By contrast, the binding site of NKG2D for the MHC-related ligand MICA (Li et al., 2001) (Figure S1C), as well as the binding site of NKG2A/CD94 for HLA-E (Petrie et al., 2008; Kaiser et al., 2008), is formed by the precise juxtaposition of two CTLD subunits. The KLRG1–EC1 structure explains our finding that the second Ig-like domain of E-cadherin (EC2) does not contribute to KLRG1 binding (Table 1), since EC2 makes no contacts with KLRG1 in a model of the interaction between full-length E-cadherin (EC1–EC5) and KLRG1 (see Discussion).
E-cadherin docks with its short A’ β-strand, the N-terminal portion of the extended peptide segment following the A’ strand, and β-strand G onto a surface of KLRG1 that roughly corresponds to the ligand-binding site of other C-type lectin-like receptors, most notably Ly49s (Dam et al., 2003; Held and Mariuzza, 2008), NKG2D (Li et al., 2001) and NKG2/CD94 (Petrie et al., 2008; Kaiser et al., 2008). In KLRG1, this site is formed by three loops (L3, L4 and L6) and β-strand 4 (Figures 3A and 5A), whereas the more extensive binding sites of these other NK receptors comprise additional structural elements of the CTLD, in particular β-strands 1 and 5.
Figure 5. Structure-Based Sequence Alignments of KLRG1 Receptors and Cadherins.
(A) Sequence alignment of KLRG1 CTLDs. Secondary structure elements for hKLRG1 are denoted by squiggles (α-helices) and arrows (β-strands). These, and the loop regions, are numbered according to Figure 2A. Residues that contact E-cadherin in the mKLRG1–hEC1 and hKLRG1–hEC1 complexes are marked with blue triangles. The paired green numbers (1–3) indicate the bonded cysteine residues in the hKLRG1 and mKLRG1 structures. White characters on a red background show strictly residues. Residues that are well conserved are drawn in red and framed in blue. The remaining residues are black.
(B) Sequence alignment of the N-terminal domain of classical cadherins. Secondary structure elements for hEC1 are indicated by squiggles (310 helix (η)) and arrows (β-strands). Strands are labeled according to Figure 3A. Residues that contact KLRG1 in the mKLRG1–hEC1 and hKLRG1–hEC1 complexes are denoted with blue triangles. Sequences were retrieved from SwissProt using the following numbers: hKLRG1, Q96E93; mKLRG1, O88713; rat KLRG1 (rKLRG1) Q64335; hEC1, P12830; mEC1, P09830; mouse NC1 (mNC1), P15116; mouse RC1 (mRC1), P39038. Sequence alignments were performed with the program ClustalW (http://www.expasy.ch).
The KLRG1–E-Cadherin Interface
The KLRG1–E-cadherin complex buries a total solvent-accessible surface of only 1139 Å2, of which 563 Å2 is contributed by KLRG1 and 576 Å2 by E-cadherin. By contrast, NK receptors that recognize MHC or MHC-like ligands bury considerably more surface: Ly49C–H-2Kb (2393 Å2) (Deng et al., 2008), NKG2D–MICA (2374 Å2) (Li et al., 2001), NKG2A/CD94–HLA-E (1975 Å2) (Petrie et al., 2008), LILRB1–HLA-A2 (1783 Å2) (Willcox et al., 2003), and KIR2D–HLA-C (1466 Å2) (Boyington et al., 2000). Indeed, the exceptionally small interface of the KLRG1–E-cadherin complex is at the lower limit of the average value of 1600 (± 400) Å2 for stable protein–protein complexes (Lo Conte et al., 1999). However, it is similar in size to the interfaces of transient protein–protein complexes that mediate weak interactions in intracellular signaling or cell–cell adhesion (Nooren and Thornton, 2003), such as the heterophilic adhesion complex between CD2 and CD58 (1154 Å2) (Wang et al., 1999).
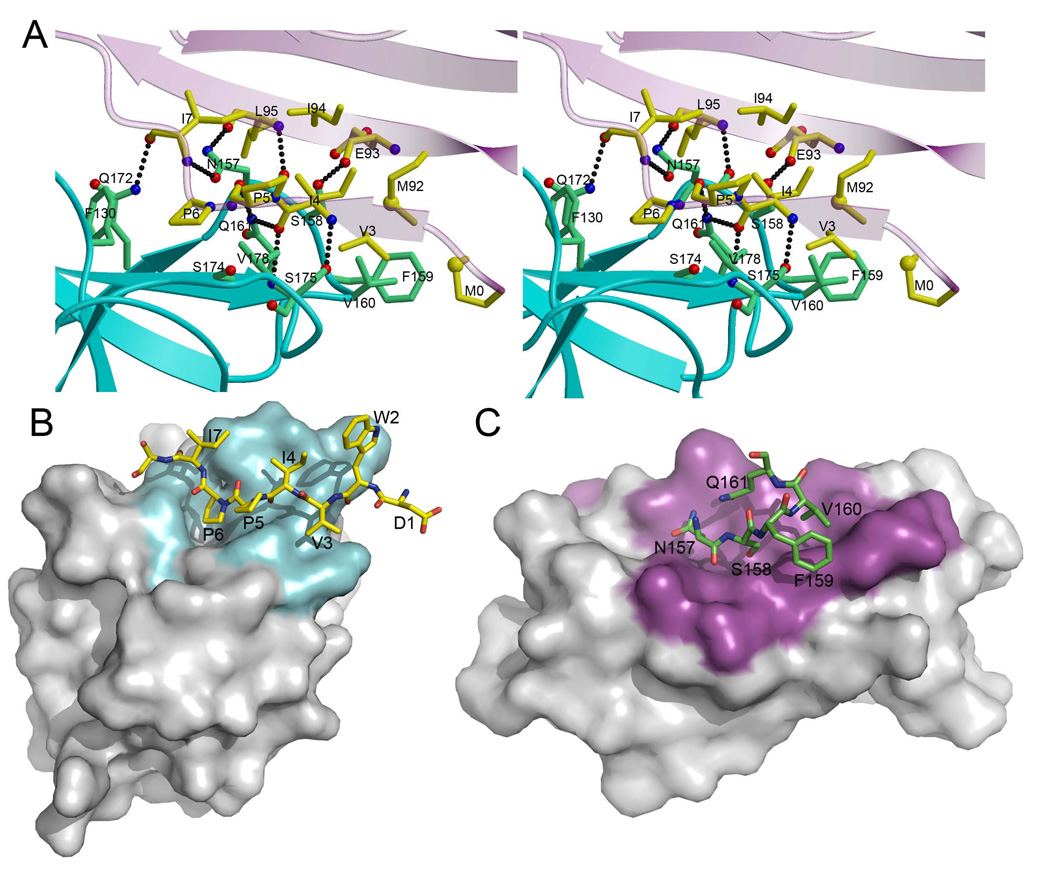
