Abstract
A growing number of non-heme-iron oxygenases and oxidases catalyze reactions for which the well-established mechanistic paradigm involving a single C-H-bond cleaving intermediate of the Fe(IV)-oxo (ferryl) type [1] is insufficient to explain the chemistry. It is becoming clear that, in several of these cases, Fe(III)-superoxide complexes formed by simple addition of O2 to the reduced [Fe(II)] cofactor initiate substrate oxidation by abstracting hydrogen [2]. This substrate-oxidizing entry route into high-valent-iron intermediates makes possible an array of complex and elegant oxidation reactions without consumption of valuable reducing equivalents. Examples of this novel mechanistic strategy are discussed with the goal of bringing forth unifying principles.
Introduction
Non-heme iron enzymes catalyze a remarkably diverse repertoire of oxidation reactions [3] using an equally remarkable breadth of reaction mechanisms [4]. In transformations of atoms lacking π or non-bonding electrons, one well-described mechanistic strategy involves input of electrons to O2 by the reduced iron(II) cofactor and a reducing co-substrate (e.g., α-ketoglutarate, tetrahydropterin, reduced nicotinamide), resulting in O-O-bond scission and generation of an Fe(IV)-oxo (ferryl) complex [5]. This intermediate then abstracts hydrogen from the substrate to initiate its oxidation. However, the members of a small subset of these enzymes have been found to oxidize electron-poor aliphatic substrates without need of reducing co-substrates. With no obvious, substrate-independent pathway to the potently oxidizing ferryl complex, these enzymes have hinted at a surprising potency and versatility of superoxo or peroxo intermediates [1,6,7] that are merely precursors to ferryl species ("pre-ferryls") in the better-studied systems. This review will cover recent developments in this area that highlight the mechanistic plasticity of these proteins.
Isopenicillin N Synthase
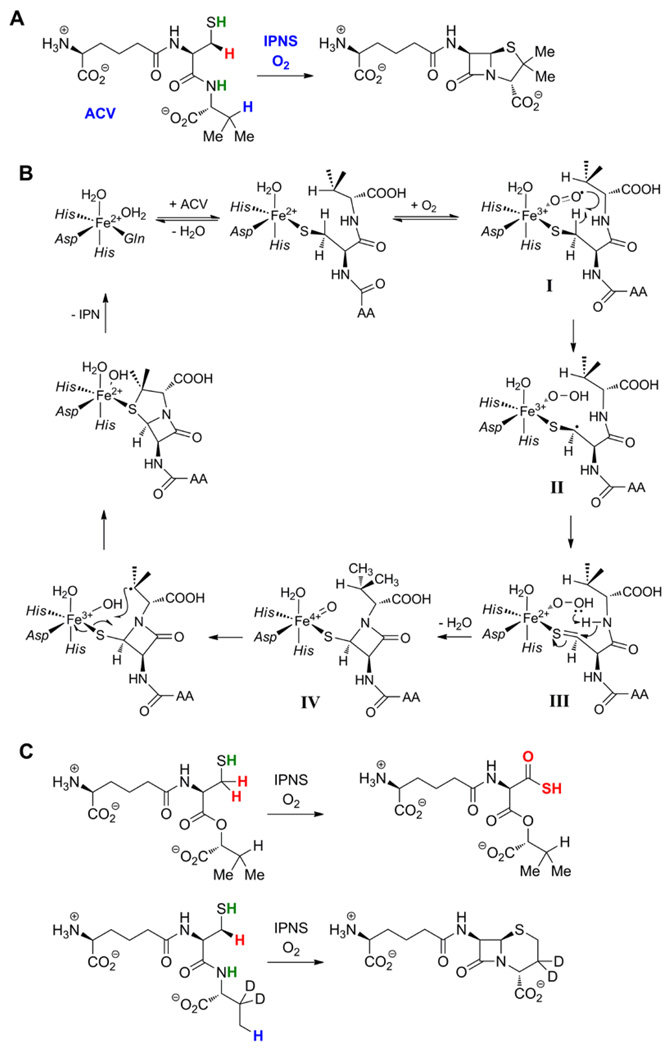
Recognition of the "pre-ferryl" type of hydrogen abstraction originated with the classic experiments of Baldwin and co-workers on isopenicillin N synthase (IPNS) [8]. This non-heme-iron oxidase forms β-lactam and thiazolidine rings from a linear tripeptide substrate (ACV; Figure 1A) to make the penicillin nucleus. In this process, two C-H bonds must be cleaved along with one N-H and one S-H bond. Substrate deuterium kinetic isotope effects revealed that cleavage of the β C-H bond of the l-cysteine residue initiating closure of the β-lactam occurs before cleavage of the β C-H bond of d-valine that initiates formation of the thiazolidine [8]. From this result, analysis of alternative reactions occurring with chemically and isotopically modified substrates, and X-ray crystal structures of numerous enzyme forms, the mechanism shown in Figure 1B was formulated [9]. By direct analogy to mechanisms previously put forth for related enzymes, a ferryl complex was proposed to cleave the d-valine C-H bond. This proposal required that the Cβ,Cys-H cleavage be effected by an intermediate at the same oxidation state as the initial Fe(II)-O2 adduct, an {FeOO}8 species in the Enemark-Feltham notation [10]. Computational studies suggest that this complex should have an FeIII-O2− (ferric superoxide) electronic structure I [11,12]. However, neither this intermediate nor the presumptive ferryl complex IV has been directly characterized yet, an important remaining goal for the field.
