Abstract
A 28-year-old man with a bicuspid aortic valve presented with facial droop and slurred speech with several months of constitutional symptoms of night sweats, weight loss and productive cough. Examination confirmed aortic regurgitation, palpable spleen and left facial droop. Multiple peripheral blood cultures were negative. Inflammatory markers, cytoplasmic staining antineutrophil cytoplasmic antibodies (cANCA) and anti-PR3 antibody were all elevated. MRI of the brain and CT of the chest and abdomen confirmed embolic infarcts to brain, kidney and spleen. Transoesophageal echocardiogram (ECG) showed valve vegetations and severe aortic regurgitation. Endocardial Wegener's granulomatosis was considered. Aortic valve replacement was performed. Grindings from aortic valve leaflets were analysed for rpoB gene, which confirmed the presence of Bartonella henselae. Serological assays demonstrated B henselae IgM 20 (normal <20) and IgG >2048 (normal < 64). The patient completely recovered after prolonged antibiotic treatment. Culture-negative infective endocarditis may mimic vasculitis and be associated with positive cANCA. Serology and molecular techniques may aid diagnosis.
Background
Elevated cytoplasmic staining antineutrophil cytoplasmic antibodies (cANCAs) are associated with Wegener's granulomatosis (WG) and other vasculitides. Cardiac involvement occurs in 6% of patients with WG.1 However, infectious diseases such as infective endocarditis (IE) may also be associated with cANCA positivity.2 Distinguishing between chronic or occult infection and ANCA-associated vasculitis (AAV) is important, particularly when immunosuppressive treatment is considered, although this can be difficult in the case of culture negative IE.
Case presentation
A 28-year-old man with symptoms of night sweats, weight loss and productive cough was seen and investigated as an outpatient over a 7-month period. He had a history of bicuspid aortic valve and moderate asymptomatic aortic regurgitation.
The patient had obtained a kitten 1 month prior to developing the above symptoms. There was no history of recent overseas travel, dental or gingival problems. There was no history of oral, aural or nasal lesions, epistaxis, haemoptysis or haematuria.
After 7 months of ongoing night sweats, weight loss and cough, he then developed a week-long history of headache, followed by sudden-onset slurred speech and left facial droop, which prompted acute admission to hospital.
On examination during his admission, the patient was afebrile with heart rate 78 bpm and blood pressure 99/49 mm Hg. Clubbing, splinter haemorrhages and other peripheral stigmata of IE were absent. Heart sounds were normal except for a diastolic decrescendo murmur. There was no evidence of cardiac failure. His spleen was palpable. Cranial nerve examination was normal except for a seventh nerve lesion with left facial droop slurred speech. Muscle tone was generally increased and plantar reflex was up going on the right. No synovitis, cutaneous, oral, aural or nasal lesions were detected.
The patient's facial droop and dysarthria resolved over 2 days. The patient remained afebrile and did not develop any further clinical features consistent with vasculitis.
Investigations
Investigations as an outpatient prior to admission
Repeated transthoracic ECGs confirmed a bicuspid aortic valve with moderate aortic regurgitation but no vegetations. Multiple peripheral blood cultures without prior antibiotic exposure were negative. Blood tests revealed positive cANCA, proteinase 3 (PR3) 100 AU/ml (<20 AU/ml), erythrocyte sedimentation rate (ESR) 57 mm/h and C reactive protein (CRP) 38 mg/l.
Investigations during admission
At the time of admission the patient's inflammatory markers remained elevated with ESR 76 mm/h and CRP 41 mg/l. A positive cANCA and PR3 >250 AU/ml were detected. Perinuclear staining ANCAs, myeloperoxidase assays, anti-nuclear antibody and tissue autoantibody screens were normal. Rheumatoid factor was elevated at 367 kIU/l (<20 kIU/l). Complement levels of C4 were low at 0.1 (0.2–0.5 g/l), but C3 levels were normal.
Full blood count revealed microcytic anaemia with haemoglobin 111 g/l (130–175 g/l), mean cell volume 77 fl (80–99 fl) and white blood cell count 3.9 × 109/l (4–11 × 109/l). Electrolytes, creatinine and liver function tests were normal.
Lupus anticoagulant was detected with lupus anticoagulant kaolin clotting time 121 s (55–120 s) and lupus anticoagulant kaolin clotting time 20% index 1.4 (<1.2). Diluted Russell viper venom was normal. Thrombophilia screen and anti-cardiolipin antibody assays were normal.
