Abstract
Myopathy, lactic acidosis and sideroblastic anaemia (MLASA) is a rare condition that combines early-onset myopathy with lactic acidosis and sideroblastic anaemia. MLASA has been associated with a missense mutation in pseudouridylate synthase 1 (PUS1), an enzyme located in both nucleus and mitochondria, which converts uridine into pseudouridine in several cytosolic and mitochondrial tRNA positions and increases the efficiency of protein synthesis in both compartments. We examined two Italian brothers with MLSA and sequenced the PUS1 gene. We found combined defects in mitochondrial respiratory chain complexes in muscle and fibroblast homogenates of both patients, and low levels of mtDNA translation products in fibroblast mitochondria. A novel, homozygous stop mutation was present in PUS1 (E220X). The stop mutation in PUS1 is likely to determine the loss of function of the protein, since it predicts the synthesis of a protein missing 208/427 amino acid residues on the C terminus, and was associated with low mtDNA translation.
BACKGROUND
Mitochondrial disorders are characterised by primary defects in the mitochondrial respiratory chain (RC).1 Biochemically, these disorders can affect single enzymatic activities or present as a combination of multiple RC defects. Genetically, defects in single enzymatic activities can be due to mutations in genes encoding individual subunits of each complex, or specific factors involved in their assembly and turnover. In adults, multiple defects in RC activities are often caused by mutations in the mtDNA encoded RNA products, mainly tRNAs, involved in mtDNA protein translation.1 These mutations are less frequently found in children. Conditions characterised by profound reduction in mtDNA (mtDNA depletion syndromes) account for a small fraction of infantile cases in which multiple defects in RC complexes are present in single or multiple tissues, such as muscle, liver or brain. Mutations in factors involved in mtDNA replication have been identified in some mtDNA depletion syndromes.2 However, in many cases the genetic and molecular bases of multiple RC defects remain undiagnosed. In a cohort of seven children with multiple defects in the RC complexes, we identified two brothers with a homozygous stop mutation in the gene encoding pseudouridylate synthase 1 (PUS1).3 Similar to the reported cluster of Persian-Jewish families in which the first PUS1 mutation was identified, our patients were affected by an infantile myopathy with lactic acidosis and sideroblastic anaemia (MLASA; MIM 600462).3 PUS1 is part of the truA family of tRNA pseudouridine synthases and converts uridine into pseudouridine in several tRNA positions encoded by either nuclear or mitochondrial genes, thereby acting in both cellular compartments.3 Here we report a new nonsense mutation causing MLASA in two brothers.
CASE PRESENTATION
The probands were two brothers with reportedly unrelated parents. Patient 1 was a boy born at term after an uneventful pregnancy. Body weight was 2610 g, length was 45.5 cm and head circumference was 32.2 cm. Body growth was consistently below the third centile and an arginine test failed to show increased secretion of growth hormone (GH). However, the child walked at 18 months and language and mental development were both normal. At age 6 months generalised hypotonia was noted, together with joint laxity, pseudoepicanthus and hypertelorism. At 5 years of age the patient was diagnosed with severe sideroblastic anaemia, unresponsive to vitamin B6 supplementation (haemoglobin (Hb) 5 g/dl, haematocrit 16%, mean corpuscular volume (MCV) 100 μm3, red blood cells (RBC) 1.6×106/mm3), requiring periodic blood transfusions with associated iron chelating treatment with desferoxamine. He also received GH supplementation for the correction of hypopituitarism and severe growth failure. Physical examination at 10 years of age revealed notable growth retardation: body weight was 18.7 kg (<<3rd centile), height was 125 cm (<3rd centile) and head circumference was 49 cm (−2 SD). Flat nose, hypertelorism and prominent cheek bones were attributed to bone marrow hyperplasia. The child had profound, generalised muscle hypotrophy and weakness, more pronounced in the hands, winging scapulae, hyperlordosis of the trunk and anserine gait with a frank Gower’s manoeuvre. No cerebellar or pyramidal signs were present. Extrinsic ocular motility was normal, with mild weakness of the upper eyelids. His IQ was 120.
The clinical course of patient 2 was much milder than that of his older brother. His weight at birth was 3280 g, and body growth has consistently remained at the lower normal limit. Motor and language milestones were reached at the appropriate age. However, his IQ (Leiter scale) was 85 at 6 years of age, and the visual-perception and visual-motor test results were <1st centile, with impaired general visual perception and visual-motor integration. Neurological examination showed mild generalised muscle hypotrophy, more severe in both hands, very mild weakness, and hypotonia, mainly in the lower limbs, but no Gower’s manoeuvre. The patient is currently 13 years old. He displays mild exercise intolerance and an initial ventilatory insufficiency. He has medium mental insufficiency: total IQ is now 53, verbal IQ 59, performance IQ 51 using WISC-R, Raven test 12/60, <5th centile. He has no pigmentary retinopathy.