In the complex, 10 KLRG1 residues interact with 9 E-cadherin residues, with no bound water molecules or Ca2+ ions in the interface (Figures 5 and 6A). The interface is characterized by high shape complementarity, based on an Sc value of 0.77, which is at the upper end of the range for protein–protein complexes (Lawrence and Colman, 1993), and greater than the Sc values of the Ly49C–H-2Kb (0.58) (Deng et al., 2008), NKG2D–MICA (0.72) (Li et al., 2001), and NKG2A/CD94–HLA-E (0.59) (Petrie et al., 2008) interfaces. On the hEC1 side of the interface, residues Ile4–Ser8, which adopt a polyproline type II conformation upon interacting with KLRG1 (Figure 4B), form a bulge on the surface of the ligand that fits snuggly into a groove composed of loops L3, L4 and L6, and strand β4 of the receptor (Figure 6B). Within the groove, hEC1 residue Pro6 is situated in a deep pocket lined by KLRG1 residues Phe130, Asn157, Gln172, Ala173 and Ser174, where it makes multiple hydrophobic contacts with Phe130 and Ser174. On the KLRG1 side of the interface, residues Asn157–Gln161 of loop L4 lie in a long groove formed by the Ile4–Ser8 segment of hEC1, and by strands A’ and G (Figure 6C). The mainly hydrophobic KLRG1–hEC1 interface contains nine hydrogen bonds (Figure 6A; Table 3), which is considerably more than expected based on the size of the interface (Wodak and Janin, 2003). All these hydrogen bonds are strictly conserved in the hKLRG1–hEC1 and mKLRG1–hEC1 complexes, and all are between main-chain atoms of hEC1 and main-chain or side-chain atoms of KLRG1 (Table 3). The high shape complementarity of the KLRG1–hEC1 interface, the complete exclusion of bulk solvent, and the relative abundance of intermolecular hydrogen bonds probably compensate for the small size of the interface to achieve sufficient affinity for inhibitory signaling.
Figure 6. The Mouse KLRG1–Human E-Cadherin Binding Interface.
(A) Stereo view of the mKLRG1–hEC1 interface. The side chains of interacting residues are shown in ball-and-stick representation, with carbon atoms in green (mKLRG1) or yellow (hEC1), nitrogen atoms in blue, and oxygen atoms in red. Hydrogen bonds are drawn as dotted black lines. In addition to residues 1–108 of hEC1, an N-terminal methionine residue (M0), introduced by the cloning methodology, is well ordered in the electron density and makes several van der Waals contacts with mKLRG1.
(B) The mKLRG1 molecular surface is shown in gray with the region contacting hEC1 colored cyan. Residues 1–8 of hEC1 are drawn in stick format and labeled.
(C) The hEC1 molecular surface is shown in gray with the region contacting mKLRG1 colored magenta. Residues 157–161 of mKLRG1 are drawn in stick format and labeled.
Table 3.
Interactions between KLRG1 and E-Cadherin
| EC1 | KLRG1 | ||
|---|---|---|---|
| Hydrogen bonds | Distance (Å) | Van der Waals contacts | |
| Val3 | Ser175, Val178 | ||
| Ile4 | Ile4(N)–Ser175(Oγ) | 2.86 | Ser158, Gln161, Ser174, Ser175 |
| Ile4(O)–Ser175(N) | 3.04 | ||
| Ile4(O)–Gln161(Nε2) | 3.01 | ||
| Pro5 | Pro5(O)–Gln161(Nε2) | 3.02 | Gln161, Ser174, Ser175, Val178 |
| Pro6 | Phe130, Asn157, Ala173, Ser174 | ||
| Ile7 | Ile7(N)–Asn157(Oδ1) | 3.04 | Asn175, Gln172 |
| Ile7(O)–Gln172(Nε2) | 2.94 | ||
| Met92 | Ser158, Phe159, Val160 | ||
| Glu93 | Glu93(O)–Ser158(Oγ) | 2.91 | Asn157, Ser158 |
| Ile94 | Asn157, Ser158 | ||
| Leu95 | Leu95(N)–Asn157(O) | 2.75 | Asn157 |
| Leu95(O)-Asn157(Nδ2) | 2.83 | ||
Hydrogen bonds were calculated using a cutoff distance of 3.5 Å. The cutoff distance for van der Waals contacts was 4.0 Å.
In addition to KLRG1, lymphocytes express another receptor for E-cadherin, the integrin αEβ7 (CD103), which mediates T cell homing and adhesion to epithelial cells (Agace et al., 2000). Although no structure is available for E-cadherin bound to αEβ7 integrin, mutational analysis of hEC1 has localized the binding site to the face formed by loops BC and FG (Taraszka et al., 2000), which is distinct from the KLRG1 binding site on E-cadherin (Figure 3A). The αEβ7 integrin binding site is also distinct from the site that mediates trans interactions between E-cadherin molecules during cell–cell adhesion.