Lumbar puncture yielded clear, colourless cerebrospinal fluid (CSF) with white cell count 40 × 106/l that was predominantly lymphocytic, CSF protein 0.45 g/l (0.15–0.45 g/l) and CSF glucose 2.5 mmol/l (2.8–4.4 mmol/l). There were no organisms on the CSF Gram stain and CSF cultures were negative. Urinalysis was normal, with no casts, white cells or red cells.
The multiple peripheral blood cultures taken during the admission prior to introduction of antibiotics were inoculated in aerobic, anaerobic and resin bottles (BioMerieux, Durham, NC, USA) and incubated up to 3 weeks in BacT Alert 3D automated system and remained negative.
A non-contrast CT scan of the patient's head on day of admission revealed multiple hypodense and hyperdense lesions. MRI and MR angiography scans of his brain on the third day of admission confirmed areas of acute infarction within the right parietal and perisylvian frontal lobes with stenosis of the proximal right middle cerebral artery and dense calcification of this region. CT of the chest, abdomen and pelvis with contrast identified recent splenic and left renal infarcts, splenomegaly of 16 cm and mild hepatomegaly.
Transoesophageal ECG on the fifth day of admission confirmed a bicuspid aortic valve, severe aortic regurgitation and well-defined echogenic masses on both cusps of the aortic valve consistent with vegetations or endocardial WG (figure 1).
Figure 1.
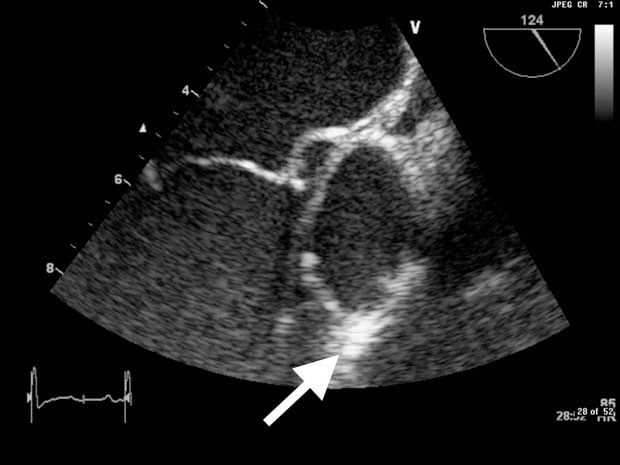
Image from transoesophageal ECG. White arrow indicates vegetation on the patient's aortic valve.
Following aortic valve replacement, histopathological assessment of the aortic valve demonstrated the presence of poorly staining coccobacilli and vegetations composed of fibrin, neutrophils and inflammatory infiltrate on the valve surface with patchy areas of necrosis and thickened fibrotic valve tissue (figure 2). Gram stain of the aortic valve specimen was negative. Culture of aortic valve leaflet performed using commercial sheep blood agar, chocolate agar and brain heart infusion agar supplemented with Vitamin K and Hemin grew Bartonella henselae in 10 days under anaerobic conditions with carbon dioxide. Susceptibility testing was not performed. PCR for B henselae was positive. Identification of species was confirmed by 16S rRNA sequencing.
Figure 2.
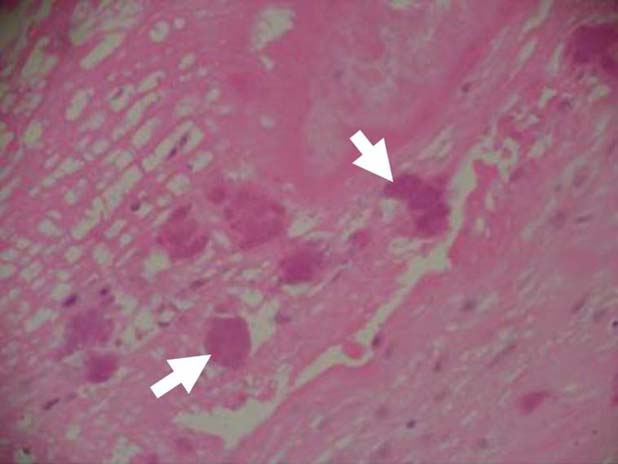
The histology from the patient's resected aortic valve. White arrows indicate the presence of bacterial colonies on aortic valve (colour).
Positive B henselae IgG (IFA) >2048 (<64) was reported during convalescence.
Differential diagnosis
The vegetations seen on transoesophageal ECG, the embolic phenomenon with multiple cerebral, left renal and splenic infarcts, and the background of several months of systemic illness with weight loss, night sweats and productive cough were consistent with a potential diagnosis of culture negative IE. Differential diagnoses included endocardial Wegener's disease or cANCA associated valvulitis since the cANCA and PR3 were positive in the absence of evidence of infectious aetiology on multiple peripheral blood cultures.
