Abstract
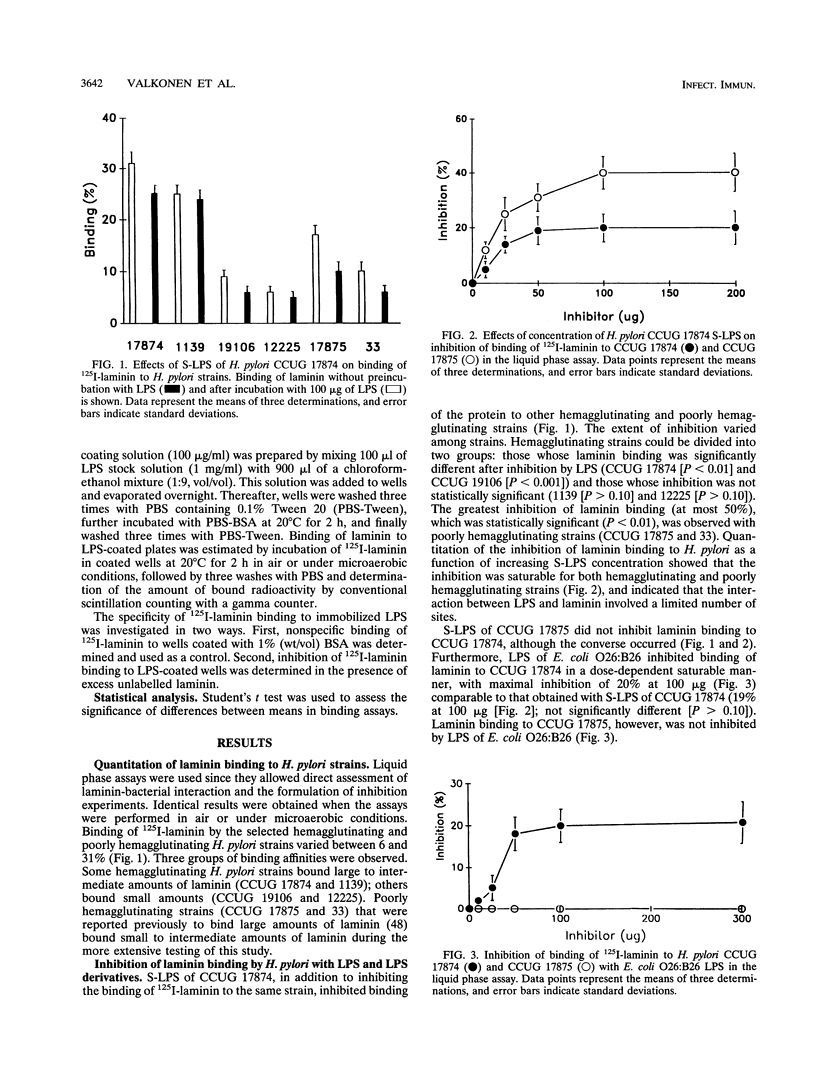
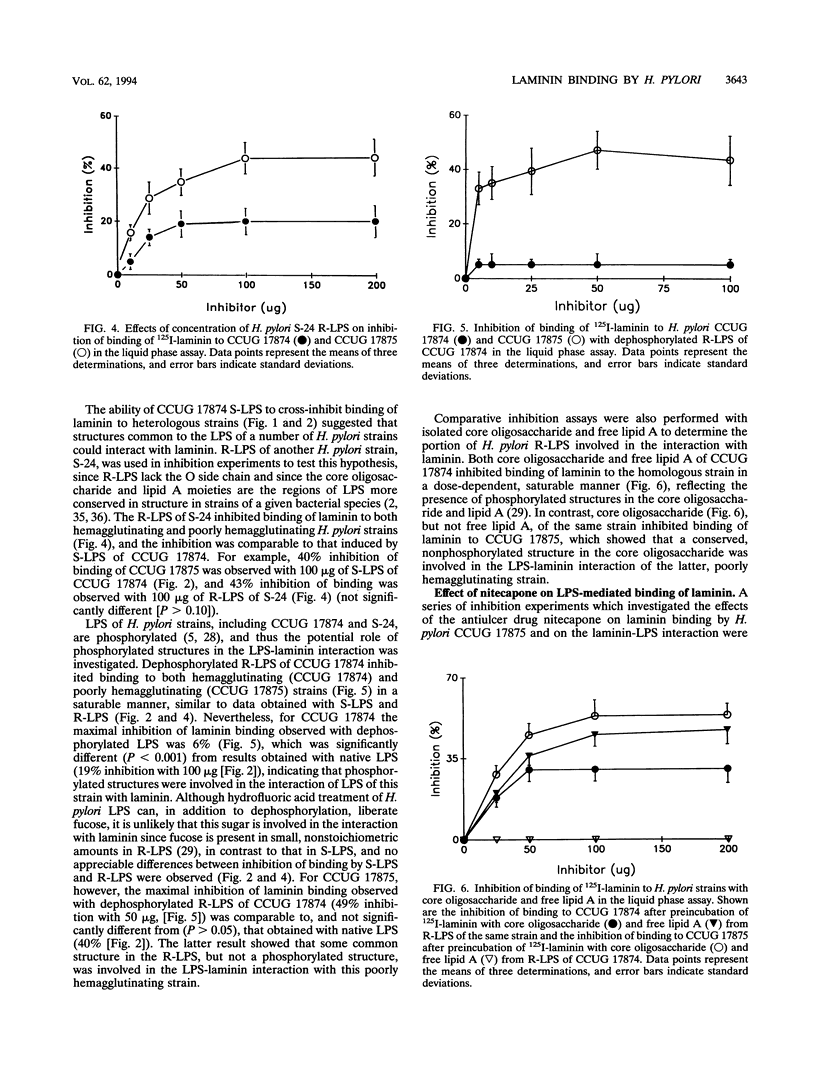
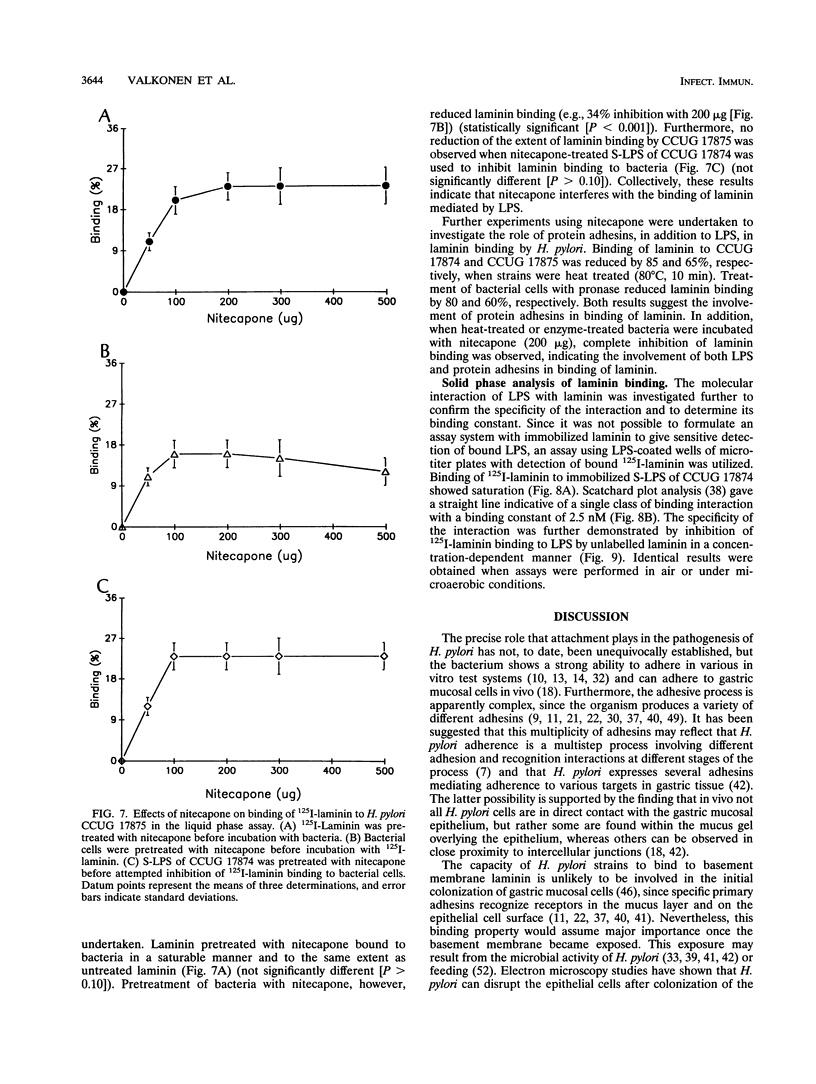
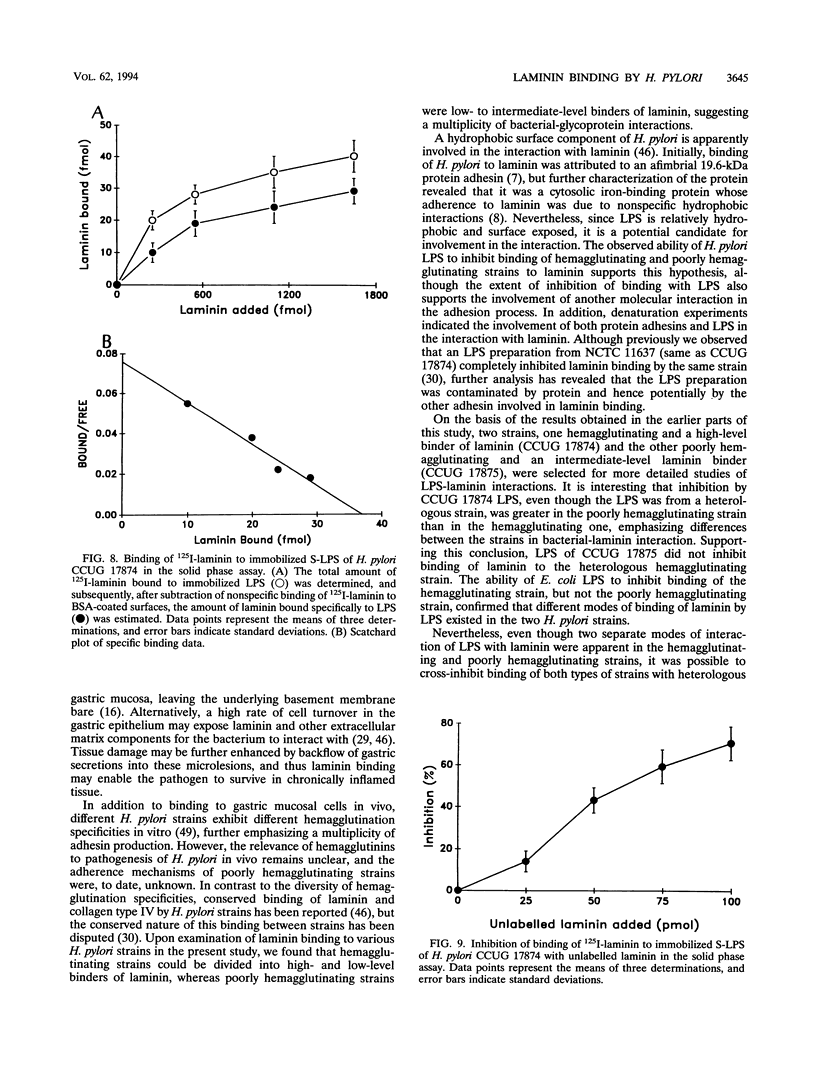
The ability of hemagglutinating and poorly hemagglutinating strains of the gastroduodenal pathogen Helicobacter pylori to bind 125I-radiolabelled laminin was quantitated in a liquid phase assay. Although all strains bound laminin, some hemagglutinating strains were good binders of laminin (maximum of 31% binding), whereas poorly hemagglutinating strains bound intermediate to small amounts of laminin (minimum of 6% binding). Since a hydrophobic component of the bacterium has been reported to be involved in binding of laminin (T. J. Trust, P. Doig, L. Emödy, Z. Kienle, T. Wadström, and P. O'Toole, Infect. Immun. 59:4398-4404, 1991), we investigated the role of lipopolysaccharide (LPS) in the interaction of both types of strains with laminin. Although the extent of inhibition varied among strains, laminin binding to hemagglutinating and poorly hemagglutinating strains was inhibited with homologous and heterologous smooth-form LPS. The ability of heterologous rough-form LPS to produce inhibition comparable to that shown by smooth-form LPS indicated that the O side chain of H. pylori LPS was not involved in the interaction. Further inhibition experiments with dephosphorylated LPS, isolated core oligosaccharide, and free lipid A suggested that a phosphorylated structure in the core oligosaccharide mediates the interaction of a hemagglutinating strain of H. pylori with laminin, whereas a conserved nonphosphorylated structure in the core oligosaccharide mediates the interaction of a poorly hemagglutinating strain. Furthermore, we showed that the interaction of H. pylori LPS with 125I-radiolabelled laminin in a solid phase