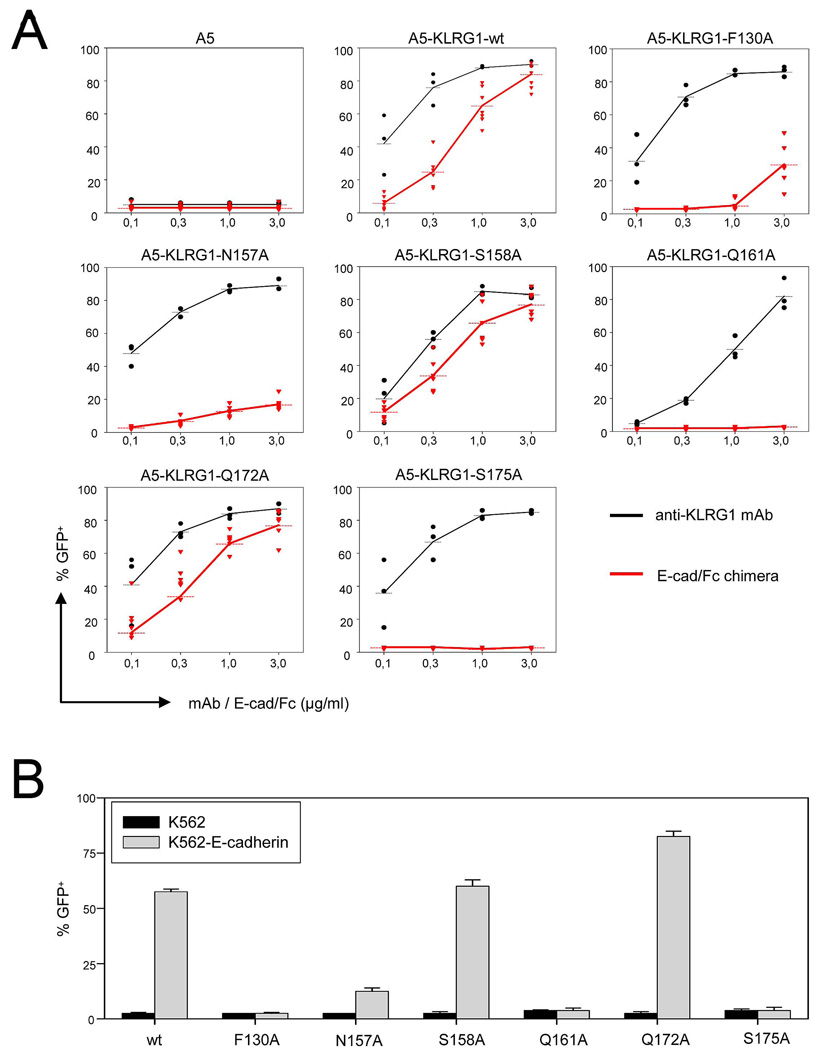
Functional Validation and Mutational Analysis of the KLRG1–E-Cadherin Interface
To functionally validate the KLRG1–E-cadherin interface observed in the crystal structures, as well as to identify key binding interactions, we prepared a panel of six alanine-substituted mutants of human KLRG1. The residues chosen (Phe130, Asn157, Ser158, Gln161, Gln172 and Ser175) are involved in hydrogen bonding and/or van der Waals contacts with hEC1 (Table 3). The reactivity of the KLRG1 mutants was determined in a reporter cell assay with A5 T cell hybridomas expressing the extracellular domain of KLRG1 fused to the intracellular domain of CD3ζ. Engagement of the chimeric KLRG1 receptor in this system results in green fluorescent protein (GFP) expression since A5 cells contain a NFAT-driven GFP-expression-cassette (Schwartzkopff et al., 2007; Andersen et al., 2001). Cell surface expression of the mutant KLRG1-CD3ζ chimeras after retroviral transduction was verified by staining with different monoclonal antibodies (mAb) specific for human KLRG1 (Figure S2). Importantly, engagement of mutant KLRG1-CD3ζ chimeras by plate-bound anti-KLRG1 mAb resulted in a robust and dose-dependent GFP induction in all cell lines (Figure 7A, black lines). The reactivity of the KLRG1-CD3ζ mutant molecules towards E-cadherin were then assayed using plate-bound E-cadherin-Fc chimeras (Figure 7A, red lines). The results revealed that alanine substitution of KLRG1 residues Gln161 and Ser175 completely abrogated the ability to trigger a response. GFP induction in alanine mutants of Phe130 and Asn157 was also strongly reduced, although some residual activity could be detected at high concentrations of E-cadherin-Fc chimeras. However, mutation of Ser158 or Gln172 did not reduce the reactivity of A5-KLRG1 reporter cells towards E-cadherin. This lack of effect may be explained by the location of Ser158 and Gln172 at the periphery, rather than center, of the protein–protein interface (Figure 6A), where solvent rearrangements are known to compensate for mutations (Sundberg and Mariuzza, 2003). In rat KLRG1, Gln172 is replaced by histidine (Figure 5A). This reactivity pattern was confirmed for all six KLRG1 mutants in a cell-based assay using K562 cells expressing E-cadherin (Figure 7B). Taken together, these results identify Phe130, Asn157, Gln161, and Ser175 as crucial residues for KLRG1 recognition of E-cadherin and validate the KLRG1–E-cadherin interface observed in the crystal structure.
Figure 7. Mutational Analysis of the KLRG1–E-Cadherin Interface.
(A) A5-KLRG1 reporter cells carrying the indicated alanine substitutions in the extracellular domain of human KLRG1 were cultured in plates pre-coated with different concentrations of anti-KLRG1 mAb (black lines) or E-cadherin-Fc chimera (red lines). Cells were harvested after 17 h and induction of GFP expression was determined by flow cytometry. Dots represent values from individual assays and data from at least three independent experiments are shown.
(B) A5-KLRG1 reporter cells were co-cultured with parental (black bars) or E-cadherin-transduced (gray bars) K562 cells. GFP expression was determined after 17 h. Results are shown as mean values including SEM derived from three independent experiments.
Basis for Binding Specificity
KLRG1 readily recognized E-, N- and R-cadherin in the reporter cell assay, but showed no reactivity towards M-, P, or VE-cadherin (Figure S3), in agreement with previous functional studies (Ito et al., 2006; Tessmer et al., 2006). The ability of KLRG1 to recognize three classical cadherins (E-, N- and R-) is readily explained by the KLRG1–hEC1 structure. Thus, hEC1 residues Val3–Ile7, which account for most of the interactions with KLRG1 (54 of 74 van der Waals contacts; six of nine hydrogen bonds), are absolutely conserved in N- and R-cadherins (Figure 5B). Although the other KLRG1-contacting region, comprising residues 92–95, is variable in E-, N- and R-cadherins, two of these residues are conservatively substituted (Glu/Asp93 and Ile/Leu94). Moreover, all three hydrogen bonds between KLRG1 and hEC1 residues 92–95 involve main-chain, rather than side-chain, atoms of the ligand: KLRG1 Ser158(Oγ)–Glu93(O) hEC1, KLRG1 Asn157(O)–Leu95(N) hEC1, and KLRG1 Asn157(Nδ2)–Leu95(O) hEC1 (Table 3). Such interactions should facilitate the accommodation of diverse amino acids at these KLRG1-contacting positions
The KLRG1–hEC1 structure also explains the inability of KLRG1 to bind two other classical cadherins, M- and P-cadherin (Ito et al., 2006). In P-cadherin, Ile4 of hEC1 is replaced by valine, whose side chain would be too short to contact Gln161 of KLRG1, a crucial residue for E-cadherin recognition (Figure 7). In addition, P-cadherin contains alanine, rather than proline, at position 5, which could alter the main-chain conformation at this site, thereby disrupting interactions with KLRG1 residues Asn157, Gln161 and Ser174 (Table 3). In the KLRG1–hEC1 complex, Met92 of hEC1 makes multiple hydrophobic contacts, through its side chain, with Ser158, Phe159 and Val160 of KLRG1 (Figure 6A). These interactions would be lost upon replacement of Met92 by threonine in M-cadherin.
