Abstract
Multi-layered poly(glycerol sebacate) (PGS) scaffolds with controlled pore microarchitectures were fabricated, combined with heart cells, and cultured with perfusion to engineer contractile cardiac muscle constructs. First, one-layered (1L) scaffolds with accordion-like honeycomb shaped pores and elastomeric mechanical properties were fabricated by laser microablation of PGS membranes. Second, two-layered (2L) scaffolds with fully interconnected three dimensional pore networks were fabricated by oxygen plasma treatment of 1L scaffolds followed by stacking with off-set laminae to produce a tightly bonded composite. Third, heart cells were cultured on scaffolds with or without interstitial perfusion for 7 days. The laser-microablated PGS scaffolds exhibited ultimate tensile strength and strain-to-failure higher than normal adult rat left ventricular myocardium, and effective stiffnesses ranging from 220 to 290 kPa. The 7 day constructs contracted in response to electrical field stimulation. Excitation thresholds were unaffected by scaffold scale up from 1L to 2L. The 2L constructs exhibited reduced apoptosis, increased expression of connexin-43 (Cx-43) and matrix metalloprotease-2 (MMP-2) genes, and increased Cx-43 and cardiac troponin-I proteins when cultured with perfusion as compared to static controls. Together, these findings suggest that multi-layered, microfabricated PGS scaffolds may be applicable to myocardial repair applications requiring mechanical support, cell delivery and active implant contractility.
Introduction
Cardiovascular disease is the leading cause of death in developed countries [1] and congenital heart disease, which affects approximately one percent of newborns world-wide, is associated with high morbidity [2]. The functional consequences of myocardial infarction (MI) and other heart defects in which muscle fibers and collagen networks are disrupted are loss of myocardial elasticity, compliance and pumping action [3]. Current myocardial regeneration strategies, while promising [4], are unable to recreate the robust mechanical and contractile properties of normal heart muscle. In particular, an effective graft for myocardial repair is a critical unmet need, where combining elasticity and strength without compromising heart cell viability and contractility have proved challenging [5–7].
In the prototypical tissue engineering approach, three-dimensional (3D) scaffolds provide the delivery vehicle for transplanting large numbers of viable cells toward a goal of tissue regeneration [8,9]. Numerous 3D biomaterials have been explored as cardiac tissue engineering scaffolds, including non-woven poly(glycolic acid) (PGA) mesh [10-12], collagen gel [13,14], collagen foam [15–19], alginate foam [20,21], chitosan foam [22], knitted poly(lactic acid) [23], knitted hyaluronan ester [24], poly-4-hydroxybutyrate foam [25], poly(lactic acid)/poly(glycolic-co-lactic acid) (PLLA/PLGA) foam [26], and composites of natural and synthetic polymers [27]. However, these scaffolds are either thermoplastic polymers, which tend to be stiffer than normal soft tissues, degrade by bulk hydrolysis, and fail under long-term cyclic loading [28], or naturally occurring materials with intrinsic variability, immunogenicity, and mechanical strength concerns [29]. Langer and colleagues [30] developed a tough bioresorbable elastomer, poly(glycerol-sebacate) (PGS), that degraded predominately by surface hydrolysis [31] and has been tested in various tissue engineering applications [32–34] including myocardial repair. The mechanical properties of the PGS elastomer, both in the context of non-porous membranes [7,35,36] and porous scaffolds [37,38], could be tailored to match those of normal heart muscle. Recently, one-layered (1L) PGS scaffolds with in-plane pore anisotropy, i.e., rectangular and accordion-like honeycomb pores produced by laser microablation of ~250 μm thick PGS membranes [37], were shown to guide the alignment of cultured neonatal rat heart cells [37] and C2C12 myoblasts [39].
Alternatives to the cell-scaffold paradigm include “scaffold-free” approaches based on transplanting cell-cell or cell-ECM grafts. As examples, engraftment and vascularization were demonstrated for heart cell patches comprised of human embryonic stem cell-derived cardiomyocytes, endothelial cells, and fibroblasts [40] and electrical and vascular integration were demonstrated after implantation of thin (~100 μm) heart cell sheets comprised of interconnected cardiomyocytes [41]. However, scalability remains a major limitation of scaffold-free approaches [9,13,42,43]. Other alternative approaches include “cell-free” biomaterials for myocardial repair. However, biomaterials used for congenital heart defect repair in pediatric patients are limited by lack of potential for growth and remodeling [44,45], and although cell-free, non-porous PGS membranes were recently shown to reduce postinfarction myocardial hypertrophy in rodents, these implants could not assist contractile function, suggesting a role for cell-PGS implants in future approaches [35].
In the present study, multi-layered elastomeric PGS scaffolds with controlled pore microarchitectures were fabricated and combined with heart cells to engineer contractile cardiac muscle constructs in vitro. Excitation threshold, gene expression, and cardiac specific marker proteins were assessed under different conditions of cell seeding and cultivation, in particular scaffold coating with laminin (LN) to promote heart cell attachment [11,38,46] and interstitial perfusion to promote heart cell viability [12,18–20,47].
Methods
Figure 1 provides an overview of methods used to microfabricate and demonstrate the multi-layered PGS scaffold.
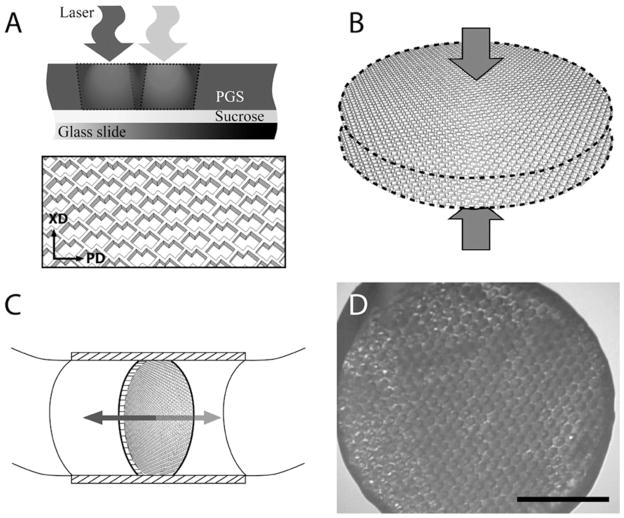
Figure 1.