assay was saturable, specific, and inhibitable with unlabelled laminin. It was postulated that the initial recognition and binding of laminin by H. pylori may occur through LPS and that subsequently a more specific interaction with a lectin-like adhesin on the bacterial surface occurs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Dubreuil D., Harel J., Girard C., Jacques M. Role of lipopolysaccharides in adherence of Actinobacillus pleuropneumoniae to porcine tracheal rings. Infect Immun. 1990 Nov;58(11):3523–3530. doi: 10.1128/iai.58.11.3523-3530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chammas R., Veiga S. S., Travassos L. R., Brentani R. R. Functionally distinct roles for glycosylation of alpha and beta integrin chains in cell-matrix interactions. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1795–1799. doi: 10.1073/pnas.90.5.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester I. R., Murray R. G. Analysis of the cell wall and lipopolysaccharide of Spirillum serpens. J Bacteriol. 1975 Dec;124(3):1168–1176. doi: 10.1128/jb.124.3.1168-1176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J. W., Waller C. A., Schirrmacher V. Identification of asparagine-linked oligosaccharides involved in tumor cell adhesion to laminin and type IV collagen. J Cell Biol. 1984 Oct;99(4 Pt 1):1416–1423. doi: 10.1083/jcb.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Austin J. W., Kostrzynska M., Trust T. J. Production of a conserved adhesin by the human gastroduodenal pathogen Helicobacter pylori. J Bacteriol. 1992 Apr;174(8):2539–2547. doi: 10.1128/jb.174.8.2539-2547.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Austin J. W., Trust T. J. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J Bacteriol. 