Figure 1.
(A) Reaction catalyzed by IPNS in the formation of the penicillin nucleus. (B) Mechanism proposed by Baldwin and co-workers for the IPNS reaction [9]. (C) Aberrant reactions occurring with ACV derivatives containing either d-α-hydroxyisovalerate [18] or 3,3-[2H]2-d-aminobutyrate in place of d-valine [8].
The IPNS reaction and proposed mechanism exemplify at least three functional themes exhibited by these pre-ferryl-utilizing non-heme-iron oxidases and oxygenases. First, the reaction is a four-electron oxidation of the substrate by a single equivalent of dioxygen [8], a redox economy shared by several other members, including myo-inositol oxygenase (MIOX) [13], 2-hydroxyethylphosphonate dioxygenase (HEPD) [14] and one of the two reactions catalyzed by the enzyme CloR [15] (see below). Second, a heteroatom (for IPNS, the l-cysteine sulfur) on the oxidation target is coordinated by the iron cofactor [16] (which acts as Lewis acid) thereby facilitating abstraction of a hydrogen from the adjacent carbon by the {FeOO}8 complex [17]. Third, subsequent to this hydrogen abstraction, another electron is harvested from the substrate (generating the thiocarbonyl in III, Figure 1B, in the case of IPNS) to permit progression to a ferryl complex. Either its distinct geometry or motion of the substrate relative to the cofactor (or both) permits the ferryl complex to target a different position of the substrate (the d-valine methine) for oxidation. Although, for IPNS, the mode of reactivity is the same for both intermediates (hydrogen abstraction), it appears that fundamentally different modes of reactivity for the pre-ferryl and ferryl intermediates are utilized in other members (e.g. HEPD; see below). The ability to wield two oxidizing intermediates to target different positions for distinct oxidation outcomes endows the pre-ferryl-utilizing enzymes with even greater catalytic versatility than the more conventional, exclusively ferryl-utilizing oxygenases and oxidases. It also leads to a bewildering array of alternative reactions on chemically or isotopically modified substrates. For example, in IPNS, substitution of the d-valine moiety of ACV with d-α-hydroxyisovalerate (converting amide to ester) reroutes the second two-electron oxidation to the l-cysteine β-carbon, resulting in production of the acyclic thiocarboxylate derivative [18] (Figure 1C). Similarly, the ACV analogue with 3,3-[2H]2-d-aminobutyrate in place of d-valine is converted largely to the corresponding cepham (containing a six-membered ring), presumably because a large intramolecular selection effect against abstraction of deuterium from the 3-methylene redirects the ferryl intermediate to the protium-containing 4-methyl (Figure 1C) [8]. Such product analyses of reactions with altered substrates can be extremely informative (and have been in the case of IPNS [8]), but, particularly for this class of enzymes, must be interpreted with caution. The flexibility required to target multiple positions, in some cases for different outcomes, makes the point of mechanistic divergence of the altered reaction from the natural reaction ambiguous. In other words, the reaction with the substrate analog can follow a profoundly different pathway, making inferences with respect to the natural pathway somewhat perilous (see discussion of HEPD below).