INVESTIGATIONS
In patient 1, blood lactate at rest was 6.1 mM (normal value 0.5–2.2 mM), blood pyruvate was 0.64 mg/dl (normal value 0.36–0.59 mg/dl) and serum creatine kinase (CK) was normal. Electrocardiogram (ECG) and ultrasound examination of the heart were both normal. The child had severe restrictive ventilatory syndrome, which was attributed to failure of the respiratory muscles, including the diaphragm. Electromyogram (EMG), electroneurogram (ENG), brain magnetic resonance imaging (MRI) and abdominal ultrasound examination were normal. A muscle biopsy from the left quadriceps showed myopathic changes and the presence of ragged-red and COX negative fibres (fig 1A). The activity of the RC complexes showed a profound deficiency of complex IV and less severe deficiency of complex I in both muscle homogenate and cultured skin fibroblasts (table 1).
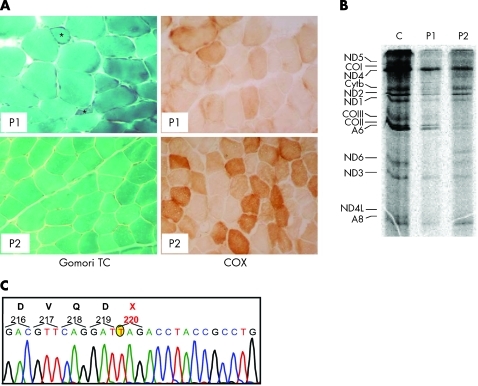
Figure 1.
Morphological, biochemical and molecular findings in patients 1 and 2. (A) Gomori trichrome (TC) and cytochrome c oxidase (COX) staining of muscle biopsies in patient 1 (P1) and patient 2 (P2). Patient 1 shows more severe abnormalities than patient 2; note the presence of ragged-red and atrophic fibres (asterisks) and diffuse reduction in COX reactivity. (B) mtDNA translation in cell culture. The bands in the autoradiograph correspond to the 13 polypeptides encoded by mtDNA genes. A, ATP synthase (complex V); CO, cytochrome c oxidase (complex IV); Cytb, cytochrome b (complex III); ND, polypeptides of NADH-CoQ reductase (complex I). (C) Electropherogram of the PUS1 automated sequence analysis in patient 1. A mutant T, which replaces a wild-type G at position 658 of the cDNA sequence, is encircled in yellow.
Table 1.
Respiratory chain (RC) activity
| Complex I* (NADH-CoQ1 red) | Complex II* (succ-CoQ1 red) | Complex III* (DBH2-cit C red) | Complex IV* (COX) | Complex V* (ATPase) | Citrate synthase† | ||
| Patient 1 | Muscle | 2.6 | 14.8 | 15 | 8.8 | 61 | 274 |
| Fibroblasts | 9.7 | 6.9 | 73 | 58 | 40 | 321 | |
| Patient 2 | Muscle | 3.7 | 16.9 | 26 | 22 | 70 | 249 |
| Fibroblasts | 6.7 | 14.4 | 110.2 | 12 | 60.6 | 136.5 | |
| Referencevalues | Muscle | 15–31 | 20–40 | 80–160 | 80–180 | 100–220 | 90–200 |
| Fibroblasts | 13–34 | 10–20 | 95–155 | 70–170 | 60–110 | 100–250 |
*Values refer to citrate synthase activity; †nmol/min/mg protein.
Patient 2 has mild sideroblastic anaemia (Hb 11.8–9.8 g/dl), and moderate elevation of blood lactate (3.9 mM) and pyruvate (1.31 mg/dl), muscle homogenate and cultured fibroblasts showed combined deficiency of complex IV and complex I. The morphological features of the muscle biopsy were similar, although less severe, than those found in patient 1.
The six exons of the PUS1 gene were screened and a homozygous G>T transversion in DNA samples from both patients was found. The parents were both heterozygous for the same mutation. Considering the A of the first ATG of the PUS1-1 ORF as nucleotide position +1, the mutation affects the G nucleotide at position 658 (658G>T). The 658G>T mutation causes the replacement of the codon for the amino acid residue E220 of isoform 1 (PUS1-1) with a stop codon (TAG). The position equivalent to the PUS1-1 E220X is E192X in the PUS1–2 isoform. For both isoforms, the mutation found in our patients predicts the synthesis of a shorter polypeptide, which encompasses approximately the N-terminal half of the wild-type protein, lacks the last 208 C-terminal amino acid residues, and is likely to be inactive. Accordingly, mtDNA-specific protein translation was reduced in fibroblasts from both patients (fig 1), which would explain the combined deficiency of complex I and complex IV found in these cells (table 1).
