Abstract
This paper reports a novel, physiologically significant, microfluidic phenomenon generated by nanomolar concentrations of drag-reducing polymers (DRP) dissolved in flowing blood, which may explain previously demonstrated beneficial effects of DRP on tissue perfusion. In microfluidic systems used in this study, DRP additives were found to significantly modify traffic of red blood cells (RBC) into microchannel branches as well as reduce the near-wall cell-free layer, which normally is found in microvessels with a diameter smaller than 0.3 mm. The reduction in plasma layer size led to attenuation of the so-called “plasma skimming” effect at microchannel bifurcations, increasing the number of RBC entering branches. In vivo, these changes in RBC traffic may facilitate gas transport by increasing the near vessel wall concentration of RBC and capillary hematocrit. In addition, an increase in near-wall viscosity due to the redirection of RBC in this region may potentially decrease vascular resistance as a result of increased wall shear stress, which promotes endothelium mediated vasodilation. These microcirculatory phenomena may explain the previously reported beneficial effects of DRP on hemodynamics in vivo observed in many animal studies. We also report here our finding that DRP additives reduce flow separations at microchannel expansions, deflecting RBC closer to the wall and eliminating the plasma recirculation zone. Although the exact mechanism of the DRP effects on RBC traffic in microchannels is yet to be elucidated, these findings may further DRP progress toward clinical use.
Keywords: Blood soluble drag-reducing polymers, microcirculation, wall shear stress, “plasma skimming” effect, flow separations, RBC traffic
1. Introduction
Certain long-chain water-soluble polymers, known as drag reducing polymers (DRP), when added in minute concentrations, have been shown to produce remarkable effects on blood circulation in vivo [6]. Blood soluble DRP injected into the vascular system of animals at nanomolar concentrations have been previously shown to increase blood flow, tissue perfusion, and tissue oxygenation and reduce vascular resistance with no direct effect on blood viscosity or blood vessel tone [6,7,22]. For example, injections of 1–2 μg/ml (1–2 ppm) of DRP in blood have resulted in significant increase in the number of functioning capillaries and capillary blood flow in diabetic rats [5], enhanced myocardial perfusion in a canine model of a severe coronary stenosis [21], and prevented mortality from severe myocardial ischemia in rats [24]. The addition of DRP to resuscitation fluids was shown to significantly increase survival in rats subjected to lethal hemorrhagic shock [14,19].
These high molecular weight polymers have a primarily linear structure with few or no branches [18]. They have been known to reduce hydrodynamic resistance in turbulent flow since BA Toms discovered this effect in 1948 [25]. It was therefore initially assumed that a similar mechanism was responsible for the observed microvascular effects [10]. However, the Reynolds number for blood flow in small blood vessels typically ranges from 0.1–100, and hence is not turbulent. An alternate mechanism was therefore suggested, motivated by in vitro observations, whereby DRP diminish flow separation and recirculation at vessel bifurcations [12,13]. The ability of DRP to reduce flow separation was first discovered (in 1988) in models of bifurcating vessels with diameters of 3–12 mm and Reynolds numbers in the range of 1–100 [12,13]. More recently, our laboratory has discovered a new microrheological phenomenon produced by DRP in blood, namely the addition of a minute concentration of DRP to red blood cell (RBC) suspensions flowing in a straight microchannel significantly reduces the thickness of the near-wall cell free plasma layer [14].
Blood flowing in microtubes or small blood vessels (less than 0.3 mm in diameter) is known to exhibit a thin layer near the wall that is depleted of red blood cells. This causes a reduction in viscosity in the vessel (Fåhraeus–Lindquist effect [3]) and produces a plasma skimming effect at bifurcations [17] that, in turn, causes a reduction of hematocrit in smaller vessel branches (Fåhraeus effect [2]). The plasma layer adjacent to the vessel wall in the parent and main daughter branch may present a barrier to oxygen diffusion to tissues [26]. In healthy subjects there are no known deleterious consequences, but clinically significant hypoxia may occur in certain pathological states such as anemia or hypovolemia. This may also be exacerbated by increased vascular resistance due to diminished production of nitric oxide caused by the reduction of endothelial shear stress by the plasma layer.
The present study aimed to microscopically investigate the mechanism responsible for the microvascular phenomena caused by DRP by observing the traffic of RBC in microfluidic circuits. Blood flow was observed in straight channels, bifurcations and expansions.