Myo-Inositol Oxygenase
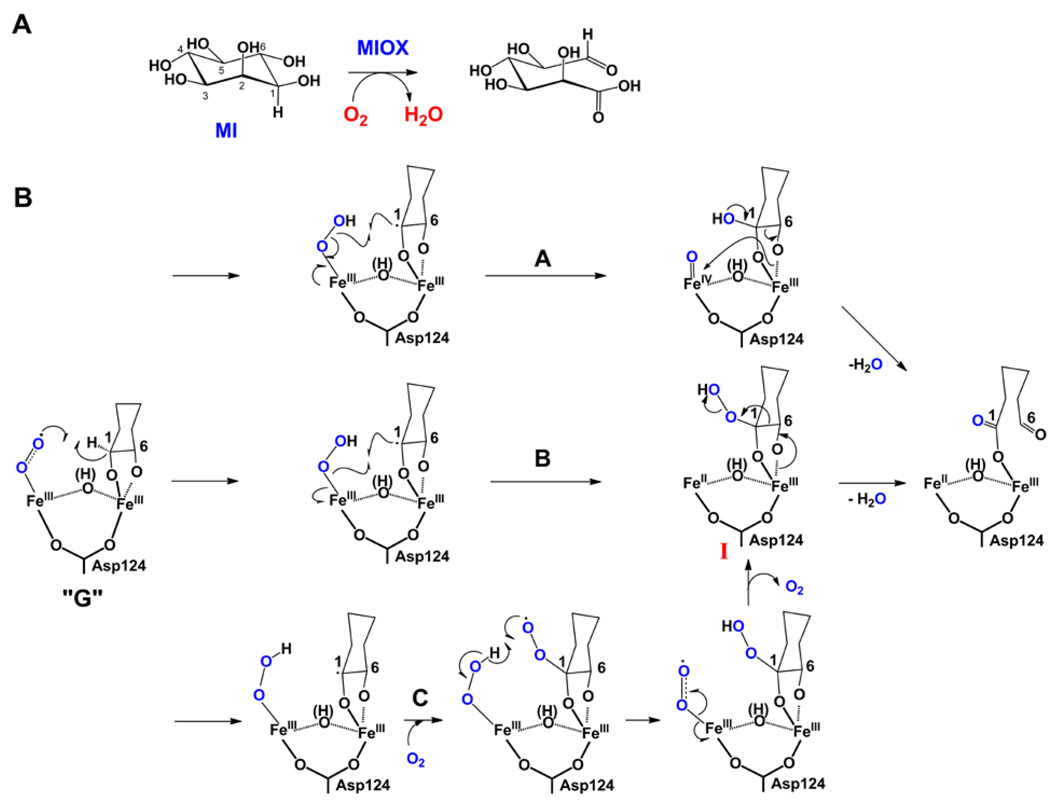
The only known pathway in mammals for degradation of myo-inositol (cyclohexan-1,2,3,5/4,6-hexa-ol, MI, Figure 2A), the carbon backbone of cell-signaling inositol (poly)phosphates, begins with its oxidation to d-glucuronate catalyzed by myo-inositol oxygenase (MIOX) [13]. MIOX is a unique non-heme diiron monooxygenase, structurally and mechanistically unrelated to the well-studied bacterial multicomponent monooxygenases (e.g., soluble methane monooxygenase [19]), which activates MI and O2 at a mixed-valent (Fe2II/III) diiron cluster. Mechanistic and structural studies have suggested that the FeIII site coordinates the substrate via its 1 and 6 hydroxyl groups and that the FeII site activates O2 [13]. An intermediate, designated G and assigned on the basis of its EPR-spectroscopic properties as a superoxo-Fe2III/III complex (Figure 2B), accumulates to ~ 0.5 equiv only when abstraction of hydrogen from C1 is slowed by deuterium substitution [20]. The deuterium kinetic isotope effect on its decay establishes that G, or a complex with which G reversibly interconverts, cleaves the C1-H/D bond. To date, MIOX remains the only enzyme for which a pre-ferryl intermediate has been directly detected and shown to abstract hydrogen [20]. Similarly direct evidence should be (and is being) sought for the other systems.
Figure 2.
(A) Reaction catalyzed by MIOX. B) Possible mechanisms for conversion of the superoxo-Fe2III/III complex, G, to the d-glucuronate product complex involving C1 hydroxylation coupled to peroxide O-O cleavage (pathway A), C1 hydroperoxylation by peroxyl-radical rebound (pathway B), or C1 hydroperoxylation by addition of a second molecule of O2 to the C1 radical (pathway C). In pathway A, C-C bond cleavage with the electrons of the incipient anion directly attacking the ferryl complex is also feasible, but this would lead to incorporation of the ferryl oxygen into the resulting hydrated aldehyde. Oxygen labeling experiments did not detect any oxygen derived from O2 in the aldehyde [45], requiring that for this mechanism to hold, either the oxygen of the ferryl intermediate underwent complete exchange, or the conversion of the initial hydrated product to the aldehyde occurred stereospecific and enzyme catalyzed.
Events subsequent to hydrogen atom abstraction from C1 leading to formation of d-glucuronate are still largely unknown. Pathways involving transfer of either a hydroxyl radical (pathway A, Figure 2B) or hydroperoxyl radical (pathway B) from the hydroperoxo-Fe2III/III complex to C1 of the myo-inositol radical have been envisaged [13]. The proposed hydroxylation step would produce a ferryl-like Fe2III/IV state of the cofactor (pathway A), which would act as electrophile toward the formal carbanion formed during C1-C6 cleavage. As discussed below, this pathway has some analogy (albeit imperfect) to one of the two mechanisms proposed for HEPD. In pathway B, the hydroperoxide I is set up for β-scission to provide the product. A third pathway not previously discussed would involve a second molecule of O2 (the first reacting with the cofactor to form G) adding to the C1 radical (Figure 2C), analogous to the oxygenation step in lipoxygenases [21]. Hydrogen transfer from the cofactor-bound hydroperoxide to the substrate-bound peroxyl radical would again give hydroperoxide I prepared to undergo β-scission. The reversibility of O2 addition to the cofactor in the formation of G [20] creates a major challenge for testing the idea that O2 could be involved both as cofactor (in mediating the initial 1-H abstraction) and as co-substrate (adding to the C1 radical).