DISCUSSION
Antigen-specific receptors involved in adaptive immunity, notably antibodies and TCRs, are both highly diverse and highly discriminatory. By contrast, MHC-specific NK receptors involved in missing self recognition, including Ly49s and the KIRs, show limited diversity and are only moderately discriminatory (Yokoyama and Plougastel, 2003; Lanier, 2005). Thus, individual Ly49s recognize multiple (but not all) H-2D and H-2K alleles, independently of the bound peptide, by engaging MHC-I at a site underneath the peptide-binding platform formed by β2-microglobulin and mainly non-polymorphic residues of the MHC-I heavy chain (Deng and Mariuzza, 2006). KIR receptors, whose docking mode onto MHC-I resembles that of TCRs, bind multiple HLA-A, -B and -C alleles by contacting mostly invariant residues of the α1 and α2 helices while minimizing interactions with the bound peptide (Boyington et al., 2000; Fan et al., 2001). Similarly, KLRG1 recognizes three of seven classical cadherins (E-, N- and R-) by binding to a highly conserved site on these self ligands, including a nearly invariant motif (VIPPI; residues 3–7) in their N-terminal Ig-like domain. However, just as individual Ly49 and KIR receptors display a degree of MHC allelic specificity (Yokoyama and Plougastel, 2003; Lanier, 2005), KLRG1 discriminates among classical cadherins through additional interactions involving a considerably more variable region of these molecules (M/I/T/V-E/N/D/P-I/L-T/L/S/V/Y/E; residues 92–95). In this way, NK cells bearing a single KLRG1 receptor are able to specifically monitor expression of several cadherins on target cells, resulting in MHC-independent missing self recognition.
The interaction of KLRG1 with cadherins shows parallels but also differences to the 2B4–CD48 receptor–ligand system with regard to the regulation of NK cell function by non-MHC ligands. In humans, 2B4 acts mainly as an activating receptor, whereas in mice it is inhibitory (McNerney et al., 2005a). In distinction, KLRG1 functions as an inhibitory receptor in both species. The inhibitory function of mouse 2B4 has been shown in various assay system in vitro. Moreover, 2B4-mediated inhibition of CD48+ tumor cell clearance and protection of bone marrow cells from NK cell attack in the absence of self-MHC-I has been demonstrated in vivo (Lee et al., 2004; McNerney et al., 2005b). In the KLRG1–E-cadherin system, analogous in vivo data are not available, but KLRG1-mediated inhibition of NK and T cell function has been demonstrated by several groups (Robbins et al., 2002; Gründemann et al., 2006; Ito et al., 2006). In these in vitro assays, KLRG1-mediated inhibition of lymphocyte function appears to be less pronounced compared to the 2B4 system, which might be related to the low affinity interaction of KLRG1 with cadherins. Missing self recognition should involve a ligand that is down-regulated during a disease process and we are not aware of any data suggesting that malignant or infected cells down-regulate CD48 to escape NK cell control. By contrast, the importance of E-cadherin down-regulation during tumor progression and its role as a tumor suppressor is very well established (Jeanes et al., 2008). However, direct in vivo data about the functional role the KLRG1–E-cadherin interaction are missing, since KLRG1-deficient mice have not yet been published.
E-cadherin function is often abrogated during development of human carcinomas (Takeichi, 1993; Cowin et al., 2005; Jeanes et al., 2008). For example, somatic mutations of the E-cadherin gene are found in ~50% of diffuse-type gastric carcinomas. Most of the observed gene alterations are splice-site mutations resulting in in-frame loss of exon 8 or 9 and produce deletions in EC2 or EC3 (Becker et al., 1994). These tumor-associated mutations have been shown to interfere with binding to KLRG1 and to lower the threshold for NK cell triggering (Schwartzkopff et al., 2007). Since we have shown that KLRG1 recognizes E-cadherin through EC1, with no direct involvement of EC2–EC5, deletions in EC2 or EC3 may indirectly alter the conformation of the KLRG1-binding site of EC1, or disrupt the overall structure of the cadherin ectodomain, whose integrity depends on Ca2+ ions bound specifically at the interfaces between individual Ig-like domains (Pokutta and Weis, 2007).
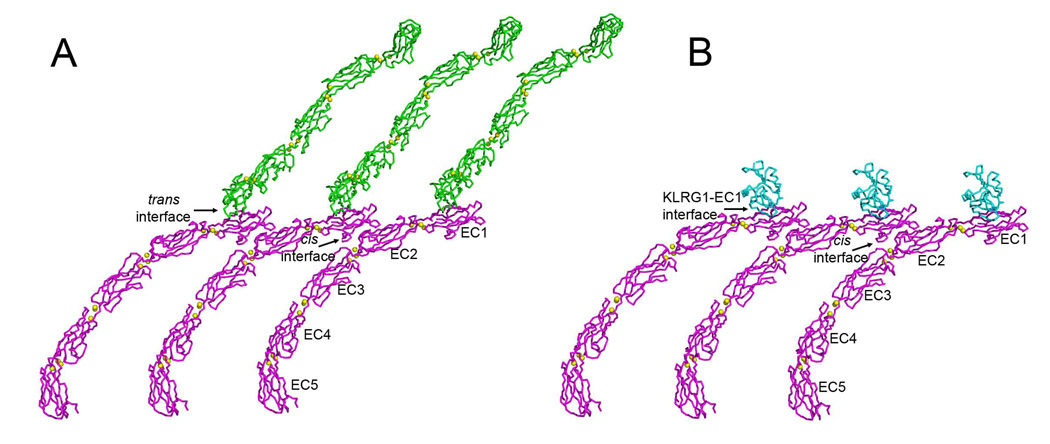
The affinity of KLRG1 for cadherins (KD~150 µM), as measured here by SPR, is significantly stronger than the affinity between cadherin monomers in adhesion dimers (700 µM) (Häussinger et al., 2004). However, it is 10–100-fold weaker than for any other NK receptor–ligand pair characterized to date, including Ly49A–H-2Dd (KD~5 µM) (Wang et al., 2002), KIR2D–HLA-C (10 µM) (Boyington et al., 2000), NKG2D–MICA (1 µM) (Lengyel et al., 2007), NKG2A/CD94–HLA-E (1 µM) (Petrie et al., 2008; Kaiser et al., 2008), and 2B4–CD48 (5 µM) (Velikovsky et al., 2007). We propose that KLRG1 compensates for its exceptionally low monomeric affinity for cadherins through multipoint attachment to cadherin molecules on the target cell surface. In addition to trans interactions between cadherin molecules on opposing cells responsible for cell–cell adhesion, cadherins emanating from the same cell membrane are believed to form lateral (cis) interactions (Figure 8A) (Pokutta and Weis, 2007). In the five-domain C-cadherin ectodomain structure (Boggon et al., 2002), EC1 of one molecule contacts EC2 of another; this cis interaction is also present in several two-domain structures of E-cadherin (Pokutta and Weis, 2007). Based on the KLRG1-hEC1 structure, cadherin molecules that are not engaged in trans should be available to bind KLRG1 receptors on the NK cell. A model of KLRG1–cadherin interactions at the NK cell–target cell interface may be constructed by superposing the KLRG1–hEC1 complex onto cadherin molecules arrayed in parallel on the target cell surface via cis interactions (Figure 8B). In this way, KLRG1–cadherin recognition could be achieved through the cooperativity of multiple associations, rather than by relying on the stability of individual complexes, while still allowing for dissociation of the complexes during transient NK cell–target cell encounters. Additionally, the ability of KLGR1 to form disulfide-linked dimers (Corral et al., 2000), or even multimers (Rosshart et al., 2008), on the cell surface may further increase the avidity of KLRG1–cadherin interactions through multivalent binding.