OUTCOME AND FOLLOW-UP
Progressive worsening of the muscle weakness and sideroblastic anaemia led to the death of patient 1 at 12 years of age from respiratory failure. Patient 2 is now 18 years old.
DISCUSSION
The haematological and neurological presentation varied in our patients, in spite of the common parental origin and identical PUS1 mutation. The mitochondrial biochemical defect was equally severe in the two patients in both muscle and fibroblasts. The mutation affecting PUS1 in these patients predicts severe damage of the protein with the likely complete loss of enzymatic activity. Variability of clinical features has been reported in other MLASA families in the past, including the presence of additional symptoms such as microcephaly, micrognathia, dysthichiasis, high philtrum, high palate, and mental retardation4,5 What is the molecular basis of this clinical heterogeneity? PUS1 has been shown to modify several tRNA positions of both nuclear and mitochondrial origin. Pseudouridylation increases the efficiency of protein translation in both cytoplasm and mitochondria. Some of the clinical features of MLASA that were found in our patients are clearly attributable to mitochondrial failure, including the myopathy with ragged red fibres. Complex II deficiency was seen in cultured fibrobasts from patient 1. Complex II is not encoded by mtDNA, which suggests that its defect could be due to either abnormal cytosolic protein synthesis or to secondary destabilisation of the entire RC within the mitochondrial inner membrane. Other abnormalities reported in some MLASA patients, such as psychiatric symptoms and facial dysmorphisms, are uncommon in mitochondrial disorders and are also likely due to abnormalities in cytosolic protein synthesis. Thus, the clinical features of MLASA may be due to failure of both cytoplasmic and mitochondrial function of PUS1.
It is clear that an important source of variability depends on the dual spatial distribution of the PUS1 protein, and its activity on two physically and functionally separated translation machineries. Regulation of the differential expression of the PUS1 isoforms may in fact play a relevant role in controlling and coordinating cytosolic versus mitochondrial translation in normal and, possibly, disease conditions. An interesting possibility is that deleterious mutations affecting different parts of PUS1 could determine the onset of clinical presentations different from typical MLASA, due to defective translation in the cytoplasmic but not in the mitochondrial compartments and vice a versa.
LEARNING POINTS
Myopathy, lactic acidosis and sideroblastic anaemia (MLASA) is a mitochondrial disorder presenting with myopathy, lactic acidosis and sideroblastic anaemia.
MLSA shows clinically significant variability in its phenotype.
PUS1 protein is alternately spliced to produce nuclear/cytoplasmic and mitochondrial isoforms.
Different PUS1 mutations may differentially affect nuclear/cytoplasmic and mitochondrial functioning, contributing to phenotypic variability in MLSA.
Acknowledgments
This article has been adapted with permission from Fernandez-Vizarra E, Berardinelli A, Valente L, Tiranti V, Zeviani M. Nonsense mutation in pseudouridylate synthase 1 (PUS1) in two brothers affected by myopathy, lactic acidosis and sideroblastic anaemia (MLASA). J Med Genet 2007;44:173–80.
Footnotes
Competing interests: none.
REFERENCES
- 1.DiMauro S, Hirano M. Mitochondrial encephalomyopathies: an update. Neuromuscul Disord 2005; 15: 276–86 [DOI] [PubMed] [Google Scholar]
- 2.Jacobs HT, Turnbull DM. Nuclear genes and mitochondrial translation: a new class of genetic disease. Trends Genet 2005; 21: 312–14 [DOI] [PubMed] [Google Scholar]
- 3.Patton JR, Bykhovskaya Y, Mengesha E, et al. Mitochondrial myopathy and sideroblastic anemia (MLASA): missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J Biol Chem 2005; 280: 19823–8 [DOI] [PubMed] [Google Scholar]
- 4.Inbal A, Avissar N, Shaklai M, et al. Myopathy, lactic acidosis, and sideroblastic anemia: a new syndrome. Am J Med Genet 1995; 55: 372–8 [DOI] [PubMed] [Google Scholar]
- 5.Zeharia A, Fischel-Ghodsian N, Casas K, et al. Mitochondrial myopathy, sideroblastic anemia, and lactic acidosis: an autosomal recessive syndrome in Persian Jews caused by a mutation in the PUS1 gene. J Child Neurol 2005; 20: 449–52 [DOI] [PubMed] [Google Scholar]