MIOX largely conforms to the functional themes outlined above for IPNS. The reaction is a four-electron oxidation by a single equivalent of dioxygen. As with IPNS, this redox economy is made possible by obviating reductive cleavage of O2 prior to hydrogen abstraction, the modus operandi of the ferryl-utilizing enzymes. In addition, coordination of the hydroxyl at C1 to the FeIII site almost certainly leads to deprotonation, activating the C1-H bond for cleavage [17]. Whether extraction of a second electron from the formal ketyl radical allows progression to a high-valent, ferryl-like state (pathway A), analogous to the mechanisms proposed above for IPNS and below for HEPD, remains an open question.
2-Hydroxyethyl Phosphonate Dioxygenase
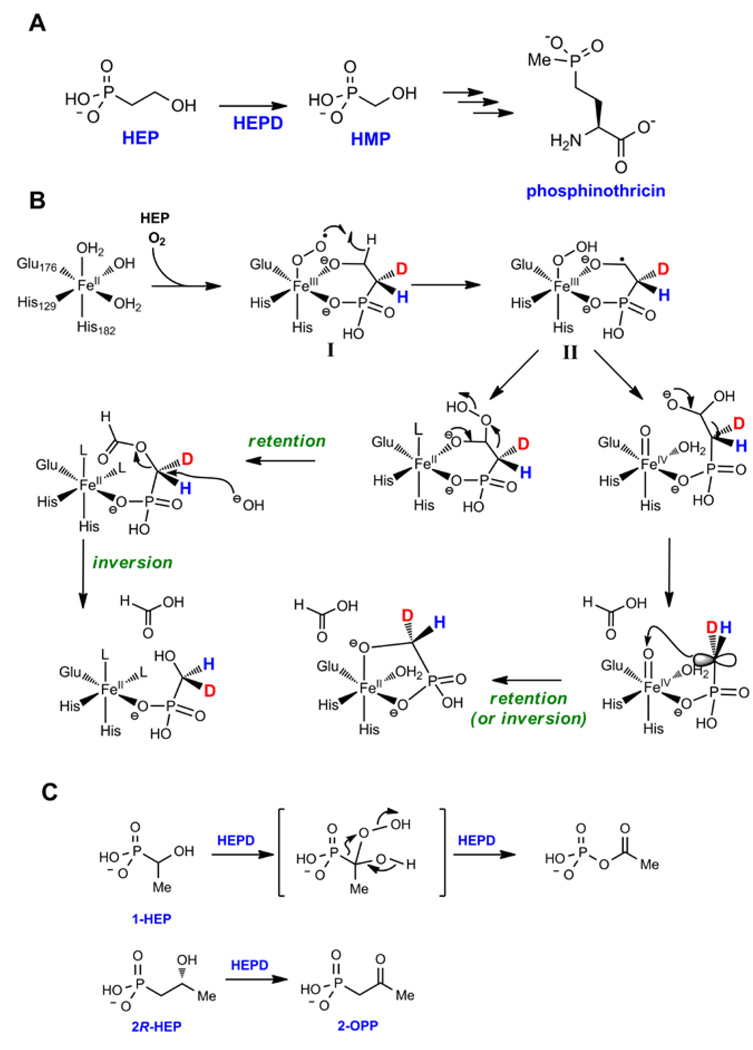
During the biosynthesis of phosphinothricin, the active ingredient in various herbicides that have found widespread use in combination with transgenic crops, 2-hydroxyethyl phosphonate dioxygenase (HEPD) converts 2-hydroxyethyl phosphonate (2-HEP) to hydroxymethylphosphonate (HMP) (Figure 3A). As discussed for IPNS and MIOX, HEPD does not require external reductants or cofactors: all four electrons required for reduction of O2 are provided by 2-HEP. During the transformation, the excised carbon is converted to formate and the hydrogens on C1 of HEP are retained in HMP [14]. Experiments using 18O2 demonstrated that both HMP and formate contained label, but surprisingly, the label in HMP was sub-stoichiometric indicating exchange with solvent during catalysis. In support of this hypothesis, conducting the reaction in H218O also resulted in incorporation of 18O into HMP [14].