Figure 8. Hypothetical Model of KLRG1–E-Cadherin Interactions at the NK Cell–Target Cell Interface.
(A) Model for classical cadherin interactions at adherens junctions. In the C-cadherin crystal (1L3W) (Boggon et al., 2002), C-cadherin ectodomains (EC1–EC5), joined by both cis and trans interactions, are arrayed as if projecting from juxtaposed cell surfaces. Molecules from either cell surface are shown in blue or pink. Bound calcium ions are represented as yellow spheres. The cis interface between C-cadherin molecules is formed between a face of EC1 distal from the site of strand exchange and the bottom of EC2, such that the cadherins are arranged front-to-back in a continuous line on the cell surface.
(B) Model of KLRG1–E-cadherin interactions at the NK cell–target cell interface. The model was constructed by superposing the structure of the KLRG1–hEC1 complex onto the structure of the whole ectodomain of C-cadherin (EC1–EC5) in (A).
Besides NK cells, KLRG1 is expressed on subsets of T lymphocytes (Voehringer et al., 2001), where it has been used to discriminate short-lived effector T cells from long-lived memory cell precursors (Joshi et al., 2008). Certain T lymphocytes also express αEβ7 integrin, which mediates T cell homing and adhesion to epithelial cells via interactions with E-cadherin (Agace et al., 2000). These T cells provide immunosurveillance against infected, damaged, or transformed epithelial cells, and contribute to tissue repair. Recently, the interaction of αEβ7 integrin on tumor-infiltrating lymphocytes with E-cadherin on ICAM-1-negative tumor cells was shown to promote cytotoxic T lymphocyte activity by triggering lytic granule polarization and exocytosis (Le Floc’h et al., 2007). Because of their advanced differentiation as effector T cells, tumor-infiltrating lymphocytes probably also express KLRG1. If so, KLRG1 would compete with αEβ7 integrin for binding to E-cadherin, resulting in transmission of opposite signals to the T cell. Apart from competition, however, the possibility also exists of co-engagement of KLRG1 and αEβ7 integrin by a single E-cadherin molecule (Colonna, 2006). Indeed, the KLRG1–hEC1 structure reported here, together with previous mutational studies of the αEβ7–E-cadherin interaction (Taraszka et al., 2000), indicate that KLRG1 and αEβ7 recognize distinct sites on E-cadherin, such that binding of one receptor may not preclude binding of the other. Such co-engagement may allow cross-talk between KLRG1 and αEβ7, whereby KLRG1 inhibits activation of αEβ7 integrin, thus modulating its control over lymphocyte migration and function.
EXPERIMENTAL PROCEDURES
Protein Preparation
The C-type lectin-like domains of mouse (residues Cys75–Tyr189) and human (residues Cys75–Lys186) KLRG1 were cloned into the expression vector pET-26b (Novagen) and expressed as inclusion bodies in BL21(DE3) Escherichia coli cells (Novagen). The inclusion bodies were washed with 50 mM Tris-HCl (pH 8.0) containing 5% (v/v) Triton X-100, then dissolved in 6 M guanidine, 50 mM Tris-HCl (pH 8.0), and 10 mM DTT. For in vitro folding, the inclusion bodies were diluted into ice-cold folding buffer containing 0.4 M L-arginine-HCl, 50 mM Tris-HCl (pH 8.0), 1 mM EDTA, 3 mM reduced glutathione, and 0.9 mM oxidized glutathione to a final protein concentration of 40 mg/l. After 72 h at 4 °C, folded proteins were separated from aggregates using a Superdex S-75 column (GE Healthcare). Further purification was carried out by MonoS (mKLRG1) or MonoQ (hKLRG1) chromatography.
For production of biotinylated mouse and human KLRG1, a 16-amino acid tag (GSLNDIFEAQKIEWHE) was added to the N-termini of both receptors. The tagged proteins were produced in the same way as the unmodified proteins. Biotinylation was performed using a Biotinylation Kit (Avidity); biotinylated KLRG1 was separated from excess biotin with a Superdex S-75 column.
The first N-terminal domain of mouse and human E-cadherin (mEC1 and hEC1; residues Asp1–Phe108), the first and second N-terminal domains of mouse and human E-cadherin (mEC1–EC2 and hEC1–EC2; residues Asp1–Phe221), and the first and second N-terminal domains of mouse N-cadherin (mNC1–NC2; residues Asp1–Phe223) were cloned into pET-26b. Both mEC1 and mEC1–EC2 were expressed as soluble proteins in E. coli BL21(DE3) cells. The protein were purified from cell lysates using a POROS 20 HQ column (Applied Biosystems), followed by sequential Superdex S-75 and MonoQ columns. By contrast, hEC1, hEC1–EC2, and mNC1–NC2 were expressed in inclusion bodies. Following solubilization in 4 M urea, the inclusion bodies were dialyzed overnight at 4 °C against 50 mM Tris-HCl (pH 8.0). Folded proteins were purified using Superdex S-75 and MonoQ columns.
