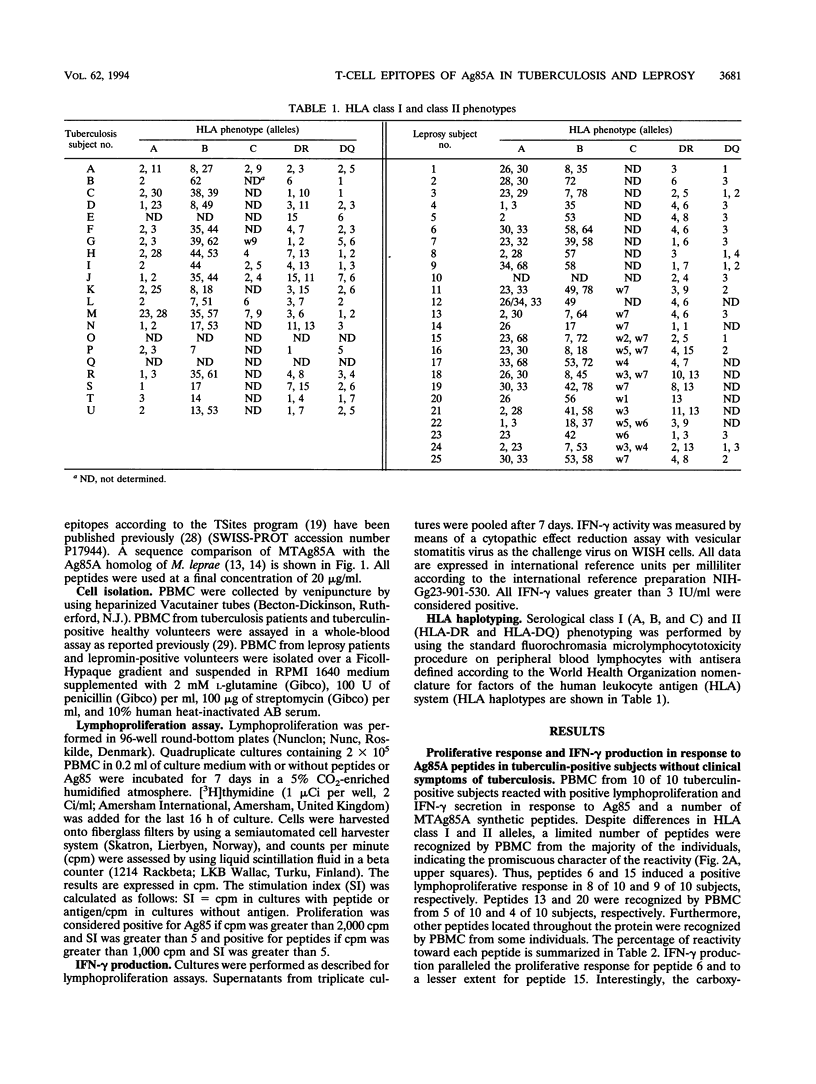
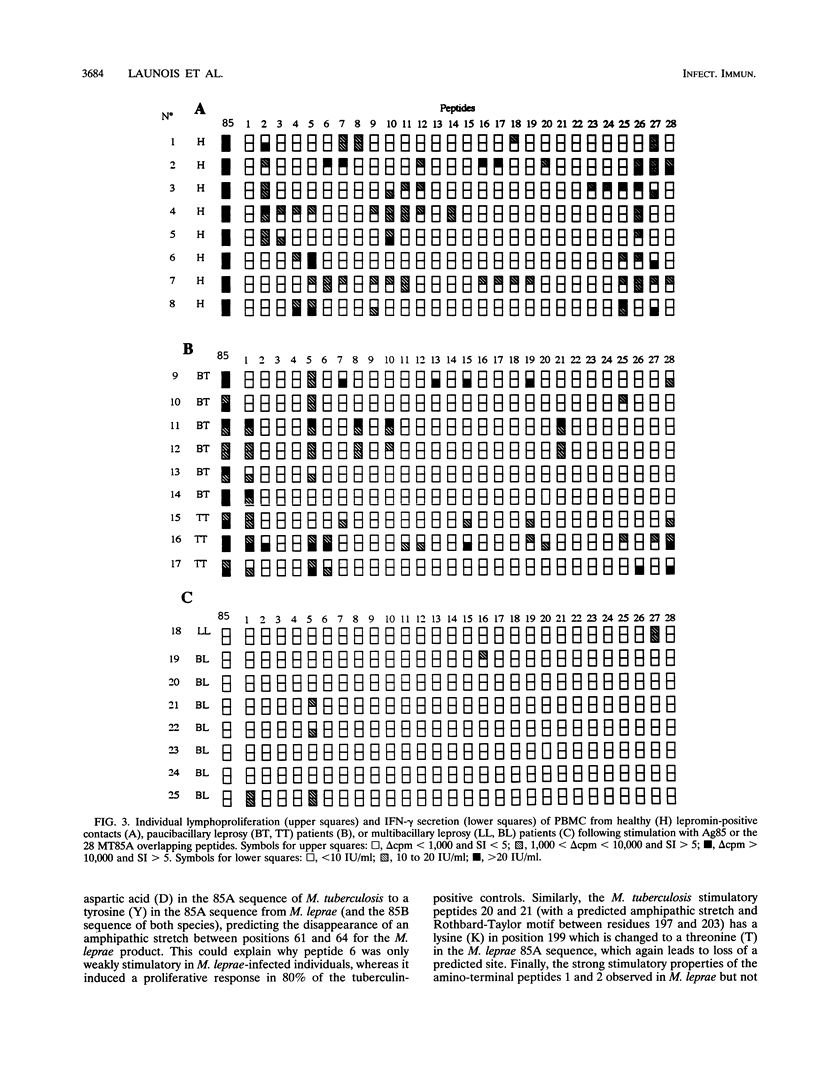
Abstract
Lymphoproliferation and gamma interferon (IFN-gamma) secretion in response to 28 overlapping 20-mer synthetic peptides covering the complete sequence of the mature (295-amino-acid) 85A component of the major secreted, fibronectin-binding antigen 85 complex from Mycobacterium tuberculosis and Mycobacterium bovis BCG (MTAg85A) was examined by using peripheral blood mononuclear cell (PBMC) cultures from healthy tuberculin- and lepromin-positive volunteers and from patients with tuberculosis and leprosy. Peptide recognition was largely promiscuous, with a variety of human leukocyte antigen haplotypes reacting to the same peptides. PBMC from all tuberculin-positive subjects reacted to Ag85, and the majority proliferated in response to peptide 6 (amino acids 51 to 70), peptides 13, 14, and 15 (amino acids 121 to 160), or peptides 20 and 21 (amino acids 191 to 220). PBMC from tuberculosis patients demonstrated a variable reactivity to Ag85 and its peptides, and the strongest proliferation was observed against peptide 7 (amino acids 61 to 80). MTAg85A peptides were also recognized by PBMC from healthy lepromin-positive volunteers and paucibacillary leprosy patients (again in a promiscuous manner), but despite a 90% homology between the 85A proteins of M. leprae and M. tuberculosis, the peptides recognized were different. PBMC from lepromin-positive healthy contacts reacted against peptide 2 (amino acids 11 to 30), peptide 5 (amino acids 41 to 60), and peptides 25 and 26 (amino acids 241 to 270). PBMC from paucibacillary patients reacted preferentially against peptide 1 (amino acids 1 to 20) and peptide 5. Multibacillary patients were not reactive to Ag85 or the MT85A peptides. IFN-gamma production was generally detected simultaneously with positive lymphoproliferative responses, although peptide 1 mostly stimulated proliferation and peptides 27 and 28 mostly elicited an IFN-gamma response. In conclusion, regions 41 to 80 and 241 to 295 demonstrated powerful and promiscuous T-cell-stimulatory properties, resulting in proliferative responses and IFN-gamma secretion, respectively, in the majority of reactive subjects tested in this study. These results could be of value in the development of a subunit vaccine for tuberculosis and leprosy.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Ratliff T. L., Wiker H. G., Harboe M., Bennedsen J., Rook G. A. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988 Dec;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H., SEGAL W. Viability and multiplication of vaccines in immunization against tuberculosis. Am Rev Tuberc. 1955 Feb;71(2):228–248. doi: 10.1164/artpd.1955.71.2.228. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Abrams J. S., Lu S., Sieling P. A., Rea T. H., Modlin R. L. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993 Jan;61(1):197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Berzofsky J. A. Influences of antigen processing on the expression of the T cell repertoire. Evidence for MHC-specific hindering structures on the products of processing. J Exp Med. 1988 Jul 1;168(1):357–373. doi: 10.1084/jem.168.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Content J., de la Cuvellerie A., De Wit L., Vincent-Levy-Frébault V., Ooms J., De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85-C of M. tuberculosis. Infect Immun. 1991 Sep;59(9):3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit J., Sampson C., Zúiga M., Smith P. G., Plata J., Silva J., Molina J., Pinardi M. E., Bloom B. R., Salgado A. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet. 1992 Feb 22;339(8791):446–450. doi: 10.1016/0140-6736(92)91056-e. [DOI] [PubMed] [Google Scholar]