2. Materials and methods
Standard photolithography and replica molding techniques were used to fabricate polydimethylsiloxane (PDMS) microchannel systems containing a series of bifurcations and expansions [20]. Channel widths ranged from 25 to 200 μm and channel height was 100 μm. A glass microchannel with a square cross section, width and height of 100 μm, and length of 2.5 cm was used in some experiments. In addition, hydrodynamic studies were performed in a round channel with a 115 μm diameter and a length of 1.3 cm, since this more closely simulated the geometry of blood vessels. Rectangular channels, however, were necessary to obtain clear images in the visualization studies.
Bovine blood was collected in containers with ACD added as an anticoagulant (10% by volume) at a local slaughterhouse. After centrifugation of blood and removal of plasma and buffy coat, RBC were washed three times with phosphate buffered saline (PBS), and resuspended at a hematocrit of 20% in PBS with 1% bovine serum albumin added to preserve biconcave shape. A hematocrit of 20% was chosen for the studies in capillary tubes and microchannels since in vivo microcirculatory hematocrit is generally assumed to be less than half of the systemic hematocrit [4,23] due to the Fåhraeus phenomenon. In addition, since the Fåhraeus effect is more pronounced at lower hematocrit for a given vessel diameter [4], use of 20% hematocrit allowed for clear visualization of the near wall plasma layer and its modification by DRP. DRP was added to the suspension at a final concentration of 10 μg/ml (10 ppm) and slowly mixed for one hour. The DRP used in this study was a polyethylene oxide with a molecular weight of 4500 kDa, PEO-4500 (Polyox WSR-301, Dow Chemical). An equal volume of saline was added to controls. The asymptotic viscosity of the prepared RBC suspensions was measured using a Brookfield cone and plate rotational rheometer (Middleboro, MA). Light microscopy was used to verify biconcave shape of the RBC after preparation but before subjecting them to flow. All tests were performed at room temperature.
The viscosity of the 10 μg/ml PEO-4500 solution was ∼1.05 cP at room temperature. Addition of 10 μg/ml PEO-4500 had no effect on density of the fluid. The asymptotic viscosity of 20% RBC in PBS was 2.0 cP, and for 20% RBC in 10 μg/ml PEO it was ∼2.2 cP. At the shear rates and hematocrits used in this study, the blood behaved as a Newtonian fluid.
A syringe pump (Harvard Apparatus) was used to generate flow of RBC suspensions through the channels at flow rates ranging from 0.01 to 0.3 ml/min. Reynolds number (Re) in the channels ranged from ∼0.25–30. In order to prevent RBC sedimentation, the suspension in the syringe was kept well mixed by placing a small magnetic stir bar inside the syringe, and manually agitating it using another magnet on the outside. The length of the connective tubing was minimized to avoid cell settling in the tubing as much as possible.
Bifurcated PDMS channels were used to study the DRP effects on the plasma skimming phenomenon. In channels containing a right angle bifurcation, hematocrit was measured in the parent channel (50 μm × 100 μm), daughter branch (25 μm × 100 μm), and feed reservoir using a standard microcapillary technique. An unsealed microcapillary tube was attached to the outlet of each channel branch, and the RBC suspension was allowed to flow through the capillary. When ∼0.5 ml of blood passed through the capillary, it was quickly detached from the outlet and sealed, and discharge hematocrit was measured for each branch. Hematocrit values from the branches were compared to each other in both DRP and control samples using an unpaired, two-sample Student's t-test assuming unequal variances with two-tail distribution.
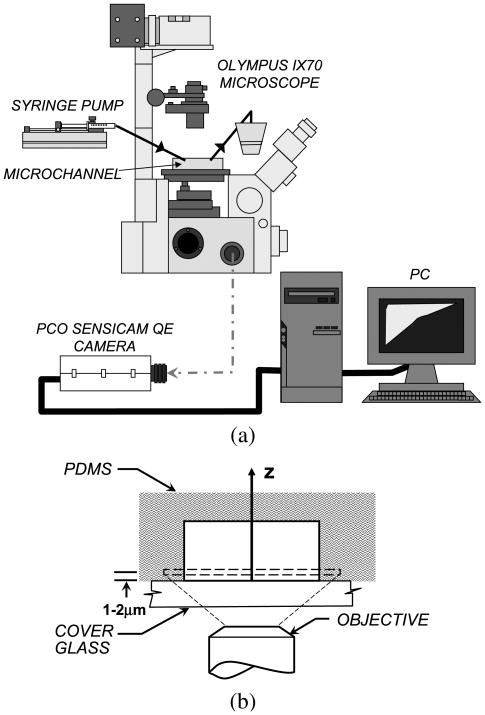
RBC flow behavior was recorded with a microscopic flow imaging system (schematically shown in Fig. 1) consisting of an inverted research microscope (IX70, Olympus, NJ), a CCD camera (PCO Sensicam QE double exposure, The Cooke Corp., Romulus, MI), and an associated image acquisition board hosted in a PC. A very short camera exposure time of 10 μs was used in order to allow for the capture of images at high flow velocity. Twenty images were recorded at each condition. Images were taken at a distance of 1–2 μm from the bottom wall of the channel in order to clearly observe the near wall plasma layer. Since this depth corresponds to the observed size of the near wall plasma layer under similar flow conditions [14], the RBC in the near wall region could be best observed at this depth of focus. Images were taken far from the channel inlet and outlet to avoid entrance and exit effects. A control RBC suspension was first run in each experiment. The suspension was then replaced with RBC samples containing DRP without moving the channel or changing the focus. Finally, the control suspension was tested again to ensure that focus had not changed. Image J (National Institutes of Health) was used to quantify the size of the near wall cell free plasma layer in each image. The cell free area adjacent to the vertical channel wall was measured and the area was divided by the length of the image to give a spatial average plasma layer width. Plasma layer widths for at least five images were then averaged to give a time average. Mean plasma layer size in the control and in the DRP tests was compared using an unpaired, two-sample Student's t-test assuming unequal variances with two-tail distribution.