Crystallization and Data Collection
Crystals of unbound mKLRG1 (4 mg/ml) were grown in hanging drops at room temperature in 0.7 M Na,KPO4 and 0.1 M Hepes (pH 8.0). For crystallization of KLRG1–cadherin complexes, hKLRG1 or mKLRG1 was mixed with hEC1 in a 1:1 molar ratio to a final protein concentration of ~10 mg/ml. The hKLGR1–hEC1 complex crystallized in 25% (w/v) polyethylene glycol monomethyl ether 4000, 200 mM ammonium acetate, and 100 mM sodium acetate (pH 4.6). The mKLRG1–hEC1 complex crystallized in 18% (w/v) polyethelene glycol 8000, 200 mM calcium acetate, and 100 mM sodium cacodylate (pH 6.5).
For data collection, hKLGR1–hEC1 crystals were transferred to a cryoprotectant solution of mother liquor containing 30% (w/v) PEG 4000, prior to flash cooling in liquid nitrogen. X-ray diffraction data were collected to 1.8 Å resolution at beamline X29 of the Brookhaven National Synchrotron Light Source (BNSLS). 100 K on a Rigaku R-axis IV++ image plate detector using CuKα radiation from a Micromax-007 rotating anode generator. The crystals belong to space group P21 with two complex molecules per asymmetric unit. Crystals of unbound mKLRG1 and mKLRG1–hEC1 were cryoprotected by soaking in mother liquor containing 20% (v/v) glycerol. For mKLRG1, diffraction data to 1.7 Å resolution were measured on a Rigaku R-axis IV++ image plate detector using CuKα radiation from a Micromax-007 rotating anode generator. The crystals belong to space group C2 with two molecules per asymmetric unit. For the mKLRG1–hEC1 complex, diffraction data to 2.0 Å resolution were recorded at BNSLS. The crystals belong to space group P212121 with two complex molecules per asymmetric unit. All data were indexed, integrated, and scaled with the program CrystalClear 1.3.6 (Rigaku) or HKL2000 (Otwinowski and Minor, 1997). Data collection statistics are summarized in Table 2.
Structure Determination and Refinement
The structures of mKLRG1, hKLRG1–hEC1 and mKLRG1–hEC1 were solved by molecular replacement using Phaser (Storoni et al., 2004). A search of the mKLRG1 sequence against the Protein Data Bank showed that mKLRG1 is most similar to human CD94 (PDB accession code 1B6E) (Boyington et al., 1999) and human CD69 (1FM5) (Natarajan et al., 2000), with 33% and 24% sequence identity, respectively. However, no molecular replacement solution was found with CD94 or CD69 alone. Phaser was then used to build an ensemble model by combining the aligned CD94 and CD69 structures. Using this ensemble search model, the two mKLRG1 molecules were located by automatic rotation and translation searches with Z-score of 7.4; initial rigid body refinement gave Rfree of 39.1% and Rwork of 38.0%. The partially refined mKLRG1 structure and a human E-cadherin domains 1 and 2 structure (2O72) (Parisini et al., 2007) were used as search models for solving the structures of hKLRG1–hEC1 and mKLRG1–hEC1. The two KLRG1 and two EC1 molecules in the asymmetric unit of both crystals were located by rotation and translation searches with Z-scores of 18.3 and 27.0 for the hKLRG1–hEC1 and mKLRG1–hEC1 complexes, respectively. All refinements were carried out using CNS1.1 (Brünger, et al., 1998), including iterative cycles of simulated annealing, positional refinement and B factor refinement, interspersed with model rebuilding into σA-weighted Fo– Fc and 2Fo– Fc electron density maps using XtalView (McRee, 1999). Refinement statistics are summarized in Table 2. Stereochemical parameters were evaluated with PROCHECK (Laskowski et al., 1993).
SPR Analysis
The interaction of KLRG1 with E- and N-cadherins was assessed by SPR using a BIAcore T100 biosensor. Typically, biotin-KLRG1 was immobilized on the sensor chip by flowing a biotin-KLRG1 solution (~20 µg/ml) containing 0.5 M NaCl over a BIAcore SA chip pre-immobilized with streptavidin at high surface density. After immobilizing 200–500 resonance units (RU), 20 µl of a 20 µM biotin solution was injected to block the remaining streptavidin sites. As the blank, biotin alone was injected. Immediately prior to SPR analysis, all cadherin preparations were purified using a Superdex S-75 column to eliminate possible aggregates. To assess cadherin binding, solutions containing different concentrations of cadherin were injected sequentially over flow cells immobilized with KLRG1. The data were analyzed using BIAevaluation 4.1 software. Dissociation constants (KDs) were determined from Scatchard analysis, after correction for nonspecific binding, by measuring the concentration of free reactants and complex at equilibrium. Standard deviations for two or more independent KD determinations were typically <20%.
Construction of Mutated KLRG1-CD3ζ Chimeras
The generation of a cDNA encoding the transmembrane and extracellular domain of human KLRG1 fused to the cytoplasmic domain of mouse CD3ζ has been described previously (Schwartzkopff et al., 2007). The KLRG1-CD3ζ cDNA cloned in the XhoI site of pcDNA3.1/Zeo (Invitrogen) was used as a template DNA for site-directed mutagenesis. The mutants were constructed by Quick-Change site-directed mutagenesis using PfuTurbo DNA polymerase (Stratagene) and the following primers: F130A: 5’-CCTCAGTGAGGCCGCTTGCTGGATTGGTCTGAGG-3’; N157A: 5’-GGATTTCTTCTGCTAGCTTTGTGCAGACATGC-3’; S158A: 5’-GGATTTCTTCTAATGCCTTTGTGCAGACATGC-3’; Q161A: 5’-CTAATAGCTTTGTGGCGACATGCGGTGC-3’; Q172A: 5’-CCATCAACAAAAATGGTCTTGCAGCCTCAAGC-3’; S175A: 5’-GGTCTTCAAGCCTCAGCCTGTGAAGTTCC-3’. PCR products were digested with DpnI and transformed into Top10 competent cells (Invitrogen). The mutations were confirmed by sequencing the pcDNA3.1-KLRG1-CD3ζ clones and sequence-verified XhoI/NotI fragments were subcloned in the pMSCV2.2 proviral vector. Retroviral transduction of A5 T cell hybridoma cells was performed as described (Schwartzkopff et al., 2007) and KLRG1-expressing cells were enriched by magnetic beads (Miltenyi Biotec).