Treatment
Aspirin and unfractionated heparin were started after MRI of the brain confirmed areas of cerebral infarction and subsequent CT of the chest, abdomen and pelvis revealed evidence of embolic infarcts at left kidney and spleen.
Due to the patient's increasing aortic regurgitation and the lack of a firm diagnosis he underwent aortic valve replacement with a 29 mm St Jude's mechanical prosthesis. Steroid and immunosuppressive treatment had been considered in the context of possible endocardial Wegener's disease and the lack of evidence for IE with multiple negative cultures, but this decision was deferred until after valve replacement.
After aortic valve replacement and the diagnosis of B henselae IE confirmed by histopathological assessment of resected aortic valve with positive B henselae PCR and serology, the patient was treated with intravenous ceftriaxone 2 g daily for 6 weeks, intravenous gentamicin 240 mg daily for 2 weeks and oral doxycycline 100 mg twice daily for 3 months. Anti-coagulation with warfarin was started.
Outcome and follow-up
The patient made a full recovery following prolonged antibiotic treatment. His inflammatory markers were normal at the time of follow-up 7 months later.
Discussion
Culture-negative endocardial disease with evidence of systemic embolisation is a diagnostic and management challenge. Immunosuppression of a non-infective inflammatory condition is usually considered.
IE mimicking AAV
Cardiac involvement has been reported in 6% of patients with WG.1 Development of vegetations, non-infectious valvulitis, endocarditis or valvular degeneration and myxomatous change in patients with AAV is well-documented.3–9 Cardio-embolic stroke following non-infectious mitral valve vegetation due to WG has been reported.3 Most patients with valvular vegetation or endocarditis due to AAV require valve replacement despite aggressive immunosuppressive treatment, although some patients have had reversal of valvulitis and endocarditis with immunosuppressive treatment.3 4 10
Patients with IE mimicking AAV with positive cANCA and antiPR3 have been reported.2 10–12 Chirinos et al10 reported a patient with IE and a positive cANCA whose blood and aortic valve cultures were positive for Enterococcus faecalis. A literature review identified eight patients with IE who were positive for cANCA: seven had peripheral blood cultures positive for viridans streptococcus and one had a negative blood culture but positive valve culture.10 Culture negative B quintana endocarditis mimicking AAV has been reported.11 Peripheral blood cultures were negative for growth but B quintana was identified by PCR amplification, restriction fragment length polymorphism analysis of genomic DNA from the resected aortic valve and positive serology for B quintana and B henselae.
B henslae endocarditis
In a large single reference laboratory, Bartonella species were the second commonest cause of true blood culture-negative IE after Coxiella burnetii.13 In this series, B quintana (aetiological agent of Trench fever) caused the majority of Bartonella IE cases (53 vs 17 cases) compared to B henselae (aetiological agent of cat-scratch disease). Globally, more than 95% of Bartonella IE is caused by either B quintana or B henselae.13 14 Clinical manifestations of Bartonella IE are generally similar to those of other causes of IE.15 However, there is a predilection for native aortic valves. Seventy-five per cent of cases involve aortic valve either singly (58%) or in combination with other valves.15 B henselae IE compared to B quintana occurs more frequently in cases with known exposure to cats or cat fleas (79% vs 57%; p<0.01), underlying valvulopathy (100% vs 53%; p<0.05) and arterial embolism (47% vs 27%; p<0.05).13 Other Bartonella species also known to cause endocarditis on rare occasions include B elizabethae, B vinsonii subsp. berkhoffii, B koehlerae and B alsatica.16 17 Prosthetic valve endocarditis is infrequently reported.18
Diagnostic tests for detection of IE caused by Bartonella species include blood and valve tissue cultures, serology and nucleic acid amplification techniques.13–15 19 Sensitivity of blood and valvular tissue culture is low (20% and 31%, respectively). Molecular identification using PCR amplification and direct sequencing of DNA from resected valve specimens can achieve high sensitivity (92–98%), although it requires expertise, specialised equipment and an invasive intervention for sample collection. Serology (generally using immunofluorescence assay or ELISA platforms) provides a rapid, sensitive, species-specific and non-invasive technique. Differentiation between B quintana and B henselae may not be possible due to cross reactions between species. Further analysis of serum samples by cross-adsorption and Western immunoblotting may overcome this limitation.13 20
Our case report highlights the role of serology and molecular techniques in diagnosis of B henselae IE along with the limitations of conventional culture techniques. Outcome of Bartonella IE has improved over recent years with reported mortality of 7% compared to 20–30% in previous reports.13 14 19 Factors possibly responsible for this include use of valve surgery, prolonged courses of combination antibiotics, such as ceftriaxone, doxycycline and aminoglycosides, and improved awareness and recognition.