Figure 3.
(A) Reaction catalyzed by HEPD in the biosynthesis of phosphinothricin. (B) Two proposed mechanisms for the conversion of 2-HEP to HMP by HEPD. The intermediates are shown with monoprotonated phosphonates but they may be fully deprotonated. The predicted stereochemical outcome at C1 is also shown. The retro-Claisen-like reaction could occur with retention of inversion. (C) Conversion of substrate analogs to various products by HEPD.
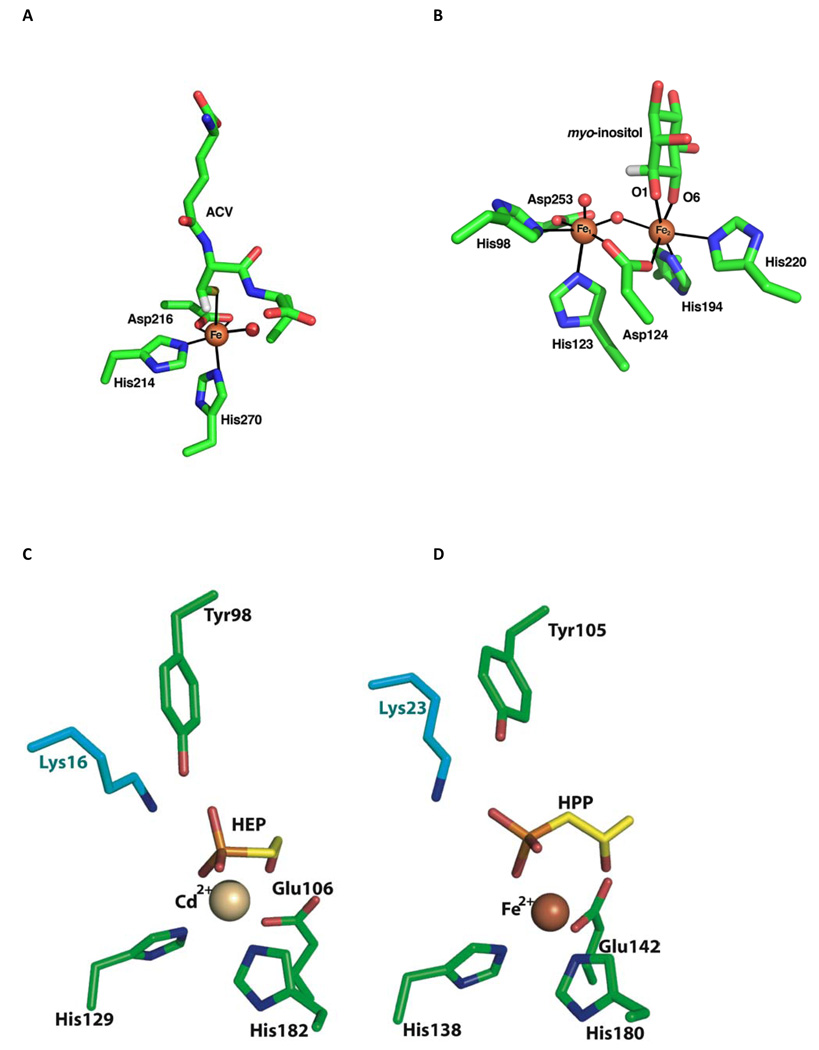
The structure of a Cd2+-substituted form of HEPD was recently solved [14] and is remarkably similar to the previously reported structure of 2-hydroxypropylphosphonate epoxidase, HppE, which is discussed below [22]. The overall fold of HEPD consists of tandem repeats of a bi-domain architecture with each repeat consisting of an all α-helical domain linked to a β-barrel fold that is characteristic of the cupin superfamily. The amino acid metal ligands, His129, Glu176, and His182, make up the usual "facial triad" characteristic of the enzyme family [23], but the spacing between the first two metal ligands is unusually long: (HX46E) in HEPD, compared to (HX1–4E/D) in other facial triad enzymes [24], including the structurally similar HppE. The substrate, 2-HEP, coordinates to the Cd2+ via its C2 hydroxyl and a phosphonate oxygen. Similarly to the situation for IPNS and MIOX, C2-O coordination and resultant deprotonation should activate the substrate for abstraction of a hydrogen from C2 [17], a necessary step in its conversion to formate; this coordination may also activate the metal for reaction with O2.
