Abstract
Embolotherapy of arteriovenous malformations (AVM) is not without risk. A 28-year-old woman underwent transcatheter selective embolisation of an AVM in the cheek using polyvinyl alcohol (PVA) microparticles. She became hypoxic and hypotensive post procedure, and had repeated cardiorespiratory arrests despite aggressive support. Resistant hypoxia with gross right heart dilatation on echocardiography suggested extensive pulmonary embolism. She died 24 h later. A postmortem confirmed widespread thrombosis and PVA particles in the pulmonary microvasculature identical to that in the treated AVM. This is the first reported death from PVA particle pulmonary embolism following therapeutic embolisation of a peripheral AVM.
BACKGROUND
Arteriovenous malformation (AVM) is a tangled collection of blood vessels with abnormal direct connections between arteries and veins.1 Treatment is indicated for lesions in the brain and for those causing large shunts or disfigurement. The goal of treatment is obliteration of the vascular nidus and this is best achieved by transarterial embolisation rather than surgical excision.2 Although therapeutic embolisation requires only minimal access, it is in no way minimally invasive. We report on a fatal pulmonary embolism of polyvinyl alcohol (PVA) particles following a therapeutic embolisation of an AVM in the cheek.
CASE PRESENTATION
A 28-year-old Sri Lankan housewife, mother of a 3-year-old child, weighing 55 kg, 158 cm in height, with American Society of Anaesthesiologists status 1, underwent the second session of transarterial embolisation of an AVM in the cheek 6 months after the first treatment. Her cheek swelling of 18-years duration had become more prominent since her pregnancy 3 years previously. Except for disfigurement, the swelling was asymptomatic and soft with an overlying bruit.
INVESTIGATIONS
Routine laboratory results were unremarkable. CT angiography showed a 3×5 cm AVM in the left cheek involving the buccinator. Diagnostic angiography confirmed an AVM supplied by the maxillary artery with drainage predominantly to the facial vein.
TREATMENT
Arterial access via right femoral puncture following infiltration with 2% lignocaine was uneventful. A 5F catheter was positioned in the left external carotid artery, and through this catheter, a microcatheter (Tracker; Target Therapeutics, Fremont, California, USA) was advanced into the maxillary artery close to the nidus of the lesion. The patient was systemically heparinised during the procedure. A total of 6 ml of 300–500 μm PVA particles (Contour PVA, Boston Scientific, Fremont, California, USA) suspended in radiographic contrast were slowly injected under fluoroscopic guidance into the AVM and slowing of flow to the lesion was noted. The catheter was then pulled back and contrast was injected to confirm devascularisation of the AVM.
OUTCOME AND FOLLOW-UP
The patient remained well during the procedure and returned to the ward 1 h later. She became breathless 3 h after injection and peripheral oxygen saturation decreased to 90% despite supplementary oxygen. Transthoracic echocardiography showed a dilated right heart with gross tricuspid regurgitation with the main pulmonary artery showing no filling defect. Severe pulmonary hypertension due to particle embolism was suspected and antithrombotic treatment was initiated with unfractionated heparin (100 IU/kg).
She subsequently became hypotensive and was resuscitated from multiple cardiac arrests. Mechanical ventilatory support was insufficient to normalise arterial oxygen. The arterial blood pressure remained at 40–70 mm Hg despite maximal inotrope support with dopamine, dobutamine and norepinephrine. She then developed coagulopathy with low platelets (30 000/microlitre) and increased d-dimers (4.8g/dl), and died 24 h later.