1993 Jan;175(2):557–560. doi: 10.1128/jb.175.2.557-560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emödy L., Carlsson A., Ljungh A., Wadström T. Mannose-resistant haemagglutination by Campylobacter pylori. Scand J Infect Dis. 1988;20(3):353–354. doi: 10.3109/00365548809032466. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Graham D. Y. Receptor-mediated adherence of Campylobacter pylori to mouse Y-1 adrenal cell monolayers. Infect Immun. 1989 Aug;57(8):2272–2278. doi: 10.1128/iai.57.8.2272-2278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Moulds J. J., Graham D. Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: a putative colonization factor antigen. Infect Immun. 1988 Nov;56(11):2896–2906. doi: 10.1128/iai.56.11.2896-2906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Karjalainen T. K., Evans D. J., Jr, Graham D. Y., Lee C. H. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993 Feb;175(3):674–683. doi: 10.1128/jb.175.3.674-683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk P., Roth K. A., Borén T., Westblom T. U., Gordon J. I., Normark S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2035–2039. doi: 10.1073/pnas.90.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauchère J. L., Blaser M. J. Adherence of Helicobacter pylori cells and their surface components to HeLa cell membranes. Microb Pathog. 1990 Dec;9(6):427–439. doi: 10.1016/0882-4010(90)90061-t. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Fomsgaard A., Mitov I., Galanos C. ELISA for antibodies to lipid A, lipopolysaccharides and other hydrophobic antigens. Infection. 1989 Sep-Oct;17(5):322–328. doi: 10.1007/BF01650719. [DOI] [PubMed] [Google Scholar]
- Hazell S. L., Lee A., Brady L., Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986 Apr;153(4):658–663. doi: 10.1093/infdis/153.4.658. [DOI] [PubMed] [Google Scholar]