Two mechanisms have been proposed for the HEPD reaction based on the X-ray structure and isotope labeling studies [14]. After bidentate binding of 2-HEP to Fe(II), O2 addition would result in the {FeOO}8 species, most likely a superoxo-FeIII intermediate (I, Figure 3B). I may abstract a hydrogen atom from C2 of HEP to generate intermediate II, similar to the mechanisms discussed above for isopenicillin N synthase (IPNS) and MIOX [9,25]. Two different pathways can then be envisioned to complete catalysis and account for the labeling studies. The substrate radical could attack the hydroperoxide generating a ferryl intermediate and a hemiacetal (Figure 3B). The latter can undergo a retro-Claisen type C-C bond scission with the incipient negative charge on C1 attacking the electrophilic ferryl, either in concerted or stepwise fashion with a carbanion intermediate stabilized by metal coordination as drawn. Such C-C bond cleavage is unprecedented, but the intermediacy of an Fe(IV)-oxo species explains the observed exchange with solvent.
In the second mechanism the substrate radical is converted to an alkyl hydroperoxide, either by attack of the radical directly on the hydroperoxo-FeIII complex, or alternatively by reaction of the alkyl radical with a second molecule of O2 and hydrogen atom transfer between the resulting alkylperoxy radical and the hydroperoxo-FeIII complex (as suggested above in pathway C for the MIOX reaction). Regardless of the details of the formation of the alkylhydroperoxide, this intermediate could undergo a Criegee-type rearrangement to yield the formate ester of HMP. Hydrolysis of this ester would normally be expected to take place by attack at the carbonyl carbon, but this would not account for the incorporation of oxygen derived from solvent into HMP. The solvent exchange can be explained if hydrolysis were to take place at C1 as shown in Figure 3B.
Experiments with substrate analogs provided support for the hydroperoxylation mechanism (Figure 3C). Incubation of HEPD with O2 and 1-hydroxyethylphosphonate (1-HEP) resulted in the formation of acetylphosphate [26], the direct product of a precedented Criegee rearrangement in which the phosphorus atom migrates [27] (Figure 3C). It is difficult to envision a mechanism involving hydroxylation that would result in the formation of this product. To provide further support for the hydroperoxylation mechanism, 2-HEP stereospecifically deuterium-labeled at C1 was prepared. The hydroperoxylation mechanism in Figure 3B predicts retention at C1 during the Criegee rearrangement but inversion during the hydrolysis at C1. Surprisingly, however, the experimental outcome showed racemization at C1, which cannot be rationalized by the mechanisms in Figure 3B and requires further experimentation [28]. Possibly, the mechanisms for 2-HEP and 1-HEP are different with hydroperoxylation for the latter but not the natural substrate [29]. If so, it would be yet another demonstration of the plasticity of this type of enzyme. Also in keeping with the other enzymes discussed here, use of substrate analogs result in very different types of chemistry (Figure 3C).
CloR
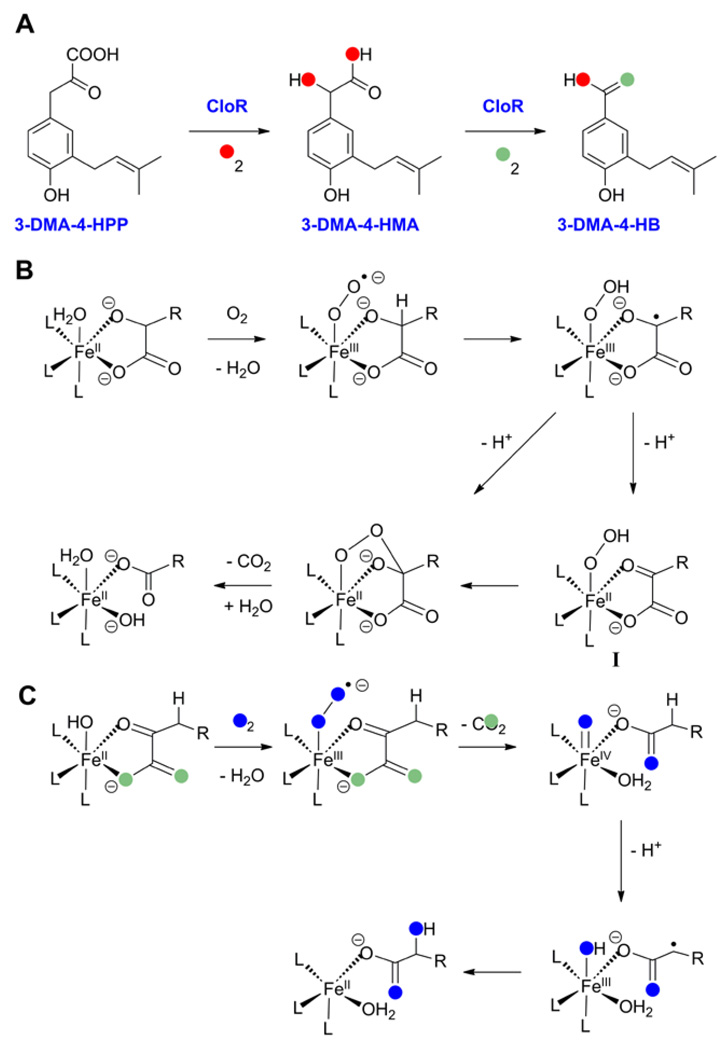