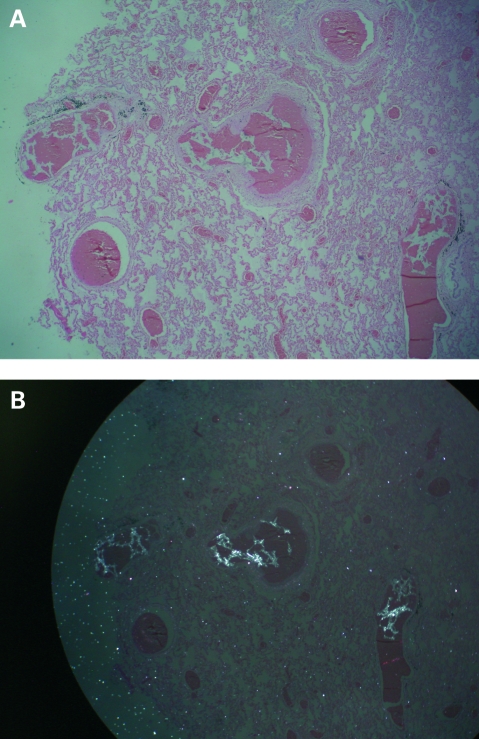
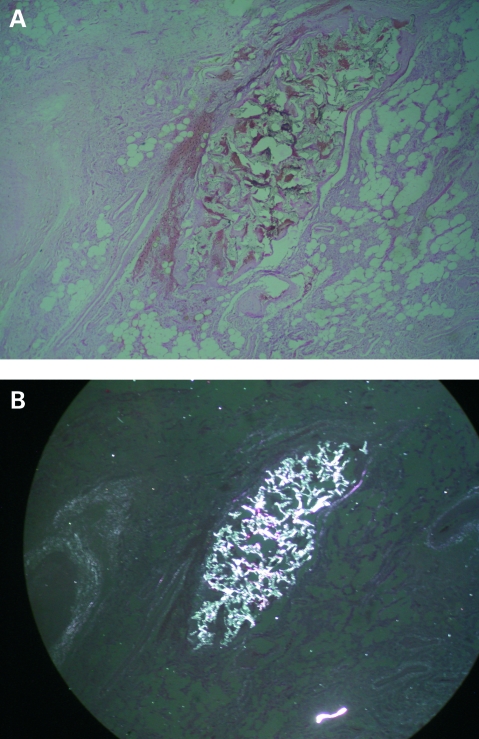
Postmortem examination showed the heart and lungs were macroscopically normal. But microscopy showed alveolar congestion and disseminated pulmonary microthrombosis (fig 1A). Foreign particles were clearly visualised under polarised light in lung tissue (fig 1B) that were identical to those in the embolised lesion in the cheek (Fig 2A,B).
Figure 1.
A. Photomicrograph of a cross-section of the lung showing extensive microthromboses (original magnification ×40). B. Photomicrograph of the same cross-section of lung visualised under polarised light showing foreign body particles among thrombi (original magnification ×40).
Figure 2.
A. Photomicrograph of a cross-section of the embolised arteriovenous malformation in the cheek showing extensive microthromboses (original magnification ×40). B. Photomicrograph of the same cross-section of the malformation visualised under polarised light showing the same foreign body particles among thrombi (original magnification ×40).
DISCUSSION
The paucity of published data on complication rates for therapeutic embolisation of peripheral AVMs points to its relative safety. Nevertheless, the manufacturer’s data sheet warns of pulmonary embolism and death unless particle size and quantity is selected appropriately.3 Although 20–25 peripheral AVMs are treated by embolisation each year at the Colombo National Hospital in Sri Lanka, this is the first case of acute pulmonary embolism and death among these patients. There is only one other report of acute pulmonary hypertension following therapeutic embolisation of a peripheral AVM in the shoulder region.4 Three deaths reported so far due to particle embolism of the pulmonary microvasculature have all been following treatment of large hepatic AVMs in children.5,6 There have also been several reports of non-fatal embolic incidents following treatment of cerebral AVMs.7,8
This report clearly illustrates that particles injected intra-arterially into the AVM nidus had passed through to the venous system and lodged in the alveolar capillaries causing extensive microthromboses. These changes explain the severe pulmonary hypertension and hypoxia that caused the patient’s death. This is the first report of death from pulmonary particle embolism following injection of particles to a peripheral AVM.
PVA is perhaps the most widely used particulate embolic agent worldwide. Its biocompatibility and clinical efficacy as a relatively permanent embolic agent is well documented.9–12 Major complications from therapeutic embolisation are due to either inadvertent forward passage or proximal reflux of injected particles, causing non-target embolism.3,13 Particles must ideally be small enough not to lodge too proximally and large enough to lodge in the nidal vasculature without passing through. Nevertheless, Kjellin et al, in a study of embolisation of cerebral AVMs in children using cyanoacrylate and platinum coils, showed that in 35% of cases studied injected material could be seen on chest x rays.8 All three reported deaths following PVA embolisation of hepatic AVMs so far have been attributed to the injection of particles that were too small.5,6 Hence proper selection of particle size seems crucial, but this is not straightforward. Firstly, angiographic assessment of the vasculature and derivation of particle size is unreliable.14 Secondly, commercially-prepared PVA particles are not homogeneous with regards to size or shape.15 The standard method for manufacturing particles explains this. A PVA sponge is ground into fine particles, dried, and separated into several size ranges using sieve sizing. Thus the advertised size is primarily a function of the particle intermediate axis, which is responsible for a given particle’s ability to pass through a square sieve hole.16 The long axes of these irregular particles are often much longer than their intermediate axes.16 Further, these PVA particles have irregular shapes, open edges and a range of pore sizes in their general structure, with the potential to fracture into smaller fragments or interlock and clump together forming larger fragments.15–18 This means that the level of embolisation is difficult to predict or control, with large aggregates causing more proximal embolisation and fractured smaller particles penetrating too far into the vascular bed, entering the venous outflow.