Method. (A-C) PGS membranes were (A) laser microablated to make one-layered scaffolds with accordion-like honeycomb pores, (B) stacked and laminated to produce two-layered scaffolds, and (C) seeded with heart cells and cultured with bi-directional interstitial perfusion. (D) Representative phase contrast micrograph of a 7-day construct, scale bar: 2 mm.
PGS scaffold fabrication
The PGS pre-polymer was prepared by adapting the methods of Wang et al [30]. In brief, poly-condensation of 0.1 mole each of glycerol (Aldrich, Milwaukee, WI) and sebacic acid (Aldrich) at 120ºC for 64 h under vacuum [48] and stored at room temperature in a desiccation chamber. Sucrose-coated glass slides (25 mm × 45 mm, Hugh Courtright, Monee, Chicago, IL) were prepared by oxygen plasma treatment (100 W for 30 s) using a Plasma Asher (PX-250, March Plasma Systems, Concord, CA), coated with sucrose (90%) (w/v), spun at 1,000 rpm for 45 s using a spray coater/spinner (EVG101, EVG, Tempe, AZ) and dried at 92°C for 2 min. The PGS pre-polymer (0.25 g) was melted on the sucrose-coated glass slides at 160ºC using a hot plate (VWR, West Chester, PA) and cured at 120ºC for 48 h at 10 mTorr in a vacuum oven (VWR) [48]. The PGS membranes were microablated using a frequency quintupled 213 nm Nd:YAG laser (LSX-213, CETAC Technologies, Omaha, NE) [39], incubated in deionized water at 60ºC for 18 h to detach scaffolds from the slides, soaked in 70% ethanol for 18 h to extract un-reacted monomers and rehydrated in de-ionized water for 18 h (Fig. 1A). One-layered (1L) discoid scaffolds (6 mm diameter by ~250 μm thick) were prepared using a dermal punch (Acuderm, Fort Lauderdale, FL). Two-layered (2L) discoid scaffolds (6 mm diameter by ~500 μm thick) were prepared by oxygen plasma treatment of 1L scaffolds (100 W for 30 s, using the Plasma Asher), stacking off-set of in-plane pore structures, and compression with a 50 g weight for 18 h, all at room temperature (Fig. 1B). Prior to cell seeding, scaffolds were autoclave sterilized at 121ºC for 20 min and incubated in culture medium containing 10% fetal bovine serum at 37ºC for 24 h.
Cardiac construct preparation and culture
All studies involving experimental animals were performed according to a protocol approved by an Institute Committee on Animal Care. Heart cells were obtained from 1–3 day old neonatal Sprague Dawley rats (8 studies totaling 100 rat pups). In brief, the ventricles were harvested, minced into ~1 mm3 pieces, and serially digested using trypsin and collagenase [10]. The freshly dissociated heart cells were plated in T-flasks, the cells that rapidly attached to the flasks were discarded, and the cells that remained unattached after 1 h of pre-plating were used to prepare constructs. This pre-plating method was previously demonstrated to yield a mixture of 63 ± 2 % cardiomyocytes, 33 ± 3 % cardiac fibroblasts, 3 % to 4 % of smooth muscle cells, and 2 % to 3% of endothelial cells [49].
Four groups of constructs were prepared using either 1L or 2L scaffolds: (i) Matrigel (M) seeding-Static culture, (ii) LN seeding-Static culture, (iii) M seeding-Bioreactor culture, and (iv) LN seeding-Bioreactor culture. In groups (i and iii), a mixture of eight million cells and 20 μL of M (BD Biosciences, catalog number 354230, San Jose, CA) was seeded onto each scaffold while working at 4ºC in a 12-well plate, and constructs were incubated at 37ºC to promote M gelation [17]. In groups (ii and iv), scaffolds were first LN-coated by incubation in 50 μg/mL of LN (BD Biosciences) at room temperature for 4 h. Eight million cells suspended in 20 μL of culture medium were then seeded onto each scaffold in a 24-well insert transwell (BD Falcon, catalog number 353096, Bedford, MA), 0.5 mL of medium were added to the bottom well, and constructs were incubated at 37ºC to promote cell attachment. In groups (i and ii), constructs were cultured statically in 12-well plates. In groups (iii and iv) constructs were cultured with bidirectional interstitial perfusion using an oscillating bioreactor [19,50]. In brief, each discoid specimen was fixed in a cylindrical specimen chamber (8 mm long, 6 mm diameter) within a loop of silicone rubber tubing (Tygon 3350, 1/4 inch diameter × 1/32 inch wall, Cole Parmer, Vernon Hills, IL). Up to 12 loops, each containing one construct and 10 mL of media, were mounted on an incubator–compatible base that slowly oscillated the chamber about its central axes at 0.05 revolutions per minute, which corresponded to a linear interstitial flow velocity of 0.2 mm/s (Fig. 1C) [19]. Culture medium was completely replaced on culture day 4, and constructs were harvested on culture day 7. A phase contrast micrograph of a representative construct, 6 mm diameter × 0.5 mm thick, is shown at low magnification in Fig. 1D.