Fig. 1.
(a) Schematic of microflow imaging system. (b) Schematic of a sample PDMS microchannel cross section and experimental setup for visualization. The focal plane is approximately 1–2 μm from bottom surface of the microchannel.
The pressure gradient was also measured in microchannels. In this case, channels with circular cross sections were used to more closely simulate physiological conditions. Wall shear stress in a channel was calculated using the formula
| (1) |
where ΔP is the pressure drop across the tube, d is tube diameter, and L is tube length.
The dimensionless friction factor was calculated using the formula:
| (2) |
which is derived from the Darcy–Weisbach equation, where λ is the friction coefficient, d is the tube diameter, ρ is the density of the fluid, Q is the volumetric flow rate, and ΔP is the pressure drop along the tube length, L. The experimental friction factor was compared to a theoretical friction factor for laminar flow given by Eq. (3):
| (3) |
which can be derived by substituting the Poiseuille equation into the Darcy–Weisbach equation. An increase in pressure gradient, and therefore friction factor, indicated attenuation of the Fåhraeus–Lindquist effect.
Measurements of plasma layer size and pressure gradient were performed in channels that were 100 μm wide. This size was optimal from the point of view of hemodynamics, cell-free layer size, and RBC sedimentation in both microchannels and in the connecting tubing. Smaller channels would have much higher resistance and would be much more difficult to clean and sterilize. Based on our experience with the experiments in which we used smaller channels, plasma layer can be observed and characterized as well in 100 μm channels as in smaller ones.
3. Results
3.1. Effects of DRP on blood flow in straight microchannels
Figure 2 shows the effect of DRP on the near wall concentration of RBC flowing in a straight 100-μm wide square channel. Following addition of 10 μg/ml (10 ppm) of polyethylene oxide (PEO) with a molecular weight of 4500 kDa (PEO-4500) to the flowing suspension, the RBC in this channel became more elongated and moved closer to the channel wall. At a flow rate of 0.1 ml/min, the plasma layer width was 5.5 ± 1.0 μm. The PEO significantly reduced the thickness of the plasma layer to 1.3 ± 0.3 μm at 0.1 ml/min (p < 0.001). When the flow was stopped, the cells redistributed evenly throughout the channel. A slight but statistically significant difference between plasma layer size in the stopped control suspension (1.68 μm) and that with PEO (1.12 μm, p < 0.001) was observed. The presence of this plasma layer was likely due to the fact that, although the pump was stopped, some flow still existed in the channel (pictures were taken immediately after the pump stop to avoid effects of RBC sedimentation). The amplification of RBC elongation with the DRP treated suspensions is likely due to the increased stress on the RBC caused by their close proximity to the channel walls. However, the possibility that the polymers themselves affect the deformability of the RBC membrane cannot be excluded. The slight difference between Control and DRP RBC alignment without flow was due to the fact that the pictures were taken immediately after flow was stopped to avoid the effect of RBC sedimentation. Figure 3 shows pictures of RBC suspensions with and without DRP taken using a light microscope. No difference can be seen between RBC in these photos.
Fig. 2.