Conceptually, a spherical particle would overcome these problems and such products have been introduced recently.16,17 Spherical particles have been shown to fit into and fill the vessel lumen more completely and also penetrate further into lesions compared with standard irregular particles, making these procedures more controlled and predictable.19 But their ability to penetrate further into vascular malformations has resulted in concerns about an increased potential for pulmonary embolisation.20 Thus, maximal safety and efficacy during therapeutic embolisation of AVMs requires injected particles to be traceable irrespective of their size and shape. This is not possible with conventional x rays because PVA particles are not radio-opaque. Neither barium impregnation21 nor contrast suspension22 of particles has made them adequately traceable. Further, there is evidence that the flow of contrast does not necessarily correlate with the eventual embolisation site of simultaneously injected embolisation particles.22 In contrast, scintigraphic imaging of 99m technetium sulfur colloid labelled PVA particles has provided for precise localisation.23,24 In addition, selection of appropriate particle size for embolisation is based on an actual physiological challenge of the vascular integrity of the AVM. Inadvertent embolisation can be detected immediately and steps can be taken to change particle size or terminate the procedure. These techniques have been instrumental in preventing complications from inadvertent pulmonary and peripheral.23,24
Further, it has been reported that a multistep procedure decreases morbidity and increases efficacy of AVM embolisation therapy.25 However, the recruitment of collateral vessels and formation of direct arteriovenous fistulae following a patient’s first embolisation procedure can result in previously safe particles being too small and dangerous during a subsequent embolisation.25 We postulate that this might have been the case with our patient, who was undergoing her second embolisation procedure.
An unusual aspect of our case is the delay of 3 h from the therapeutic injection to onset of symptoms. We attribute this phenomenon to the fact that PVA particles perform their task by adhering to vessel walls rather than by plugging them shut, and vessel occlusion is only brought about by subsequent thrombosis.26 This was shown by the gross reduction in platelets and a rise in d-dimers in our patient.
Standard management with mechanical ventilation, inotrope support and anticoagulation proved insufficient for our patient. The report on the successful use of venoarterial extracorporeal membrane oxygenation (ECMO) in a patient similar to ours following therapeutic embolisation of an AVM in the shoulder region4 alludes to a further extension to our therapeutic armamentarium.
LEARNING POINTS
This report emphasises that therapeutic embolisation of an arteriovenous malformation (AVM), despite being a minimal access procedure, is not minimally invasive and can prove fatal.
With the increasing use of therapeutic embolisation for disfiguring benign lesions, interventionists must be acutely aware of such potential for complication and take maximal precautions.
When polyvinyl alcohol (PVA) particles are being used consider technetium sulfur colloid labelling and scintigraphic monitoring of the procedure
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Allison DJ, Kennedy A. ABC of vascular diseases. Peripheral arteriovenous malformations. BMJ 1991; 303: 1191–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SK, Do YS, Shin SW, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther 2006; 13: 527–38 [DOI] [PubMed] [Google Scholar]
- 3.Boston Scientific Manufacturers data sheet: Contour PVA Embolization Particles. http://www.bostonscientific.com/templatedata/imports/collateral/Radiology/spec_contrpva_01_us.pdf (accessed 1 June 2009) [Google Scholar]
- 4.Haller I, Kofler A, Lederer W, et al. Acute pulmonary artery embolism during transcatheter embolization: successful resuscitation with veno-arterial extracorporeal membrane oxygenation. Anesth Analg 2008; 107: 945–7 [DOI] [PubMed] [Google Scholar]