The bifunctional non-heme iron oxygenase CloR catalyzes two consecutive oxidative decarboxylations during the biosynthesis of the aminocoumarin antibiotic clorobiocin [15]. The enzyme first converts 3-dimethylallyl-4-hydroxyphenylpyruvate (3-DMA-4-HPP) into 3-dimethylallyl-4-hydroxymandelic acid (3-DMA-4-HMA), and subsequently oxidizes the latter product to 3-dimethylallyl-4-hydroxybenzoate (3-DMA-4-HB) (Figure 4A). As with most of the other enzymes discussed in this review, CloR does not have any sequence similarity with known oxygenases. Instead the protein shows similarity to class II aldolases. The oxidative decarboxylations catalyzed by CloR do not require α-ketoglutarate, but the assays were performed with excess FeII and ascorbate, which may have provided additional electrons. Experiments with 18O2 demonstrated incorporation of the two oxygen atoms into 3-DMA-4-HMA with one oxygen residing in the carboxyl moiety and one in the new hydroxyl group. Conversely, only one oxygen in 3-DMA-4HB is derived from O2 when unlabeled 3-DMA-4-HMA was incubated with CloR and 18O2 (Figure 4A) [15]. Collectively, these studies suggest that the substrates for CloR have the roles of α-ketoacid co-substrate and hydroxylation substrate combined in a single compound. A proposed mechanism for the transformation of 3-DMA-4-HMA to 3-DMA-4-HB is shown in Figure 4B [15,30]. The first few steps, in which the superoxo-FeIII pre-ferryl complex oxidizes the α-hydroxyl moiety to a carbonyl and the resulting hydroperoxo-FeII complex I then attacks the carbonyl as nucleophile, are strikingly analogous to the mechanism proposed by Baldwin and co-workers for thiocarboxylate formation in the IPNS reaction with the AC-d-α-hydroxyisovaleric acid substrate analogue [31] (Figure 1C). The energetic accessibility of this pathway was recently supported by DFT calculations [32]. Moreover, recent model studies have succeeded in reproducing such oxidative decarboxylation of an α-hydroxy-carboxylate substrate by an Fe(II) catalyst in the presence of O2 [30]. Mechanistic studies supported the catalytic cycle shown in Figure 4B. A hypothetical mechanism for the first reaction catalyzed by CloR based on the understanding of α-ketoglutarate dependent hydroxylations [33–36] is shown in Figure 4C.
Figure 4.
(A) Reactions catalyzed by CloR. The results of oxygen labeling studies are indicated. (B) Proposed mechanism for the second reaction catalyzed by CloR. (C) Proposed mechanism for the first reaction catalyzed by CloR.
2-Hydroxypropylphosphonate Epoxidase
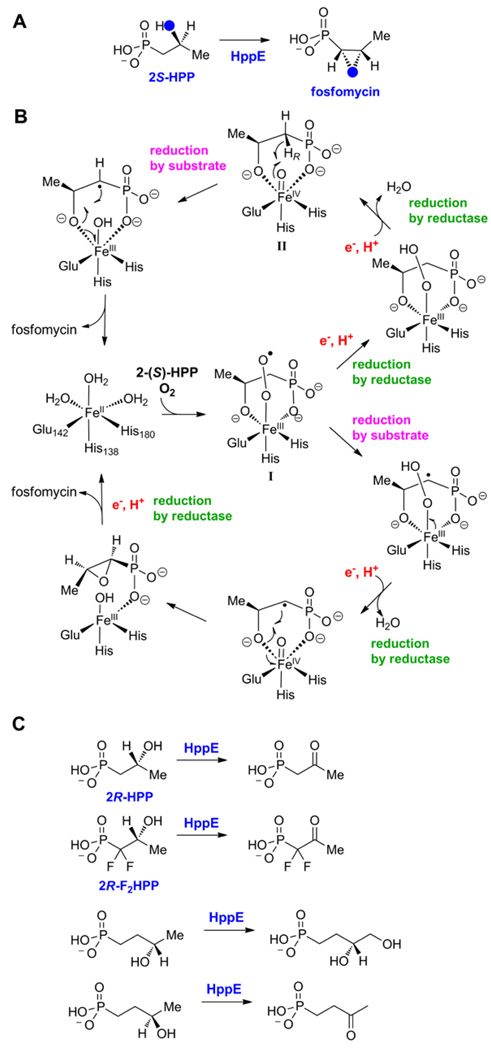
In the last step of the biosynthesis of fosfomycin, an FDA-approved drug for the treatment of uncomplicated acute cystitis (urinary tract infections), (S)-2-hydroxypropylphosphonate (2-HPP) is converted to the mature antibiotic (Figure 5A). Feeding studies with isotopically labeled precursors demonstrated that the epoxide oxygen is not derived from molecular oxygen but from the hydroxyl group in 2-HPP [37]. These studies suggested an unprecedented mechanism of epoxide formation involving dehydrogenation of a secondary alcohol (Figure 5A). Liu and coworkers reconstituted the activity of the HPP epoxidase (HppE) in vitro [38] and showed that the enzyme requires Fe(II), O2, an electron carrier (either a general reductase or catalytic amounts of FMN) and NADH as reductant, but not α-ketoglutarate. Subsequent spectroscopic, mechanistic, and structural investigations established that the enzyme is a mononuclear non-heme-iron-dependent oxidase [22,39–41].