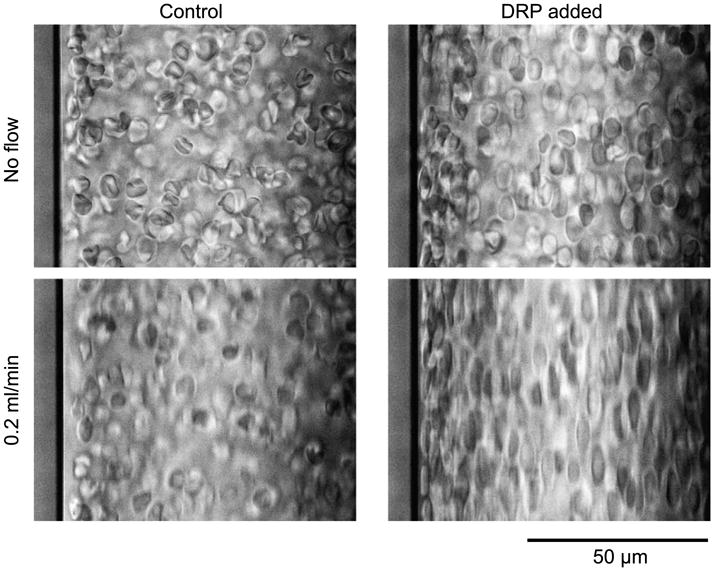
RBC suspension in a 100-μm wide rectangular straight channel. Addition of 10 ppm PEO-4500 caused a perceptible alignment of RBC in the non-flowing samples, and a dramatic elongation of RBC aligned with the flow direction (top to bottom) at 0.2 ml/min, Re ∼ 20. The PEO effectively eliminated the cell-depleted layer observed in the control sample. The slight difference between Control and DRP cell alignment at the no flow condition was due to the fact that the pictures were taken right after flow was stopped before the RBC started to sediment.
Fig. 3.
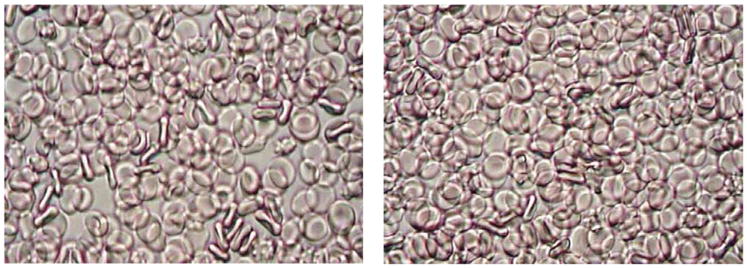
Suspensions of RBC in PBS with 1% albumin and with 20 ppm PEO (0.02 ml of PEO solution with a concentration of 1000 ppm per 1 ml of RBC suspension, right). Saline (0.02 ml per 1 ml of RBC suspension) was added to control suspension (left). Pictures (400× magnification) were taken using a light microscope (Nikon, Japan).
Similar results were obtained with other DRP such as high molecular weight (MW) hyaluronic acid and natural polysaccharides extracted from aloe vera with MW of 6–8 million Da. However, lower MW formulations of these polymers (below 1000 kDa), which do not possess drag-reducing property, did not produce these effects. This can be seen in Fig. 4, which compares two RBC suspensions flowing in the same microchannel at the same flow rate containing PEO of 200 kDa (left) and 4500 kDa (right).
Fig. 4.
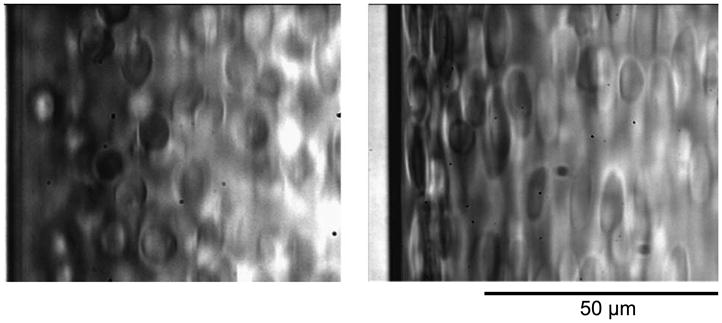
Dependence of plasma layer size on polymer molecular weight. RBC in a 100-μm straight channel with 10 ppm of 200 kDa MW PEO (left) and with 10 ppm of 4500 kDa MW PEO (right). Flow rate = 0.5 ml/min, Re = 27.8.