Reporter Cell Assay
Assays using plate-bound stimulation were performed in 96-well high-binding polystyrene flat bottom EIA/RIA plates (Corning) pre-coated overnight at 4 °C with the indicated concentration of anti-KLRG1 mAb (clone 13F12F2, 13A2 or 1A12) (Voehringer et al., 2001; Marcolino et al., 2004) or recombinant fusion proteins consisting of all five extracellular domains of human E-cadherin fused to the Fc portion of human IgG1 (RD Systems). After washing with PBS, A5-KLRG1 reporter cells (5 × 104 in 100µl culture medium/well) were added and cultured for 17 h before flow cytometric analysis. In cell-based assays, 105 A5-KLRG1 reporter cells (105/well) were co-cultured with parental or E-cadherin-transduced K562 cells (105/well) in 1 ml culture medium in 24-well plates for 17 h. Afterwards, cells were harvested, stained with anti-mouse CD4 mAb (e-Bioscience) to distinguish A5 reporter cells from K562 cells and analyzed for GFP expression by flow cytometry.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (AI47990 to R.A.M) and by the Deutche Forschungsgemeinschaft (SFB620 TP2 to H.P.). We thank H. Robinson (Brookhaven National Synchrotron Light Source) for X-ray data collection. Support for beamline X29 comes from the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy, and from the National Center for Research Resources of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Codes
Atomic coordinates and structure factors have been deposited in the Protein Data Bank: mKLRG1, 3FF9; hKLRG1–hEC1, 3FF7; and mKLRG1–hEC1, 3FF8.
REFERENCES
- Agace WW, Higgins JM, Sadasivan B, Brenner MB, Parker CM. T-lymphocyte-epithelial-cell interactions: integrin αEβ7 (CD103), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 12:563–568. doi: 10.1016/s0955-0674(00)00132-0. [DOI] [PubMed] [Google Scholar]
- Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting Edge: Lectin-like transcript 1 is a ligand for the CD161 receptor. J. Immunol. 2005;175:7791–7795. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- Andersen PS, Geisler C, Buus S, Mariuzza RA, Karjalainen K. Role of T cell receptor ligand affinity in T cell activation by bacterial superantigens. J. Biol. Chem. 2001;276:33452–33457. doi: 10.1074/jbc.M103750200. [DOI] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H. E-cadherin mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994;54:3845–3852. [PubMed] [Google Scholar]
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature. 2000;405:537–543. doi: 10.1038/35014520. [DOI] [PubMed] [Google Scholar]
- Boyington JC, Riaz AN, Patamawenu A, Coligan JE, Brooks AG, Sun PD. Structure of CD94 reveals a novel C-type lectin fold: implications for the NK cell-associated CD94/NKG2 receptors. Immunity. 1999;10:75–82. doi: 10.1016/s1074-7613(00)80008-4. [DOI] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography and NMR systems: a new software suite for macromolecular structure determination. Acta Crystallogr. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr. Opin. Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc. Natl. Acad. Sci. USA. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Cytolytic responses: cadherins put out the fire. J. Exp. Med. 2006;203:261–264. doi: 10.1084/jem.20052559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral L, Hanke T, Vance RE, Cado D, Raulet DH. NK cell expression of the killer cell lectin-like receptor G1 (KLRG1), the mouse homolog of MAFA, is modulated by MHC class I molecules. Eur. J. Immunol. 2000;30:920–930. doi: 10.1002/1521-4141(200003)30:3<920::AID-IMMU920>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr. Opin. Cell Biol. 2005;17:499–508. doi: 10.1016/j.ceb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Dam J, Guan R, Natarajan K, Dimasi N, Chlewicki LK, Kranz DM, Schuck P, Margulies DH, Mariuzza RA. Variable MHC class I engagement by Ly49 NK receptors revealed by the crystal structure of Ly49C bound to H-2Kb. Nat. Immunol. 2003;12:1213–1222. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- Deng L, Mariuzza RA. Structural basis for recognition of MHC and MHC-like ligands by natural killer cell receptors. Semin. Immunol. 2006;18:159–166. doi: 10.1016/j.smim.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Cho S, Malchiodi EL, Kerzic MC, Dam J, Mariuzza RA. Molecular architecture of the MHC-binding site of Ly49 natural killer cell receptors. J. Biol. Chem. 2008;283:16840–16849. doi: 10.1074/jbc.M801526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QR, Long EO, Wiley DC. Crystal structure of the human natural killer inhibitory receptor KIR2DL1-HLA-Cw4 complex. Nat. Immunol. 2001;2:452–460. doi: 10.1038/87766. [DOI] [PubMed] [Google Scholar]
- Gründemann C, Bauer M, Schweie O, von Oppen N, Lässing U, Saudan P, Becker K-F, Karp K, Hanke T, Bachmann M, Pircher H. Cutting Edge: Identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1 (KLRG1) J. Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Guthman MD, Tal M, Pecht I. A secretion inhibitory signal transduction molecule on mast cells is another C-type lectin. Proc. Natl. Acad. Sci. USA. 1995;92:9397–9401. doi: 10.1073/pnas.92.20.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke T, Corral L, Vance RE, Raulet DH. 2F1 antigen, the mouse homolog of the rat “mast cell function-associated antigen”, is a lectin-like type II transmembrane receptor expressed by natural killer cells. Eur. J. Immunol. 1998;28:4409–4417. doi: 10.1002/(SICI)1521-4141(199812)28:12<4409::AID-IMMU4409>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Häussinger D, Ahrens T, Aberle T, Engel J, Stetefeld J, Grzesiek S. Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. EMBO J. 2004;23:1699–1708. doi: 10.1038/sj.emboj.7600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W, Mariuzza RA. Cis interactions of immunoreceptors with MHC and non-MHC ligands. Nat. Rev. Immunol. 2008;8:269–278. doi: 10.1038/nri2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Koutis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J. Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat. Immunol. 2003;4:801–807. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- Ito M, Maruyama T, Saito N, Koganei S, Yamamoto K, Matsumoto N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J. Exp. Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;24:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2008;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser BK, Pizarro JC, Kerns J, Strong RK. Structural basis for NKG2A/CD94 recognition of HLA-E. Proc. Natl. Acad. Sci. USA. 2008;105:6696–6701. doi: 10.1073/pnas.0802736105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: A program to check the stereo chemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- Lawrence MC, Colman PM. Shape complementarity at protein-protein interfaces. J. Mol. Biol. 1993;234:946–950. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- Lebbink RJ, de Ruiter T, Adelmeijer J, Brenkman AB, van Helvoort JM, Koch M, Farndale RW, Lisman T, Sonnenberg A, Lenting PJ, Meyaard L. Collagens are functional, high affinity ligands for the inhibitory immune receptor LAIR-1. J. Exp. Med. 2006;203:1419–1425. doi: 10.1084/jem.20052554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J. Exp. Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floc’h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel CS, Willis LJ, Mann P, Baker D, Kortemme T, Strong RK, McFarland BJ. Mutations designed to destabilize the receptor-bound conformation increase MICA-NKG2D association rate and affinity. J. Biol. Chem. 2007;282:30658–30666. doi: 10.1074/jbc.M704513200. [DOI] [PubMed] [Google Scholar]