- Izhar M., Nuchamowitz Y., Mirelman D. Adherence of Shigella flexneri to guinea pig intestinal cells is mediated by a mucosal adhesion. Infect Immun. 1982 Mar;35(3):1110–1118. doi: 10.1128/iai.35.3.1110-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lelwala-Guruge J., Ljungh A., Wadström T. Haemagglutination patterns of Helicobacter pylori. Frequency of sialic acid-specific and non-sialic acid-specific haemagglutinins. APMIS. 1992 Oct;100(10):908–913. [PubMed] [Google Scholar]
- Lingwood C. A., Huesca M., Kuksis A. The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect Immun. 1992 Jun;60(6):2470–2474. doi: 10.1128/iai.60.6.2470-2474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A. P., Helander I. M., Kosunen T. U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992 Feb;174(4):1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman-Simha V., Mégraud F. In vitro model for Campylobacter pylori adherence properties. Infect Immun. 1988 Dec;56(12):3329–3333. doi: 10.1128/iai.56.12.3329-3333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. Helicobacter pylori and associated gastroduodenal diseases. Review article. APMIS. 1991 Aug;99(8):677–695. [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Natomi H., Zhao W. L., Okuzumi K., Sugano K., Iwamori M., Nagai Y. Identification of glycolipid receptors for Helicobacter pylori by TLC-immunostaining. FEBS Lett. 1991 May 6;282(2):385–387. doi: 10.1016/0014-5793(91)80519-9. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Rajiah G., Slomiany A. Inhibition of gastric mucosal laminin receptor by Helicobacter pylori lipopolysaccharide: effect of nitecapone. Gen Pharmacol. 1991;22(6):1063–1069. doi: 10.1016/0306-3623(91)90578-t. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Piotrowski J., Samanta A., VanHorn K., Murty V. L., Slomiany A. Campylobacter pylori colonization factor shows specificity for lactosylceramide sulfate and GM3 ganglioside. Biochem Int. 1989 Oct;19(4):929–936. [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Mechanism of Helicobacter pylori pathogenesis: focus on mucus. J Clin Gastroenterol. 1992;14 (Suppl 1):S114–S121. [PubMed] [Google Scholar]
- Speziale P., Hök M., Wadström T., Timpl R. Binding of the basement membrane protein laminin to Escherichia coli. FEBS Lett. 1982 Sep 6;146(1):55–58. doi: 10.1016/0014-5793(82)80704-7. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Trust T. J., Doig P., Emödy L., Kienle Z., Wadström T., O'Toole P. High-affinity binding of the basement membrane proteins collagen type IV and laminin to the gastric pathogen Helicobacter pylori. Infect Immun. 1991 Dec;59(12):4398–4404. doi: 10.1128/iai.59.12.4398-4404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkonen K. H., Ringner M., Ljungh A., Wadström T. High-affinity binding of laminin by Helicobacter pylori: evidence for a lectin-like interaction. FEMS Immunol Med Microbiol. 1993 Jun;7(1):29–37. doi: 10.1111/j.1574-695X.1993.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Valkonen K. H., Veijola J., Dagberg B., Uhlin B. E. Binding of basement-membrane laminin by Escherichia coli. Mol Microbiol. 1991 Sep;5(9):2133–2141. doi: 10.1111/j.1365-2958.1991.tb02143.x. [DOI] [PubMed] [Google Scholar]
- Westerlund B., Merenmies J., Rauvala H., Miettinen A., Järvinen A. K., Virkola R., Holthöfer H., Korhonen T. K. The O75X adhesin of uropathogenic Escherichia coli: receptor-active domains in the canine urinary tract and in-vitro interaction with laminin. Microb Pathog. 1987 Aug;3(2):117–127. doi: 10.1016/0882-4010(87)90070-2. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]