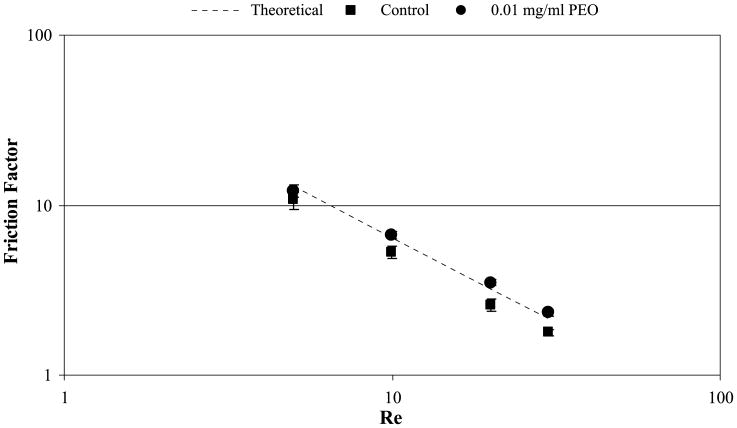
The pressure drop in the flowing RBC suspensions was measured along a 115 μm diameter channel with circular cross section. A significant effect was seen in this channel where the addition of 10 μg/ml (10 ppm) PEO-4500 to the flowing RBC suspension caused an increase in pressure drop, and thus in wall shear stress, of 24%. Pressure gradients were normalized using solution viscosity in order to account for the slight difference in viscosity between control and PEO solutions. Figure 5 shows friction factor vs. Reynolds number for flow of RBC in this channel. In the control case, the experimental friction factor is significantly lower than the theoretical due to lower near wall viscosity caused by the formation of a cell free plasma layer near the channel wall. The experimental friction factor increased significantly (p < 0.01 at all tested Re) with the addition of 10 ppm DRP due to RBC relocating to the near wall space resulting in values close to the theoretical friction factor calculated using the viscosity of the RBC suspension (assuming no cell free layer). Similar results were obtained in the 100 μm square channel. In larger tubes, where the Fåhraeus–Lindquist phenomenon did not occur (0.86 and 1.3-mm diameter), the DRP had no effect on the wall shear stress.
Fig. 5.
Friction factor vs. Re for RBC with 0 and 10 ppm PEO-4500 flowing in a 115-μm diameter microchannel with a circular cross-section.
3.2. Effects of DRP on blood flow in bifurcated microchannels
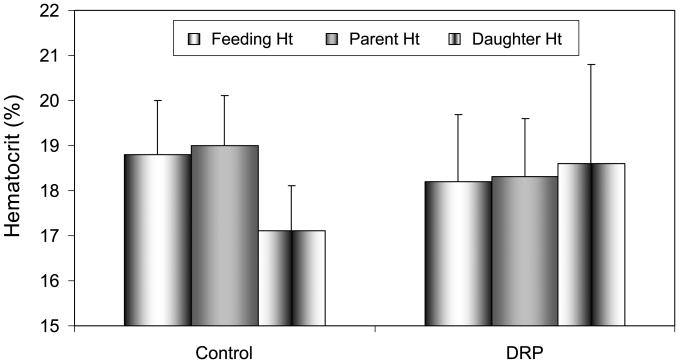
Experiments conducted in bifurcated channels also revealed interesting effects of DRP due to their modulation of the plasma layer. This was evaluated by measuring the hematocrit in both the parent and daughter branch as compared to that in the feeding syringe. Figure 6 shows these values for a microfluidic device containing a right angle bifurcation (50–25 μm). The control sample exhibited a significant reduction of hematocrit in the daughter branch compared to that in the parent (p < 0.001), demonstrating the skimming of plasma from the near-wall layer of the parent into the daughter branch. Parent branch hematocrit was 19.0% ± 1.1%, while the daughter branch hematocrit was 17.1% ± 1%. When 10 ppm PEO-4500 was added, however, no difference was observed between hematocrit values in parent and daughter branches implying that the plasma skimming effect was effectively eliminated by DRP. In the DRP case, no significant difference was observed between parent and daughter branch hematocrit (p = 0.6). The hematocrits in the parent channel and daughter channel were 18.3% ± 1.3% and 18.6% ± 2.2%, respectively. The feed hematocrit was 18.6% ± 1.3%, and no significant difference between feed hematocrit in the control and polymer containing RBC suspensions was observed (p > 0.1).
Fig. 6.
Hematocrit values in the feeding syringe and parent and daughter microchannel branches of 90 degree bifurcation (50–25 μm). Plasma skimming in the control sample was evidenced by significantly reduced hematocrit in the daughter branch; however this effect was eliminated with the addition of nanomolar concentration of DRP. p-values for parent vs. daughter hematocrit are <0.001 in Controls and >0.6 in DRP tests.