Mechanical testing
PGS scaffolds and specimens of adult rat left ventricular myocardium (LV) were tested as previously described [37]. In brief, the PGS scaffolds, which were dry when tested, were glued to a manila paper frame that was fixed between stainless steel grips (n=4 per test direction, 5 mm gauge length, 3 mm width), while the LV specimens, which were wet when tested, were fixed directly between steel grips (n=9 per test direction, 5 mm gauge length, 4 mm width). Specimen thicknesses were measured using a dial gauge (accuracy 0.01 mm; L.S. Starrett Co., Athol, MA). Specimens were mounted on an Electroforce ELF 3200 mechanical tester (Bose-Enduratec, Framingham, MA) controlled by WinTest software and fitted with a 250 g load cell (model 31-1435-03; Sensotech, Inc., Columbus, OH). Specimens were strained to failure at a rate of approximately 1 percent per second (0.1 V/s). Independent specimens were tested in two different directions: a preferred direction (PD), where stretch was applied along the long pore axis of the scaffold or the circumferential axis of the heart in the LV group, and an orthogonal cross-preferred direction (XD), where stretch was applied along the orthogonal scaffold pore axis or the longitudinal (apex-to-base) axis of the heart in the LV group [37]. Effective stiffness (E) was determined by a linear regression within the initial linear region of the curve up to a strain of 0.1. Ultimate tensile strength (UTS) and strain-to-failure (εf) were taken as the maximum stress and strain measured prior to the onset of failure, respectively.
Electrophysiology
Construct response to electrical field stimulation was assessed as previously described [19]. In brief, specimens (4 to 7 specimens per group) were placed in environmentally controlled test chambers fitted with two ¼-inch diameter carbon rod electrodes (Ladd Research, Williston, VT) separated by 1.5 cm and connected to platinum wire leads. An electrical pulse generator (Astro-Med Inc. West Warwick, RI) was used to apply monophasic square pulses at 1 Hz, and excitation threshold (ET) was determined by incrementally increasing the voltage until each stimulus was followed by a synchronous contraction of the construct, as observed at 10X magnification using a Nikon Diaphot microscope.
Scanning electron microscopy (SEM)
Representative scaffolds and constructs were gradually dehydrated in a series of ethanol solutions (35, 50, 70, 80, 90 and 100%) and then completely dried with hexamethyldisilazane solution. The dehydrated specimens were sputter-coated with Au-Pd alloy using a 108auto Sputter Coater (Cressington Scientific Instruments, Watford, UK) and examined using a Hitachi S3500 SEM (Hitachi High Technologies America, Pleasanton, CA). Cellular dehydration during processing for SEM made it difficult to evaluate cell morphology.
Confocal and light microscopy
Specimens (2 or 3 specimens per group) were fixed in 10% neutral buffered formalin for 24 h. Specimens for confocal microscopy were permeabilized using 0.1% Triton X-100, blocked with 0.1% bovine serum albumin, stained with phalloidin-fluorescein isothiocyanate conjugate (Sigma, St. Louis, MO), counterstained with DRAQ5 nuclear stain (Axxora LLC, San Diego, CA), and examined using a Zeiss LSM 510 laser scanning confocal microscope as previously described [37]. Other specimens for histological analyses were paraffin-embedded and sectioned to 5 μm thickness, stained, and examined using a Zeiss Axiovert 200M microscope [19]. Apoptosis was assessed by the terminal deoxynucleotidyl transferase biotin 2’-deoxyuridine 5’-triphosphate nick end labeling (TUNEL) assay [19]. Cardiac troponin-I (Tn-I) and connexin-43 (Cx-43) were assessed by incubation with an appropriate primary antibody (AB1627 for Tn-I; 05-763 for Cx-43, Millipore, Billerica, MA) and secondary antibody, stained with a Standard Elite ABC kit (Vector, Peterborough, UK), and counterstained with hematoxylin [19].
Real time polymerase chain reaction (PCR) and deoxyribonucleic acid (DNA) assays
To quantify construct gene expression levels, real time PCR analyses were performed using Taqman® Universal PCR Master Mix (Applied Biosystems, Foster City, CA). In brief, total RNA was isolated (10 specimens per group) by homogenization in Trizol (Invitrogen, Carlsbad, CA) followed by extraction in chloroform and centrifugation (20,800 g, 4°C, 20 min). The RNA was precipitated using a RNeasy Mini kit (Qiagen, Valencia, CA). The cDNA was synthesized by reverse transcription using the Superscript First-Strand Synthesis System (Invitrogen) with a PCR Sprint Thermal cycler (Thermo Electron Co., Waltham, MA). The gene-specific primers for Cx-43, matrix metalloprotease-2 (MMP-2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were respectively Rn 01433957_s1, Rn 02532334_s1, and Rn99999916_s1 (Assays-on demand ™ products, Applied Biosystems). Gene expression levels were normalized to GAPDH and then relative expression was calculated. Construct DNA content was assessed using the Quant-iT Picogreen dsDNA assay kit (Invitrogen, Carlsbad, CA).
Statistical analysis
Data were calculated as mean ± standard error and analyzed by analysis of variance in conjunction with Tukey’s post hoc test using Statistica (Tulsa, OK). Statistical significance was established at P values < 0.05.
Results
Scaffold microfabrication
To produce 1L scaffolds with accordion-like honeycomb pores in PGS membranes, we adapted our previously described method [37] for use with a frequency quintupled 213 nm Nd:YAG laser. A program was written in Visual Basic for Applications to generate sequences of coordinates and laser parameters, and suitably formatted for uploading into the software controlling this laser (DigiLaz II, v.3.1.0; CETAC Technologies) such that a specified in-plane pore microarchitecture could be automatically created in a specified number of rows and columns. By using this laser’s standard square aperture and demagnification objective lens and focusing on the top surface of the PGS membrane, the spot size was 150 μm × 150 μm. Operating parameters selected for laser microablation of 250 μm thick PGS membranes were 100% energy (4.3 mJ), 20 Hz, and 150 shots per hole. By overlapping two square pores oriented at 45 degrees, 1L scaffolds were produced with accordion-like pores that extended from the top to the bottom of the PGS membrane (Fig. 1A). The pores were on average ~250 μm long × 150 μm wide and the intervening structural elements (i.e., struts) were on average ~40 μm wide (Fig. 2A).
Figure 2.