- 5.Repa I, Moradian GP, Dehner LP, et al. Mortalities associated with use of a commercial suspension of polyvinyl alcohol. Radiology 1989; 170: 395–9 [DOI] [PubMed] [Google Scholar]
- 6.Brown KT. Fatal pulmonary complications after embolization with 40–120 micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol 2004; 15:197–200 [DOI] [PubMed] [Google Scholar]
- 7.Kline JN, Ryals TJ, Galvin JR, et al. Pulmonary embolization and infarction. An iatrogenic complication of transcatheter embolization of a cerebral arteriovenous malformation with polyvinyl alcohol sponge. Chest 1993; 103: 1293–5 [DOI] [PubMed] [Google Scholar]
- 8.Kjellin IB, Boechat MI, Vinuela F, et al. Pulmonary emboli following therapeutic embolization of cerebral arteriovenous malformations in children. Pediatr Radiol 2000; 30: 279–83 [DOI] [PubMed] [Google Scholar]
- 9.Tadavarthy SM, Moller JH, Amplatz K. Polyvinyl alcohol (Ivalon): a new embolic material. AJR Am J Roentgenol 1975; 125: 609–16 [DOI] [PubMed] [Google Scholar]
- 10.Adavarthy SM, Coleman CC, Hunter D, et al. Polyvinyl alcohol (Ivalon) as an embolic agent. Semin Intervent Radiol 1984; 1: 101–9 [Google Scholar]
- 11.Lanman TH, Martin NA, Vinters HV. The pathology of encephalic arteriovenous malformations treated by prior embolotherapy. Neuroradiology 1988; 30: 1–10 [DOI] [PubMed] [Google Scholar]
- 12.White RI, Strandberg JV, Gross GS, et al. Therapeutic embolization with long-term occluding agents and their effects on embolized tissues. Radiology 1977; 125: 677–87 [DOI] [PubMed] [Google Scholar]
- 13.Miller FJ, Mineau DE. Transcatheter arterial embolization - major complications and their prevention. Cardiovasc Intervent Radial 1983; 6:141–9 [DOI] [PubMed] [Google Scholar]
- 14.Kerber CW. Flow-controlled therapeutic embolization: a physiologic and safe technique. AJR Am J Roentgenol 1980; 134: 557–61 [DOI] [PubMed] [Google Scholar]
- 15.Herrera M, Rysavy J, Kotula F, et al. Ivalon shavings: technical considerations of a new embolic agent. Radiology 1982; 144: 638–40 [DOI] [PubMed] [Google Scholar]
- 16.Derdeyn CP, Moran CJ, Cross DT III, et al. Polyvinyl alcohol particle size and suspension characteristics. AJNR Am J Neuroradiol 1995; 16: 1335–43 [PMC free article] [PubMed] [Google Scholar]
- 17.Jack CR, Forbes G, Dewanjee MK, et al. Polyvinyl alcohol sponge for embolotherapy: particle size and morphology. AJNR Am J Neuroradiol 1985; 6: 595–7 [PMC free article] [PubMed] [Google Scholar]
- 18.Kerber CW, Bank WO, Horton JA. Polyvinyl alcohol foam: prepackaged emboli for therapeutic embolization. AJR Am J Roentgenol 1978; 130:1193–4 [DOI] [PubMed] [Google Scholar]
- 19.Yamammoto A, Imai S, Kobatake M, et al. Evaluation of tris-acryl gelatin microsphere with monochromatic x-rays: comparison with polyvinyl alcohol particles. J Vasc Interv Radiol 2006; 17:1789–802 [DOI] [PubMed] [Google Scholar]
- 20.Derdeyn CP, Graves VB, Salamat MS, et al. Collagen-coated acrylic microspheres for embolotherapy: in vivo and in vitro characteristics. AJNR Am J Neuroradiol 1997; 18: 647–53 [PMC free article] [PubMed] [Google Scholar]
- 21.Szwarc IA, Carrasco CH, Wallace S, et al. Radiopaque suspension of polyvinyl alcohol foam for embolization. AJR Am J Roentgenol 1986; 146: 591–2 [DOI] [PubMed] [Google Scholar]
- 22.Rodari A, Benfanti G, Garbagnati F, et al. Microsphere angiography in hepatic artery infusion for cancer. Eur J Nucl Med 1981; 6: 473–6 [DOI] [PubMed] [Google Scholar]
- 23.Sirr SA, Johnson TK, Stuart DD, et al. An improved radiolabeling technique of Ivalon and its use for dynamic monitoring of complications during therapeutic transcatheter embolization. J Nucl Med 1989; 30: 1399–404 [PubMed] [Google Scholar]
- 24.Whiting JH, Morton KA, Datz FL, et al. Embolization of hepatic arteriovenous malformations using radiolabeled and nonradiolabeled polyvinyl alcohol sponge in a patient with hereditary hemorrhagic telangiectasia: case report. J Nucl Med 1992; 33: 260–2 [PubMed] [Google Scholar]
- 25.Gomes AS, Mali WP, Oppenheim WL. Embolization therapy in the management of congenital arteriovenous malformations. Radiology 1982; 144: 41–9 [DOI] [PubMed] [Google Scholar]
- 26.Davidson GS, Terbrugge KG. Histologic long–term follow-up after embolization with polyvinyl alcohol particles. AJNR Am J Neuroradiol 1995; 16: 843–6 [PMC free article] [PubMed] [Google Scholar]