3.3. Effects of DRP on blood flow in microchannel expansions
Experiments performed in a microfluidic flow expansion from 50 to 200 μm exhibited a redistribution of RBC caused by DRP in the downstream flow separation region (see Fig. 7). Considerable flow separation was observed with the control blood samples causing formation of cell-depleted pockets within the microchannel expansion. When 10 ppm of PEO-4500 was added, the streamlines of RBC were redirected to conform to the contour of the expansion, eliminating the plasma pockets at the expansion, thus reducing or eliminating the flow separation and recirculating flow. This effect became more apparent as flow rate was increased, and no difference was seen between the control and DRP case when flow was stopped. As was already mentioned in Introduction, it was previously shown that DRP diminish flow separation and recirculation at bifurcations in experiments performed with water (no RBC) flowing through models of bifurcating vessels at Reynolds numbers in the range of 1–100 [12,13].
Fig. 7.
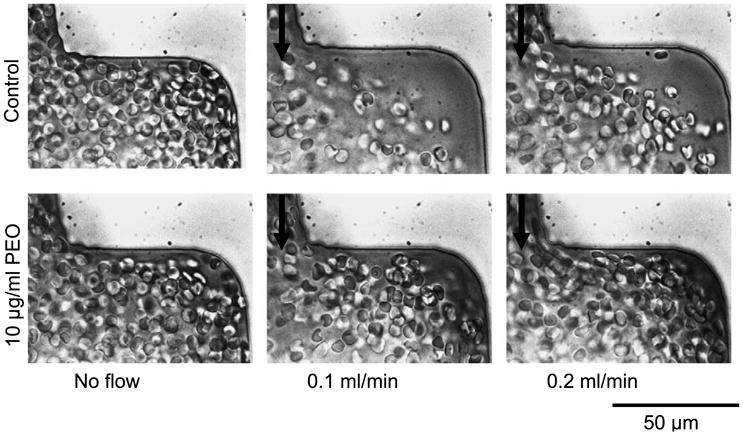
RBC flow in a 50–200-μm expansion exhibits dramatic effect of RBC traffic with addition of DRP. Cell-depleted pockets observed in control samples are virtually eliminated by 10 ppm of PEO-4500. Flow direction is from top to bottom.
4. Discussion
The redistribution of RBC traffic and reduction of plasma layer/skimming observed in these experiments imply a potentially beneficial effect in the microcirculation. The rise in the near-wall hematocrit will increase the local viscosity, thereby loading the endothelial cells with higher shear stress, which can promote vasodilatation, decreasing vascular resistance and potentially increasing number of functioning capillaries. In addition, reduction in plasma skimming would significantly increase traffic of RBC to vessel branches and ultimately to capillaries, which would be beneficial in cases of hypovolemia and might be considered as an “autotransfusion”.
The observations of this study confirmed the influence of the drag-reducing polymer molecules dissolved in blood on the near-wall cell-depleted layer development; however, the effect is contrary to intuition based on the traditional classification of the polymers. The cell depleted layer is known to reduce rather than increase pressure drop in microvessels. Therefore, its elimination cannot be directly responsible for the reduction of vascular resistance observed when DRP are administered in vivo. In rigid microchannels, plasma layer reduction/elimination causes an increase in hydraulic resistance proportional to the increase in the near-wall viscosity. In vivo, however, the proximity of the RBC to the vessel wall and the resulting increase in wall shear stress may promote the release of stress dependent vasodilators, such as nitric oxide, causing local vasodilation and thus the decrease in peripheral vascular resistance observed in animal experiments.
While the current study provides significant insight into the intravascular DRP phenomenon, the mechanism by which DRP are preventing relocation of RBC toward the center of the microchannel/microvessel, still remains unknown. One hypothesis for the DRP effect on distribution of RBC is that the polymer molecules, which are stretched by flow, align along the flow paths and diminish RBC rotation, which is an important factor in the development of a near-wall plasma layer in microvessels [14]. This alignment of the DRP may form a network structure that prevents RBC from migrating away from the walls. The increase in local viscosity due to the presence of polymers around the RBC may also inhibit lateral migration of RBC. In addition, the increase in blood viscoelasticity caused by DRP may strengthen non-Newtonian patterns of the axial velocity profile, blunting it and shifting mean RBC velocity closer to that of the bulk blood flow [14]. Future work to evaluate these hypotheses would improve understanding of the mechanisms by which DRP act in the vascular system.
The reduction of flow separations and elimination of recirculating plasma pockets have clinical significance as well. It is known that thrombi are likely to form in areas of flow separation [11,15,16]. Studies by Karino and Goldsmith, performed with human platelets flowing in an expansion with a diameter ratio of 3.33 (151–504 μm), suggested that these flow patterns would have an antagonistic effect on platelets, and promote their deposition due to flow stasis and prolonged exposure to the surface [15]. Relocation of flowing RBC closer to the wall would potentially reduce/eliminate stagnation and “wash” platelets out of these recirculating zones, therefore decreasing the potential for thrombosis. This effect would be beneficial not only in blood vessels, but also in blood-contacting medical devices, where thrombosis is often a major concern.