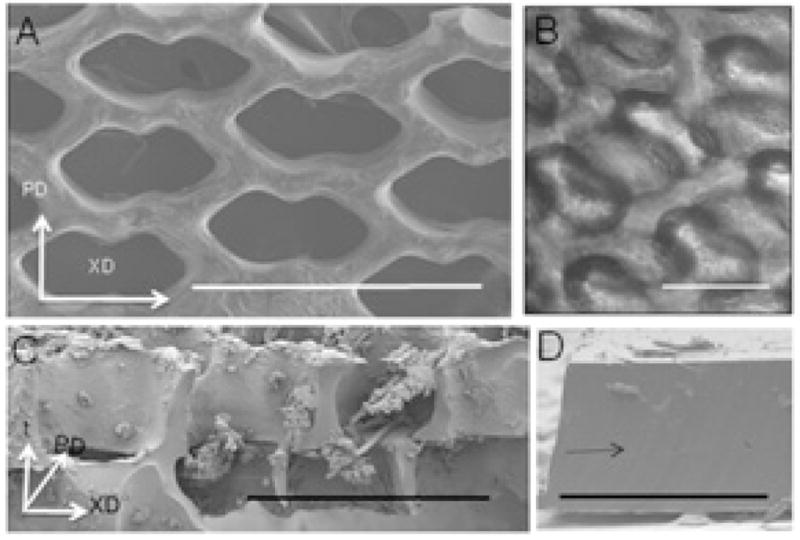
(A) One-layered scaffold, viewed from above by SEM, showing pore design. (B,C) Two-layered scaffolds seeded with cells, viewed either from above by phase contrast microscopy (B) and in cross-section by SEM (C), showing that the off-set pore structure allows cell penetration, and that cell separation from the polymer is not present by phase contrast microscopy but occurs during processing for SEM. (D) Two laminated PGS membranes showing tight bonding between layers following oxygen plasma treatment. Scaffold axes (A–C) are PD, XD, t. (D) Arrow points to region of PGS bonding. Scale bars: (A,C) 500 μm; (B) 200 μm; (D) 1 mm.
To produce two-layered (2L) scaffolds, we adapted a technique previously used for adhesive bonding of non-degradable materials [51,52] to the biodegradable PGS elastomer. Prefabricated 1L scaffolds were oxygen plasma treated (100 W for 30 s), stacked such that the pores and struts in each layer were off-set, and laminated with compression, all at room temperature. The resulting 2L scaffolds had in-plane accordion-like honeycomb pores and off-set, interconnected pores extending from the top to the bottom surface of the composite scaffold (Fig. 1B and Fig. 2B). Oxygen plasma-mediated lamination yielded a tight interface between the two bonded PGS layers that could not be distinguished by SEM (Fig. 2C,D) and was not abolished by subsequent processing steps including ethanol washing.
Effects of scaffold pore microarchitecture on heart cell seeding and culture
The main differences in pore structure between 1L scaffolds (~250 μm thick) and 2L scaffolds (~500 μm thick) was the presence of a fully interconnected 3D pore network with lateral off-set between lamina in the 2L scaffolds. This 3D pore microarchitecture allowed cells to be readily seeded throughout its full thickness (Fig. 2C) and also allowed mass transport to and from centrally located cells by interstitial perfusion (Fig. 1C). The 1L and 2L scaffolds were mechanically stable over 7 days of culture with heart cells under static and perfusion conditions. Harvested constructs exhibited good handling properties and light microscopy showed no evidence of delamination or PGS degradation over the relatively short (i.e. 7 day) culture period.
Scaffold mechanical characterization
Baseline mechanical properties of 1L PGS scaffolds and specimens of normal adult rat LV are shown in Fig. 3. Scaffolds exhibited values for E ranging from 220 to 290 kPa, UTS ranging from 200 to 225 kPa, and εf ranging from 1.5 to 1.8 (Fig. 3A, B, C, respectively). Differences in mechanical properties due to specimen type (i.e., scaffold versus LV) were statistically significant for E (p<0.0001), UTS (p<0.0001) and εf (p<0.0001), such that scaffolds were stiffer than normal LV and failed at stress values and strain values higher than normal LV. Differences in mechanical properties due to test direction (i.e., PD versus XD) were statistically significant for E (p<0.01) and UTS (p<0.05), indicating a trend toward scaffold anisotropy.
Figure 3.
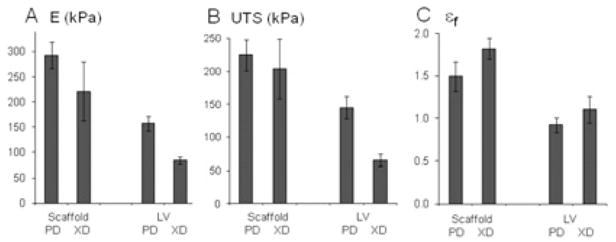
Mechanical properties of one-layered scaffolds (Scaff) and specimens of normal adult rat left ventricle (LV): (A) effective stiffnesses (E), (B) ultimate tensile strengths (UTS); (C) strains-to-failure (εf). Mechanical tests were done in two orthogonal directions (PD,XD). There were significant effects of specimen type on E (p<0.0001), UTS (p<0.0001), and εf (p<0.0001), and test direction on E (p<0.0001) and UTS (p<0.05). Data are the average ± SEM of n= 4 scaffolds and n=9 ventricles.
Effects of culture methods on excitation threshold and cell viability
The 7-day constructs based on 1L and 2L scaffolds from all groups yielded constructs that contracted in response to electrical field stimulation and could be paced at frequencies of 1 to 2 Hz. Significant interactive effects of cell seeding method (i.e., LN versus M) and cell culture method (i.e., Bioreactor versus Static) were observed (p=0.04 for 1L constructs, Fig. 4G; p=0.013 for 2L constructs, Fig. 4H). Consequently, measured values of ET were lowest for the LN-Static group, intermediate for LN-Bioreactor and M-Bioreactor groups, and highest for the M-Static group. For 1L constructs, the cells in the M-Static group appeared rounded (Fig. 4A), while cells in the LN-Static group appeared more spread and contained more actin (Fig. 4B). For 2L constructs, most cells in the M-Static group (Fig. 4C) were apoptotic by TUNEL assay, while cells in the LN-Static (Fig. 4D), M-Bioreactor (Fig. 4E), and LN-Bioreactor (Fig. 4F) groups appeared more viable.