Learning points.
-
▶
Infective endocarditis can be associated with positive cANCA and mimic endocarditis due to AAV such as Wegener's granulomatosis.
-
▶
Peripheral blood cultures are helpful in most cases where infective endocarditis mimics AAV; however, negative peripheral blood cultures do not exclude culture negative infective endocarditis.
-
▶
Serology and molecular techniques can play an important role in diagnosis of B henselae endocarditis.
Acknowledgments
We thank Dr Logan Carpenter for assistance with histopathology/microbiology photographs.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488–98 [DOI] [PubMed] [Google Scholar]
- 2.Choi HK, Lamprecht P, Niles JL, et al. Subacute bacterial endocarditis with positive cytoplasmic antineutrophil cytoplasmic antibodies and anti-proteinase 3 antibodies. Arthritis Rheum 2000;43:226–31 [DOI] [PubMed] [Google Scholar]
- 3.Jiménez Caballero PE, Segura Martín T. Cardioembolic stroke secondary to non-bacterial endocarditis in Wegener disease. Eur J Neurol 2007;14:683–5 [DOI] [PubMed] [Google Scholar]
- 4.Gerbracht DD, Savage RW, Scharff N. Reversible valvulitis in Wegener's granulomatosis. Chest 1987;92:182–3 [DOI] [PubMed] [Google Scholar]
- 5.Mishell JM. Cases from the Osler Medical Service at Johns Hopkins University: cardiac valvular lesions in Wegener's granulamatosis. Am J Med 2002;113:607–9 [DOI] [PubMed] [Google Scholar]
- 6.Leff RD, Hellman RN, Mullany CJ. Acute aortic insufficiency associated with Wegener granulomatosis. Mayo Clin Proc 1999;74:897–9 [DOI] [PubMed] [Google Scholar]
- 7.Horino T, Takao T, Taniguchi Y, et al. Non-infectious endocarditis in a patient with cANCA-associated small vessel vasculitis. Rheumatology (Oxford) 2009;48:592–4 [DOI] [PubMed] [Google Scholar]
- 8.Fox AD, Robbins SE. Aortic valvulitis complicating Wegener's granulomatosis. Thorax 1994;49:1176–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanda RJ, Guis MS, Rabkin JM. Aortic valvulitis in a patient with Wegener's granulomatosis. West J Med 1989;151:555–6 [PMC free article] [PubMed] [Google Scholar]
- 10.Chirinos JA, Corrales-Medina VF, Garcia S, et al. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol 2007;26:590–5 [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama H, Sahara M, Imai Y, et al. Infective endocarditis by Bartonella quintana masquerading as antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Cardiology 2009;114:208–11 [DOI] [PubMed] [Google Scholar]
- 12.de Corla-Souza A, Cunha BA. Streptococcal viridans subacute bacterial endocarditis associated with antineutrophil cytoplasmic autoantibodies (ANCA). Heart Lung 2003;32:140–3 [DOI] [PubMed] [Google Scholar]
- 13.Houpikian P, Raoult D. Blood culture-negative endocarditis in a reference center: etiologic diagnosis of 348 cases. Medicine (Baltimore) 2005;84:162–73 [DOI] [PubMed] [Google Scholar]
- 14.Raoult D, Fournier PE, Drancourt M, et al. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med 1996;125:646–52 [DOI] [PubMed] [Google Scholar]
- 15.Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella quintana and Bartonella henselae endocarditis: a study of 48 patients. Medicine (Baltimore) 2001;80:245–51 [DOI] [PubMed] [Google Scholar]
- 16.Raoult D, Roblot F, Rolain JM, et al. First isolation of Bartonella alsatica from a valve of a patient with endocarditis. J Clin Microbiol 2006;44:278–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avidor B, Graidy M, Efrat G, et al. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J Clin Microbiol 2004;42:3462–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikram HR, Bacani AK, DeValeria PA, et al. Bivalvular Bartonella henselae prosthetic valve endocarditis. J Clin Microbiol 2007;45:4081–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoult D, Fournier PE, Vandenesch F, et al. Outcome and treatment of Bartonella endocarditis. Arch Intern Med 2003;163:226–30 [DOI] [PubMed] [Google Scholar]
- 20.Tang YW. Duplex PCR assay simultaneously detecting and differentiating Bartonella quintana, B. henselae, and Coxiella burnetii in surgical heart valve specimens. J Clin Microbiol 2009;47:2647–50 [DOI] [PMC free article] [PubMed] [Google Scholar]