Both the Fåhraeus effect and the DRP intravascular phenomenon produce remarkable rheological and hemodynamic manifestations which can have significant relevance to treatment of circulatory disorders and design of blood-wetted medical devices. The discoveries presented in the current study have contributed to the elucidation of the mechanisms by which DRP act in the microvasculature. This is an important step that will help to translate DRP to clinical use for critical care and emergency medicine and for treating cardiovascular diseases and many other disorders in which patients may benefit from improved microcirculation. Particularly, high molecular weight hyaluronic acid (HA), which has long been known to have drag reducing ability [9] and currently has FDA approval for certain biomedical applications (unrelated to its drag reducing properties) [8], might be a desirable candidate for enhancement of blood circulation in vivo. HA was recently shown to be very effective in treatment of animals in hemorrhagic shock [1].
4.1. Study limitations
The results of this study showed that the RBC in the near wall region become significantly elongated in the presence of DRP. In the experiments presented here, we looked only at the cells in the near wall region, and therefore it is not clear whether the DRP had any effect on the shape of the RBC far from the channel wall. Further studies looking at RBC in the center of the channel, as well as at dilute RBC suspensions, could distinguish whether the increased RBC deformation is a result of their exposure to increased shear stress in the near wall region or also related to changes in RBC deformability.
The results of the studies on the effects of DRP on RBC flow at a microchannel expansion shown above are qualitative in nature. In this manuscript, we present the observed phenomenon. However, in the future, more quantitative studies would help to further explain and measure the magnitude of this effect. A recent study quantified RBC streamlines in a microchannel expansion and the platelet margination in these regions [27]. These methods could be applied to quantitatively investigate the observed DRP phenomenon in microchannel expansions.
RBC aggregation was not present in these studies due to several reasons. Most importantly, the shear rates in the channels were high enough to prevent aggregation (>1000 s–1). The applied cells were washed RBC resuspended without fibrinogen in the suspension media. However, since the cells were well deformable, near wall cell-free or cell-poor layers were clearly observed and these layers were significantly reduced or completely eliminated by the presence of DRP in RBC suspensions.
5. Conclusion
The findings of the current study provided insight on the mechanisms by which DRP act in the microvasculature. Although more studies should be done to determine the means by which DRP affect RBC traffic in microvessels, the current findings may further the progress toward clinical applications of DRP.
Acknowledgments
The study was supported by research grants to M.V.K. from the Pittsburgh Foundation, the Commonwealth of Pennsylvania and Pennsylvania Department of Health and the Department of Defense, US Army through Pittsburgh Tissue Engineering Initiative and by the University of Pittsburgh Provost's Development Fund (J.N.M.).
References
- 1.Cotoia A, Kameneva MV, Marascalco PJ, Fink MP, Delude RL. Drag-reducing hyaluronic acid increases survival in profoundly hemorrhaged rats. Shock. 2008 doi: 10.1097/SHK.0b013e31817fc434. accepted for publication. [DOI] [PubMed] [Google Scholar]
- 2.Fåhraeus R. The suspension stability of the blood. Physiol Rev. 1929;IX:241–275. [Google Scholar]
- 3.Fåhraeus R, Lindquist T. The viscosity of blood in narrow capillary tubes. Am J Physiol. 1931;96:562–568. [Google Scholar]
- 4.Goldsmith HL, Cokelet GR, Gaehtgens P. Robin Fåhraeus: Evolution of his concepts in cardiovascular physiology. Am J Physiol. 1989;257:H1005–H1015. doi: 10.1152/ajpheart.1989.257.3.H1005. [DOI] [PubMed] [Google Scholar]
- 5.Golub AS, Grigorian MR, Kameneva MV, Malkina NA, Shoshenko KA. Influence of polyethylene oxide on the capillary blood flow of diabetic rats. Soviet Physics – Doklady. 1987;32:620–621. [Google Scholar]