Figure 4.
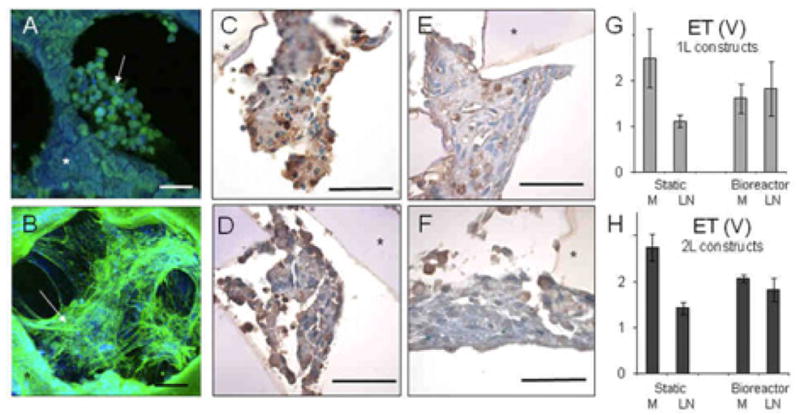
Effects of scale (1L or 2L), seeding method (M or LN), and culture method (static or bioreactor) on cell appearance and excitation threshold. (A,B) 1L constructs seeded using (A) M or (B) LN and cultured statically shown by confocal immunofluorescence microscopy after actin-phalloidin staining; arrows point to cells; asterisk indicates the scaffold. Scale bars: 50 μm. (C-F) 2L constructs based seeded using (C,D) M or (E,F) LN and cultured (C,E) statically or (D,F) with perfusion shown by light microscopy after histological sectioning and TUNEL staining; apoptotic cells appear brown; asterisk indicates the scaffold. Scale bars: 50 μm. (G,H) Excitation thresholds (ET) measured for (G) 1L and (H) 2L constructs seeded using M or LN and cultured statically or with perfusion showing significant interactive effects of seeding and culture methods (p=0.04 for 1L; p=0.013 for 2L). Data are the average ± SEM of n=4 one-layered and n=6 two-layered constructs.
Effects of culture methods on gene expression and presence of cardiac specific proteins
Gene expression for Cx-43, a gap junctional protein, depended significantly (p<0.01) on the culture method such that Cx-43 was higher in bioreactor cultures (M-Bioreactor and LN-Bioreactor) compared to static controls (M-Static and LN-Static), with an interactive effect of seeding and culture methods (p<0.05) (Fig. 5A). Likewise, gene expression for MMP-2, a gelatinase associated with extracellular matrix (ECM) remodeling, depended significantly (p<0.001) on culture method and was higher in bioreactor cultures than static controls (Fig. 5B). The presence of Cx-43 (Fig. 6A,C) and cardiac Tn-I, a subunit of the sarcomeric contractile apparatus (Fig. 6B,D) were demonstrated in 2L constructs from the LN-Static and LN-Bioreactor groups by immunohistochemistry, a qualitative assay. Consistent with gene expression data, immunostaining for Cx-43 appeared more intense in bioreactor cultures compared to static controls (Fig. 6C versus Fig. 6A). Likewise, immunostaining for Tn-I appeared more intense in bioreactor cultures than static controls (Fig. 6D versus Fig. 6B). The DNA contents of 7-day constructs were similar regardless of the cell seeding and culture method, suggesting similar cell density in all experimental groups (data not shown).
Figure 5.

Effects of culture methods on gene expression in 2L constructs. Real time PCR data for (A) Connexin-43, showing a significant increase due to perfusion (p<0.01) and an interactive effect of seeding and culture methods (p<0.05) and (B) matrix metalloprotease-2, showing a significant increase due to perfusion (p<0.001). Data are the average ± SEM of n=10-13 constructs.
Figure 6.
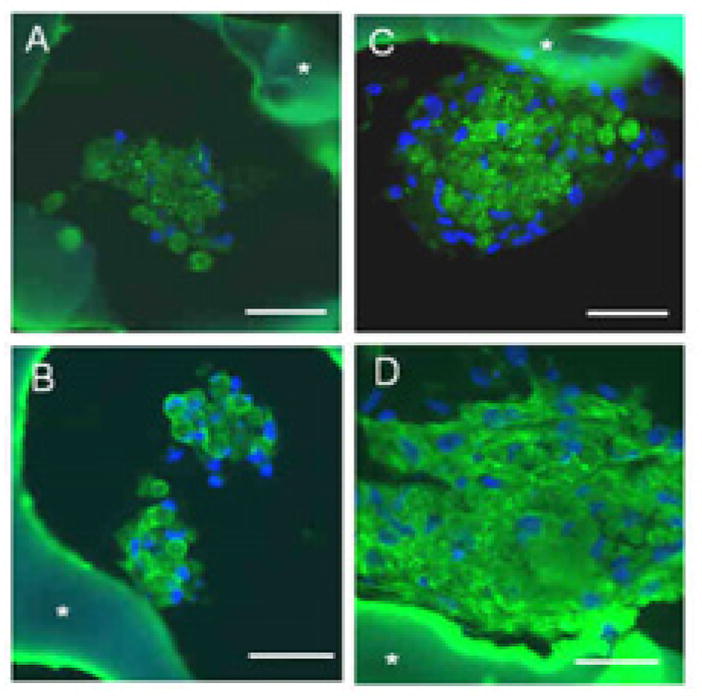
Effects of culture methods on presence of cardiac marker proteins in 2L constructs. Constructs based on LN-coated scaffolds and cultured for 7 days either statically (A,B) or in bioreactors (C,D) were assessed histologically by (A,C) immunostaining for connexin-43, which appears as punctuate dots, and (B,D) immunostaining for cardiac troponin-I; asterisk shows autofluorescent scaffold. Scale bars: 50 μm.