- Cooper A. M., Dalton D. K., Stewart T. A., Griffin J. P., Russell D. G., Orme I. M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D. K., Pitts-Meek S., Keshav S., Figari I. S., Bradley A., Stewart T. A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993 Mar 19;259(5102):1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- Doherty T. M., Bäckström B. T., Prestidge R. L., Love S. G., Harding D. R., Watson J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae. Similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J Immunol. 1991 Mar 15;146(6):1934–1940. [PubMed] [Google Scholar]
- Drowart A., Launois P., De Cock M., Huygen K., De Bruyn J., Yernault J. C., Van Vooren J. P. An isoelectric focusing method for the study of the humoral response against the antigen 85 complex of Mycobacterium bovis BCG in the different forms of leprosy. J Immunol Methods. 1991 Dec 15;145(1-2):223–228. doi: 10.1016/0022-1759(91)90330-i. [DOI] [PubMed] [Google Scholar]
- Ehlers S., Mielke M. E., Blankenstein T., Hahn H. Kinetic analysis of cytokine gene expression in the livers of naive and immune mice infected with Listeria monocytogenes. The immediate early phase in innate resistance and acquired immunity. J Immunol. 1992 Nov 1;149(9):3016–3022. [PubMed] [Google Scholar]
- Faith A., Moreno C., Lathigra R., Roman E., Fernandez M., Brett S., Mitchell D. M., Ivanyi J., Rees A. D. Analysis of human T-cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology. 1991 Sep;74(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Feller D. C., de la Cruz V. F. Identifying antigenic T-cell sites. Nature. 1991 Feb 21;349(6311):720–721. doi: 10.1038/349720a0. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haanen J. B., de Waal Malefijt R., Res P. C., Kraakman E. M., Ottenhoff T. H., de Vries R. R., Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991 Sep 1;174(3):583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Leyva-Cobian F., Unanue E. R. Mechanisms of antigen processing. Immunol Rev. 1988 Dec;106:77–92. doi: 10.1111/j.1600-065x.1988.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Ho P. C., Mutch D. A., Winkel K. D., Saul A. J., Jones G. L., Doran T. J., Rzepczyk C. M. Identification of two promiscuous T cell epitopes from tetanus toxin. Eur J Immunol. 1990 Mar;20(3):477–483. doi: 10.1002/eji.1830200304. [DOI] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Huygen K., Abramowicz D., Vandenbussche P., Jacobs F., De Bruyn J., Kentos A., Drowart A., Van Vooren J. P., Goldman M. Spleen cell cytokine secretion in Mycobacterium bovis BCG-infected mice. Infect Immun. 1992 Jul;60(7):2880–2886. doi: 10.1128/iai.60.7.2880-2886.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Lozes E., Gilles B., Drowart A., Palfliet K., Jurion F., Roland I., Art M., Dufaux M., Nyabenda J. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994 Feb;62(2):363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huygen K., Van Vooren J. P., Turneer M., Bosmans R., Dierckx P., De Bruyn J. Specific lymphoproliferation, gamma interferon production, and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand J Immunol. 1988 Feb;27(2):187–194. doi: 10.1111/j.1365-3083.1988.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Janson A. A., Klatser P. R., van der Zee R., Cornelisse Y. E., de Vries R. R., Thole J. E., Ottenhoff T. H. A systematic molecular analysis of the T cell-stimulating antigens from Mycobacterium leprae with T cell clones of leprosy patients. Identification of a novel M. leprae HSP 70 fragment by M. leprae-specific T cells. J Immunol. 1991 Nov 15;147(10):3530–3537. [PubMed] [Google Scholar]
- Kleinhenz M. E., Ellner J. J. Antigen responsiveness during tuberculosis: regulatory interactions of T cell subpopulations and adherent cells. J Lab Clin Med. 1987 Jul;110(1):31–40. [PubMed] [Google Scholar]
- Launois P., Huygen K., De Bruyn J., N'Diaye M., Diouf B., Sarthouj L., Grimaud J., Millan J. T cell response to purified filtrate antigen 85 from Mycobacterium bovis Bacilli Calmette-Guérin (BCG) in leprosy patients. Clin Exp Immunol. 1991 Nov;86(2):286–290. doi: 10.1111/j.1365-2249.1991.tb05811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launois P., Niang M. N., De Bruyn J., Sarthou J. L., Rivier F., Drowart A., Van Vooren J. P., Millan J., Huygen K. The major secreted antigen complex (Ag 85) from Mycobacterium bovis bacille Calmette-Guérin is associated with protective T cells in leprosy: a follow-up study of 45 household contacts. J Infect Dis. 1993 May;167(5):1160–1167. doi: 10.1093/infdis/167.5.1160. [DOI] [PubMed] [Google Scholar]
- Launois P., Niang M. N., Sarthou J. L., Rivier F., Drowart A., Van Vooren J. P., Millan J., Huygen K. T-cell stimulation with purified mycobacterial antigens in patients and healthy subjects infected with Mycobacterium leprae: secreted antigen 85 is another immunodominant antigen. Scand J Immunol. 1993 Aug;38(2):167–176. doi: 10.1111/j.1365-3083.1993.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Lima L. de M., Content J., van Heuverswyn H., Degrave W. Nucleotide sequence of the gene coding for the 85-B antigen of Mycobacterium leprae. Nucleic Acids Res. 1991 Oct 25;19(20):5789–5789. doi: 10.1093/nar/19.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manca F., Rossi G., Valle M. T., Lantero S., Li Pira G., Fenoglio D., De Bruin J., Costantini M., Damiani G., Balbi B. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun. 1991 Feb;59(2):503–513. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough K. A., Kress Y., Bloom B. R. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun. 1993 Jul;61(7):2763–2773. doi: 10.1128/iai.61.7.2763-2773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Orme I. M. Immunity to mycobacteria. Curr Opin Immunol. 1993 Aug;5(4):497–502. doi: 10.1016/0952-7915(93)90029-r. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessolani M. C., Brennan P. J. Mycobacterium leprae produces extracellular homologs of the antigen 85 complex. Infect Immun. 1992 Nov;60(11):4452–4459. doi: 10.1128/iai.60.11.4452-4459.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönnighaus J. M., Fine P. E., Sterne J. A., Wilson R. J., Msosa E., Gruer P. J., Jenkins P. A., Lucas S. B., Liomba N. G., Bliss L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992 Mar 14;339(8794):636–639. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Burggraaf J. D., Yong S., Faber W. R., Thole J. E., Harboe M. Heterogeneity of monoclonal antibody-reactive epitopes on mycobacterial 30-kilodalton-region proteins and the secreted antigen 85 complex and demonstration of antigen 85B on the Mycobacterium leprae cell wall surface. Infect Immun. 1992 Dec;60(12):5172–5181. doi: 10.1128/iai.60.12.5172-5181.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambukkana A., Das P. K., Krieg S., Faber W. R. Association of the mycobacterial 30-kDa region proteins with the cutaneous infiltrates of leprosy lesions. Evidence for the involvement of the major mycobacterial secreted proteins in the local immune response of leprosy. Scand J Immunol. 1992 Jul;36(1):35–48. doi: 10.1111/j.1365-3083.1992.tb02938.x. [DOI] [PubMed] [Google Scholar]
- Reece J. C., Geysen H. M., Rodda S. J. Mapping the major human T helper epitopes of tetanus toxin. The emerging picture. J Immunol. 1993 Dec 1;151(11):6175–6184. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rinke de Wit T. F., Bekelie S., Osland A., Wieles B., Janson A. A., Thole J. E. The Mycobacterium leprae antigen 85 complex gene family: identification of the genes for the 85A, 85C, and related MPT51 proteins. Infect Immun. 1993 Sep;61(9):3642–3647. doi: 10.1128/iai.61.9.3642-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Sampaio E. P., Moreira A. L., Kaplan G., Alvim M. F., Duppre N. C., Miranda C. F., Sarno E. N. Mycobacterium leprae-induced interferon-gamma production by household contacts of leprosy patients: association with the development of active disease. J Infect Dis. 1991 Nov;164(5):990–993. doi: 10.1093/infdis/164.5.990. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Guttinger M., Kilgus J., Doran D. M., Matile H., Etlinger H., Trzeciak A., Gillessen D., Pink J. R. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988 Dec 22;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- Svetić A., Jian Y. C., Lu P., Finkelman F. D., Gause W. C. Brucella abortus induces a novel cytokine gene expression pattern characterized by elevated IL-10 and IFN-gamma in CD4+ T cells. Int Immunol. 1993 Aug;5(8):877–883. doi: 10.1093/intimm/5.8.877. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsicopoulos A., Hamid Q., Varney V., Ying S., Moqbel R., Durham S. R., Kay A. B. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992 Apr 1;148(7):2058–2061. [PubMed] [Google Scholar]
- Vordermeier H. M., Harris D. P., Friscia G., Román E., Surcel H. M., Moreno C., Pasvol G., Ivanyi J. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38-kDa antigen in patients with active disease. Eur J Immunol. 1992 Oct;22(10):2631–2637. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- Walker K. B., Butler R., Colston M. J. Role of Th-1 lymphocytes in the development of protective immunity against Mycobacterium leprae. Analysis of lymphocyte function by polymerase chain reaction detection of cytokine messenger RNA. J Immunol. 1992 Mar 15;148(6):1885–1889. [PubMed] [Google Scholar]
- Wiker H. G., Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992 Dec;56(4):648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]