- 6.Grigorian SS, Kameneva MV. Resistance-reducing polymers in the blood circulation. In: Chernyi GG, Regirer SA, editors. Contemporary Problems of Biomechanics. Mir Publishers, CRC Press; Moscow, Boca Raton, FL: 1990. pp. 99–110. [Google Scholar]
- 7.Grigorian SS, Kameneva MV, Shakhnazarov AA. Effect of high molecular weight compounds dissolved in blood on hemodynamics. Soviet Physics – Doklady. 1976;21:702–703. [PubMed] [Google Scholar]
- 8.Hamburger MI, Lakhanpal S, Mooar PA, Oster D. Intra-articular hyaluronans: A review of product-specific safety profiles. Semin Arthritis Rheum. 2003;32:296–309. doi: 10.1053/sarh.2002.50008. [DOI] [PubMed] [Google Scholar]
- 9.Hoyt JW. Symposium on Rheology. ASME; New York: 1965. [Google Scholar]
- 10.Hoyt JW. Blood transfusion fluids having reduced turbulent friction properties. U.S Patent 3,590,124. 1971 June 29;
- 11.Jordan A, David T, Homer-Vanniasinkam S, Graham A, Walker P. The effects of margination and red cell augmented platelet diffusivity on platelet adhesion in complex flow. Biorheology. 2004;41:641–653. [PubMed] [Google Scholar]
- 12.Kameneva MV, Polyakova MS, Fedoseeva EV. Effect of drag-reducing polymers on the structure of the stagnant zones and eddies in models of constricted and branching blood vessels. Fluid Dyn. 1990;25:956–959. [Google Scholar]
- 13.Kameneva MV, Polyakova MS, Gvozdkova IA. Nature of the influence of polymers that lower hydrodynamic resistance on blood circulation. Proc Acad Sci USSR Biophysics Section. 1988:22–24. [PubMed] [Google Scholar]
- 14.Kameneva MV, Wu ZJ, Uryash A, Repko B, et al. Blood soluble drag-reducing polymers prevent lethality from hemorrhagic shock in acute animal experiments. Biorheology. 2004;41:53–64. [PubMed] [Google Scholar]
- 15.Karino T, Goldsmith HL. Aggregation of human platelets in an annular vortex. Microvasc Res. 1979;17:217–237. doi: 10.1016/s0026-2862(79)80001-1. [DOI] [PubMed] [Google Scholar]
- 16.Karino T, Goldsmith HL. Adhesion of human platelets to collagen on the walls distal to a tubular expansion. Microvasc Res. 1979;17:238–262. doi: 10.1016/s0026-2862(79)80002-3. [DOI] [PubMed] [Google Scholar]
- 17.Krogh A. The Anatomy and Physiology of Capillaries. Yale University Press; New Haven: 1922. [Google Scholar]
- 18.Kulicke WM, Kotter M, Grager H. Drag reduction phenomenon with special emphasis on homogeneous polymer solutions. Adv Polym Sci. 1989;89:1–68. [Google Scholar]
- 19.Macias CA, Kameneva MV, Tenhunen JJ, Puyana JC, Fink MP. Survival in a rat model of lethal hemorrhagic shock is prolonged following resuscitation with a small volume of a solution containing a drag-reducing polymer derived from aloe vera. Shock. 2004;22:151–156. doi: 10.1097/01.shk.0000131489.83194.1a. [DOI] [PubMed] [Google Scholar]
- 20.McDonald JC, Duffy DC, Anderson JR, Chiu DT, et al. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 21.Pacella JJ, Kameneva MV, Lu E, Csikari M, et al. Effect of drag reducing polymers on myocardial perfusion during coronary stenosis. Eur Heart J. 2006;19:2362–2369. doi: 10.1093/eurheartj/ehl165. [DOI] [PubMed] [Google Scholar]
- 22.Polimeni PI, Ottenbreit BT. Hemodynamic effects of a poly(ethylene oxide) drag-reducing polymer, Polyox WSR N-60K, in the open-chest rat. J Cardiovasc Pharmacol. 1989;14:374–380. doi: 10.1097/00005344-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Popel AS, Johnson PC. Microcirculation and hemorheology. Ann Rev Fluid Mech. 2005;37:43–69. doi: 10.1146/annurev.fluid.37.042604.133933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakai T, Repko B, Griffith BP, Waters JH, Kameneva MV. I.V. infusion of a drag-reducing polymer extracted from aloe vera prolonged survival time in a rat model of acute myocardial ischaemia. Br J Anaesth. 2007;98:23–28. doi: 10.1093/bja/ael307. [DOI] [PubMed] [Google Scholar]
- 25.Toms BA. 1st Int Congr Rheology. Vol. 2. Amsterdam: 1948. Some observations on the flow of linear polymer solution through straight tubes at large Reynolds numbers; pp. 135–141. [Google Scholar]
- 26.Wang C, Popel A. Effect of red blood cell shape on oxygen transport in capillaries. Math Biosci. 1993;116:89–110. doi: 10.1016/0025-5564(93)90062-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao R, Marhefka JN, Shu F, Hund SJ, et al. Micro-flow visualization of red blood cell-enhanced platelet concentration at sudden expansion. Ann Biomed Eng. 2008;36:1118–1129. doi: 10.1007/s10439-008-9494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]