Discussion
Tissue engineered cardiac muscle remains challenged by cell sourcing, mass transport, and scaffold limitations [4,7]. Recent advances in PGS microfabrication have permitted the design of biodegradable, elastomeric scaffolds with precisely defined pore microarchitectures amenable to both cardiomyogenesis and predictive mathematical modeling. Toward scaling-up our previous 1L accordion-like honeycomb PGS scaffolds for cardiac tissue engineering [37], laser microablated PGS membranes were stacked and bonded to produce a fully three dimensional, mechanically stable pore architecture.
Precise control over structural features distinguished this scaffold from many previous scaffolds fabricated by freeze-drying [15,17–21,53], porogen-leaching [25,26,34,54] and textile manufacturing [10–12,23,24] processes. A relatively simple bench-top laser microablation system was used herein to fabricate 1L scaffolds with in-plane accordion-like honeycomb pores in a highly compliant elastomer (Fig. 2A). This solid-state Nd:YAG laser did not require a gaseous lasing medium, in contrast to the excimer laser we used previously which required hazardous fluorine and krypton [37]. A scalable, room temperature process was used herein to produce 2L scaffolds that involved stacking and bonding of pre-fabricated 1L scaffolds with offset lamina, yielding in-plane accordion-like honeycomb pores and off-set, interconnected pores from the top to the bottom surface. In particular, surface treatment by activated gas plasma, a method used previously for adhesive bonding [51] and for prototyping of microfluidic systems in poly(dimethylsiloxane) [52], was used herein to fabricate the multi-layered tissue engineering scaffolds.
In principle, this technique can be used to produce multi-layered elastomeric scaffolds with other in-plane pore designs and any number of individual layers, in contrast to the method we explored previously to produce a bilaminar scaffold [37]. In our previous trial, one PGS membrane with an in-plane array of continuous channels was combined with a second PGS membrane and the composite was laser microablated to produce square pores from the top to the bottom surface and then stabilized by thermal cross-linking. However the previous approach had two disadvantages: first, it was not readily scalable to scaffolds more than a few hundred micrometers in thickness due to pore tapering associated with laser drilling [55] and second, it relied on elevated temperature to effect PGS bonding, which can increase PGS stiffness [54] thereby making it more difficult to target specific scaffold mechanical properties.
In the present report, values measured for UTS, εf and E for the PGS scaffolds were directly compared to control specimens of myocardium harvested from normal adult rat LV (Fig. 3). Scaffolds exhibited values for UTS and εf, and E values of 220 to 290 kPa, that were higher than corresponding values measured for rat LV, suggesting that these scaffolds may be applicable to myocardial repair applications requiring mechanical support, i.e., compliance to permit passive diastolic relaxation and robust elasticity to withstand cyclic loading. Of note, Stuckey et al [35] recently tested the hypothesis that cell-free solid PGS membranes implanted post-MI could have a mechanotherapeutic effect by temporarily freeing the injured area of stress. Specifically, the investigators designed PGS membranes to match a “structural modulus” (i.e., a membrane tensile modulus) of the normal rat heart wall (E of 300 kPa; thickness of 0.39 mm), implanted these membranes as epicardial patches in adult rats post-MI, and demonstrated a reduction in post-infarction myocardial hypertrophy, in contrast to mechanically mismatched materials that were tested as controls [35]. However, these PGS membranes did not improve contractile function post-MI, suggesting that cell-PGS combinations might further improve myocardial repair [35].
The 3D pore microarchitecture of the multi-layered elastomeric scaffolds allowed heart cells to be readily seeded throughout the full thickness (Fig. 2C) and, in bioreactor groups, permitted perfusion-enhanced convective transport to cells located at the construct center (Fig. 1C). Scaffolds were mechanically stable over 7 days of in vitro cell culture, consistent with our previous report in which mechanical properties were quantified under various conditions including cyclic mechanical stretch [37]. Previous in vivo studies showed that PGS degraded by surface hydrolysis with a gradual, linear decrease in mechanical strength and preserved geometry [31,56,57]. Further studies of scaffold mechanical stability are required, since PGS is being proposed as part of a tissue engineering strategy for myocardial regeneration where it will be critically important to consider the rate of scaffold degradation vis-à-vis the rate of tissue remodeling originating from transplanted cells and from the host.
In the present work, different cell seeding and cell culture methods were compared in four experimental groups (M-Static, LN-Static, M-Bioreactor, LN-Bioreactor), whereas in our previous study [37] cells were seeded in mixed culture tubes and thereafter cultured statically. In the present work, histomolecular characterizations included quantifying gene expression and special histological staining for apoptosis and cardiac specific marker proteins, whereas our previous study [37] included only staining for actin and for general histological appearance. In the present work, imaging of cells within the multi-layered constructs proved difficult. Specifically, in SEM and some histological sections, the cells were separated from the PGS, presumably due to a dehydration artifact wherein shrinkage of the cell mass exceeded that of the PGS. To address this limitation, frozen sectioning and hard resin embedding are currently being explored.
Interstitial perfusion increased cardiac gene expression in association with increased cardiac protein and improved heart cell viability, as demonstrated by direct comparison of 2L constructs cultured in bioreactors with static controls. Specifically, perfusion significantly (p<0.01) increased expression of Cx-43 gene (Fig. 5A), in association with qualitative observations of increased Cx-43 (Fig. 6C versus Fig. 6A) and Tn-I (Fig. 6D versus Fig. 6B) proteins, and reduced staining for apoptosis (Fig. 4E,F versus Fig. 4C,D). Likewise, other studies of cells cultured on different biomaterial scaffolds [12,18-21,47] showed that interstitial perfusion improved cardiomyocyte differentiation and viability. Perfusion was presumed to mediate expression of contractile and gap junctional proteins via mechanotransduction, although the precise mechanisms were not well understood. In one study, changes in cardiomyocyte gene expression were associated with fluid shear stress exerted by the interstitial flow [20]; in other studies, changes in cardiomyocyte differentiation were associated with cyclic mechanical stretch [16,58,59] and electrical stimulation [53,60]. In the present study, perfusion significantly (p<0.001) increased MMP-2 gene expression as compared to statically cultured controls. Likewise, others previously related increased MMP expression with fluid shear stress and interstitial flow [61,62], and we reported increased cell-to-cell network formation in association with increased MMP-2 expression in engineered cardiac tissue [63]. Perfusion was presumed to improve cell survival by enhanced convective transport of oxygen, since hypoxia induces apoptosis in cardiomyocytes [64]; of note the bioreactor used in the present study was entirely made of gas permeable silicone rubber such that entire device served as an oxygenator [19].