- Li P, Morris DL, Willcox BE, Steinle A, Spies T, Strong RK. Complex structure of the activating immunoreceptor NKG2D and its MHC class I-like ligand MICA. Nat. Immunol. 2001;2:443–451. doi: 10.1038/87757. [DOI] [PubMed] [Google Scholar]
- Lo Conte LL, Chothia C, Janin J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999;285:2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- Marcolino I, Przybylski GK, Koschella M, Schmidt CA, Voehringer D, Schlesier M, Pircher H. Frequent expression of the natural killer cell receptor KLRG1 in human cord blood T cells: correlation with replicative history. Eur. J. Immunol. 2004;34:2672–2680. doi: 10.1002/eji.200425282. [DOI] [PubMed] [Google Scholar]
- McNerney ME, Lee KM, Kumar V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol. Immunol. 2005a;42:489–494. doi: 10.1016/j.molimm.2004.07.032. [DOI] [PubMed] [Google Scholar]
- McNerney ME, Guzior D, Kumar V. 2B4 (CD244)-CD48 interactions provide a novel MHC class I-independent system for NK-cell self-tolerance in mice. Blood. 2005b;106:1337–1340. doi: 10.1182/blood-2005-01-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRee DE. XtalView/Xfit-A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Natarajan K, Sawicki MW, Margulies DH, Mariuzza RA. Crystal structure of human CD69: a C-type lectin-like marker of hematopoietic cells. Biochemistry. 2000;39:14776–14786. doi: 10.1021/bi0018180. [DOI] [PubMed] [Google Scholar]
- Nooren IMA, Thornton JM. Structural characteristics and functional significance of transient protein-protein interactions. J. Mol. Biol. 2003;325:991–1018. doi: 10.1016/s0022-2836(02)01281-0. [DOI] [PubMed] [Google Scholar]
- Ortega Soto E, Pecht I. A monoclonal antibody that inhibits secretion from rat basophilic leukemia cells and binds to a novel membrane component. J. Immunol. 1988;141:4324–4332. [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Parisini E, Higgins JMG, Liu J, Brenner MB, Wang J. The crystal structure of human E-cadherin domains 1 and 2, and comparison with other cadherins in the context of adhesion mechanism. J. Mol. Biol. 2007;373:401–411. doi: 10.1016/j.jmb.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr. Opin. Struct. Biol. 2003;13:690–698. doi: 10.1016/j.sbi.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J. 1999;18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, Heroux A, Hoare HL, Beddoe T, Reid HH, Wilce MC, Brooks AG, Rossjohn J. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. J. Exp. Med. 2008;205:725–735. doi: 10.1084/jem.20072525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu. Rev. Cell Dev. Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: Inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J. Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- Robbins SH, Terrizzi SC, Sydora BC, Mikayama T, Brossay L. Differential regulation of killer cell lectin-like receptor G1 expression on T cells. J. Immunol. 2003;170:5876–5885. doi: 10.4049/jimmunol.170.12.5876. [DOI] [PubMed] [Google Scholar]
- Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting Edge: Lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J. Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- Rosshart S, Hofmann M, Schweier O, Pfaff A-K, Yoshimoto K, Takeuchi T, Molnar E, Schamel WW, Pircher H. Interaction of KLRG1 with E-cadherin: new functional and structural insights. Eur. J. Immunol. 2008 Nov 13; doi: 10.1002/eji.200838690. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Schubert W-D, Urbanke C, Ziehm T, Beier V, Machner MP, Domann E, Wehland J, Chakraborty T, Heinz DW. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Schwartzkopff S, Gründemann C, Schweier O, Rosshart S, Karjalainen KE, Becker K-F, Pircher H. Tumor-associated E-cadherin mutations affect binding to the killer cell lectin-like receptor G1 in humans. J. Immunol. 2007;179:1022–1029. doi: 10.4049/jimmunol.179.2.1022. [DOI] [PubMed] [Google Scholar]
- Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- Sundberg EJ, Mariuzza RA. Molecular recognition in antigen-antibody complexes. Adv. Protein Chem. 2003;61:119–160. doi: 10.1016/s0065-3233(02)61004-6. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr. Opin. Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Taraszka KS, Higgins JMG, Tan K, Mandelbrot DA, Wang J, Brenner MB. Molecular basis for leukocyte integrin αEβ7 adhesion to epithelial (E)-cadherin. J. Exp. Med. 2000;191:1555–1567. doi: 10.1084/jem.191.9.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmer MS, Fugere C, Stevenaert F, Naidenko OV, Chong HJ, Leclercq G, Brossay L. KLRG1 binds cadherins and preferentially associates with SHIP-1. Int. Immunol. 2007;4:391–400. doi: 10.1093/intimm/dxm004. [DOI] [PubMed] [Google Scholar]
- Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, Rickinson AB, Rowland-Jones SL, Blum HE, Pircher H. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Blaser P, Brawand P, Raulet DH, Hanke T, Pircher H. Viral infections induce adundant numbers of senescent CD8 T cells. J. Immunol. 2001;167:4838–4843. doi: 10.4049/jimmunol.167.9.4838. [DOI] [PubMed] [Google Scholar]
- Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectin-like receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- Wang JH, Smolyar A, Tan K, Liu JH, Kim M, Sun ZY, Wagner G, Reinherz EL. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell. 1999;97:791–803. doi: 10.1016/s0092-8674(00)80790-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Whitman MC, Natarajan K, Tormo J, Mariuzza RA, Margulies DH. Binding of the NK inhibitory receptor Ly49A to its MHC-I ligand: crucial contacts include both H-2Dd and β2-microglobulin. J. Biol. Chem. 2002;277:1433–1442. doi: 10.1074/jbc.M110316200. [DOI] [PubMed] [Google Scholar]
- Willcox BE, Thomas LM, Bjorkman PJ. Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat. Immunol. 2003;4:913–919. doi: 10.1038/ni961. [DOI] [PubMed] [Google Scholar]
- Wodak SJ, Janin J. Structural basis of macromolecular recognition. Adv. Protein Chem. 2003;61:9–73. doi: 10.1016/s0065-3233(02)61001-0. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Plougastel BFM. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.