There was an interactive effect of cell seeding method and cell culture method on ET and, unexpectedly, the lowest threshold was measured for the LN-Static group (Fig. 4G and Fig. 4H). This finding may be rationalized by biophysical cues introduced by either the presence of perfusion or hydrogel and acting in such a manner as to attenuate mechanical and microstructural cues provided by the PGS scaffold. Specifically, cell embedding in Matrigel, a hydrogel too soft to promote heart cell spreading [65,66] or myogenesis by stem cells [67], may have attenuated mechanical cues provided by the PGS in the M-Bioreactor and M-Static groups. Moreover, fluid shear stresses caused by interstitial cross-perfusion may have attenuated in-plane microstructural cues provided by the accordion-like honeycomb pores in the LN-Bioreactor group. Hence, this study provided new insight into why previous studies that used Matrigel or cross-perfusion [12,18–21,38,47] may not have achieved the desired heart cell differentiation. To further explore this hypothesis will require adapted in vitro models in which scaffold-based cell guidance features and biophysical cues are better integrated such that effects of these two stimuli on cell differentiation can act in concert instead of in opposition.
Ongoing work involves further optimization of laser microablation conditions, including the use of different apertures, since the dimensions (and associated volume fraction) of the PGS struts are critical determinants of scaffold structural and mechanical properties and the in-plane anisotropy recently shown to be associated with heart cell guidance [37,39]. Other efforts involve mathematical modeling and simulation of the elastomeric mechanical properties of the multi-layered elastomeric scaffolds, based on the in-plane tessellation of accordion-like honeycomb pores [68].
Conclusion
Multi-layered elastomeric PGS scaffolds with controlled pore microarchitectures were fabricated by laser ablation and oxygen plasma-mediated lamination, seeded with heart cells, and cultured with interstitial perfusion. The laser-microablated PGS exhibited UTS and εf higher than normal rat left ventricular myocardium and stiffnesses ranging from 220 to 290 kPa. Heart cell culture on these scaffolds yielded cardiac muscle constructs. Excitation thresholds were unaffected by scaffold scale up from 1L to 2L. The 2L constructs exhibited reduced apoptosis, increased expression of Cx-43 and MMP-2 genes, and qualitative increases in Cx-43 and Tn-I proteins when cultured with perfusion as compared to static controls. Together, these findings suggest that multi-layered microfabricated PGS scaffolds may be applicable to myocardial repair applications requiring mechanical support, cell delivery and active implant contractility.
Acknowledgments
This work was funded by the American Recovery and Reinvestment Act (ARRA), Award 1-R01-HL086521-01A2 (to LEF) from the National Heart, Lung and Blood Institute (NHLBI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or NIH. We are indebted to R. Langer for general advice, J. Wang and J. Hsiao for help with polymer synthesis, processing, and SEM, N. Watson for help with microscopy, M.G. Moretti and G. Talo and E. Kim for help with the bioreactor, and A. Jean for helpful discussions regarding scaffold mechanics and modeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
H. Park, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
B.L. Larson, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
M.D. Guillemette, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
S.R. Jain, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
C. Hua, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, 02139 USA
G.C. Engelmayr, Jr., Department of Bioengineering, The Pennsylvania State University, University Park, PA, 16802 USA
L.E. Freed, Biomedical Engineering Group, C.S. Draper Laboratory, Cambridge, MA, 02139 USA and MIT-Affiliated Research Scientist, Cambridge, MA 02139USA.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54:1–116. [PubMed] [Google Scholar]
- 3.Fomovsky GM, Holmes JW. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol. 2010;298:H221–8. doi: 10.1152/ajpheart.00495.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–7. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 5.Boethig D, Thies WR, Hecker H, Breymann T. Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. Eur J Cardio-Thorac Surg. 2005;27:58–66. doi: 10.1016/j.ejcts.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Bautista-Hernandez V, Marx GR, Gauvreau K, Pigula FA, Bacha EA, Mayer JE, Jr, et al. Coarctectomy reduces neoaortic arch obstruction in hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2007;133:1540–6. doi: 10.1016/j.jtcvs.2006.12.067. [DOI] [PubMed] [Google Scholar]
- 7.Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater Sci Eng R-Reports. 2008;59:1–37. [Google Scholar]
- 8.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–13. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277:H433–44. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 11.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–78. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 12.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175–88. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 13.Eschenhagen T, Zimmermann WH. Engineering myocardial tissue. Circ Res. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 15.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100:II63–9. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 16.Akhyari P, Fedak PW, Weisel RD, Lee TY, Verma S, Mickle DA, et al. Mechanical stretch regimen enhances the formation of bioengineered autologous cardiac muscle grafts. Circulation. 2002;106:I137–42. [PubMed] [Google Scholar]
- 17.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–14. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 18.Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507–16. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 19.Cheng M, Moretti M, Engelmayr GC, Freed LE. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng Part A. 2009;15:645–53. doi: 10.1089/ten.tea.2008.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dvir T, Levy O, Shachar M, Granot Y, Cohen S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007;13:2185–93. doi: 10.1089/ten.2006.0364. [DOI] [PubMed] [Google Scholar]
- 21.Dvir T, Benishti N, Shachar M, Cohen S. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Eng. 2006;12:2843–52. doi: 10.1089/ten.2006.12.2843. [DOI] [PubMed] [Google Scholar]
- 22.Blan NR, Birla RK. Design and fabrication of heart muscle using scaffold-based tissue engineering. J Biomed Mater Res A. 2008;86:195–208. doi: 10.1002/jbm.a.31642. [DOI] [PubMed] [Google Scholar]
- 23.Matsubayashi K, Fedak PW, Mickle DA, Weisel RD, Ozawa T, Li RK. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation. 2003;108(Suppl 1):II219–25. doi: 10.1161/01.cir.0000087450.34497.9a. [DOI] [PubMed] [Google Scholar]
- 24.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, et al. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 2005;11:1122–32. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Sodian R, Fu P, Luders C, Lemke T, Du J, et al. In vitro fabrication of a tissue engineered human cardiovascular patch for future use in cardiovascular surgery. Ann Thorac Surg. 2006;81:57–63. doi: 10.1016/j.athoracsur.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–25. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 27.Park H, Radisic M, Lim JO, Chang BH, Vunjak-Novakovic G. A novel composite scaffold for cardiac tissue engineering. In Vitro Cell Dev Biol Anim. 2005;41:188–96. doi: 10.1290/0411071.1. [DOI] [PubMed] [Google Scholar]
- 28.Engelmayr GC, Jr, Hildebrand DK, Sutherland FW, Mayer JE, Jr, Sacks MS. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials. 2003;24:2523–32. doi: 10.1016/s0142-9612(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 29.Feng Z, Matsumoto T, Nakamura T. Measurements of the mechanical properties of contracted collagen gels populated with rat fibroblasts or cardiomyocytes. J Artif Organs. 2003;6:192–6. doi: 10.1007/s10047-003-0230-z. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Kim YM, Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2003;66:192–7. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 32.Pritchard CD, Arner KM, Neal RA, Neeley WL, Bojo P, Bachelder E, et al. The use of surface modified poly(glycerol-co-sebacic acid) in retinal transplantation. Biomaterials. 2009;31:2153–62. doi: 10.1016/j.biomaterials.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemppainen JM, Hollister SJ. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J Biomed Mater Res A. 2010;94:9–18. doi: 10.1002/jbm.a.32653. [DOI] [PubMed] [Google Scholar]
- 34.Crapo PM, Gao J, Wang Y. Seamless tubular poly(glycerol sebacate) scaffolds: high-yield fabrication and potential applications. J Biomed Mater Res. 2008;86:354–63. doi: 10.1002/jbm.a.31598. [DOI] [PubMed] [Google Scholar]
- 35.Stuckey DJ, Ishii H, Chen QZ, Boccaccini AR, Hansen U, Carr CA, et al. Magnetic resonance imaging evaluation of remodeling by cardiac elastomeric tissue scaffold biomaterials in a rat model of myocardial infarction. Tissue Eng Part A. 2010;16:3395–402. doi: 10.1089/ten.TEA.2010.0213. [DOI] [PubMed] [Google Scholar]
- 36.Chen QZ, Bismarck A, Hansen U, Junaid S, Tran MQ, Harding SE, et al. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29:47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsano A, Maidhof R, Wan LQ, Wang Y, Gao J, Tandon N, et al. Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs. Biotechnol Progress. 2010;26:1382–90. doi: 10.1002/btpr.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guillemette MD, Park H, Hsiao JC, Jain SR, Larson BL, Langer R, et al. Combined technologies for microfabricating elastomeric cardiac tissue engineering scaffolds. Macromol Biosci. 2010;10:1330–7. doi: 10.1002/mabi.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568–73. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–12. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 42.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. Tissue Eng. 2006;12:499–507. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- 44.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 45.Delius RE, Walters HL., 3rd Re-operative surgery in pediatric patients. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003;6:108–15. doi: 10.1053/pcsu.2003.50018. [DOI] [PubMed] [Google Scholar]
- 46.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288:C30–8. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 47.Maidhof R, Marsano A, Lee EJ, Vunjak-Novakovic G. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26:565–72. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neeley WL, Redenti S, Klassen H, Tao S, Desai T, Young MJ, et al. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008;29:418–26. doi: 10.1016/j.biomaterials.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I77–8. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 50.Valonen PK, Moutos FT, Kusanagi A, Moretti MG, Diekman BO, Welter JF, et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(epsilon-caprolactone) scaffolds. Biomaterials. 2010;31:2193–200. doi: 10.1016/j.biomaterials.2009.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall JR, Westerdahl CAL, Devine AT, Bodnar MJ. Activated gas plasma surface treatment of polymers for adhesive bonding. J Appl Polym Sci. 1969;13:2085–96. [Google Scholar]
- 52.Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal Chem. 1998;70:4974–84. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- 53.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006;12:917–25. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 55.Dahotre NB, Harimkar SP. Laser fabrication and machining of materials. New York: Springer Science Business Media LLC; 2008. [Google Scholar]
- 56.Sundback CA, Shyu JY, Wang Y, Faquin WC, Langer RS, Vacanti JP, et al. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials. 2005;26:5454–64. doi: 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Pomerantseva I, Krebs N, Hart A, Neville CM, Huang AY, Sundback CA. Degradation behavior of poly(glycerol sebacate) J Biomed Mater Res A. 2009;91:1038–47. doi: 10.1002/jbm.a.32327. [DOI] [PubMed] [Google Scholar]
- 58.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. FASEB J. 2000;14:669–79. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 59.Birla RK, Huang YC, Dennis RG. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 2007;13:2239–48. doi: 10.1089/ten.2006.0359. [DOI] [PubMed] [Google Scholar]
- 60.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–73. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiol Heart Circ Physiol. 2008;295:H2087–97. doi: 10.1152/ajpheart.00281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi ZD, Ji XY, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am J Physiol Heart Circ Physiol. 2009;297:H1225–34. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nichol JW, Engelmayr GC, Jr, Cheng M, Freed LE. Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun. 2008;373:360–5. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118–25. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 65.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–60. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 66.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–87. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jean A, Engelmayr GC., Jr Finite element analysis of an accordion-like honeycomb scaffold for cardiac tissue engineering. J Biomech. 2010 doi: 10.1016/j.jbiomech.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]