Figure 5.
(A) Reaction catalyzed by HppE illustrating retention of the hydroxyl oxygen in the epoxide of fosfomycin. (B) Two proposed mechanisms for the conversion of 2S-HPP to fosfomycin. (C) Conversion of substrate analogs to various products by HppE.
A series of crystal structures of the enzyme have provided a solid foundation for mechanistic analysis. As noted above, HppE is a member of the cupin superfamily with its characteristic jelly roll domain that contains the typical 2 His/1 Glu facial triad iron ligand set [22]. A structurally unique smaller domain made up of 5 short α-helices completes the structure. The mode of substrate binding in these structures varies from monodentate coordination of 2-HPP through one of the phosphonate oxygens to bidentate ligation of both the phosphonate and hydroxyl groups. The latter binding mode is associated with a more closed conformation that appears to shield the active site from solvent and may help protect reactive intermediates. Further support for productive bidentate HPP binding has been provided by EPR studies with 17O-labeled substrate and using NO as mimic of oxygen [41]. The bidentate binding of HPP resembles the binding geometry of α-ketoglutarate (α-KG) in other members of the cupin family [42,43], and analogously the bidentate binding by HPP may serve to facilitate the normally unfavorable reaction of ferrous iron with molecular oxygen [22].
The two most likely mechanisms for the HppE reaction are shown in Figure 5B [41]. They differ in the timing of the entry of the two electrons required for the overall reaction, and therefore in whether either a (pre-ferryl) Fe(III)-superoxide complex (I) or a ferryl complex (II) abstracts the hydrogen atom from C1 to permit epoxide formation; alternatively, this removal of the hydrogen at C1 may occur via proton-coupled electron transfer (not shown). In both mechanisms, the resulting substrate radical at C1 subsequently carries out an unprecedented intramolecular rebound-like reaction. Recent kinetic isotope effects studies on kcat/Km(O2) using 16O2 or 18O2 suggest the formation of an FeIII–OOH species in the rate-limiting step of O2 activation, either by hydrogen atom abstraction or H+-coupled ET from the substrate or by electron transfer from the presumptive reductase coupled to local proton transfer [44]. The former possibility is, from the perspective of this review, quite provocative, as it would include HppE among the small group of pre-ferryl-utilizing oxygenases and oxidases, despite the fact that it deviates from two of the general themes to which the other enzymes conform. First, the substrate lacks a coordinating heteroatom α to the carbon targeted for H-abstraction. Thus, it is unclear whether the C1-H bond can be sufficiently activated to make abstraction by a pre-ferryl complex (I) thermodynamically feasible. Second, the reaction is only a two-electron oxidation, requiring two exogenous electrons. A conventional pathway to an H-abstracting ferryl complex (II), involving sequential delivery of two electrons first to the initial FeIII-O2− adduct and then to the resulting FeIII–OO(H) complex by the reductase (Figure 5B), seems completely plausible. It is possible that the unique rebound-like reaction, in which the epoxide forms by formal transfer of a coordinated alkoxyl radical to the substrate radical, precludes such a conventional ferryl mechanism. Perhaps the alternative, pre-ferryl pathway, in which the alkoxyl radical transfer occurs from the ferryl rather than the ferric state of the cofactor, provides a lower, more surmountable activation barrier to the strained ring. Alternatively, it is possible that the need to avoid a hydroxylation outcome by the conventional hydroxyl-radical rebound provides the rationale for use of the pre-ferryl pathway. These intriguing mechanistic issues make resolution of the pathway in HppE an important goal for the field. Transient state kinetic experiments may be capable of distinguishing the two main possibilities by resolving the timing of electron delivery and hydrogen abstraction. However, the fact that neither the presumptive reductase protein nor a facile chemical reductant has been identified has, to date, thwarted these efforts. Similar to the observations with IPNS and HEPD, the use of substrate analogs results in various types of diverted chemistry [39,41] (Figure 5C).
Summary and outlook
The ability of this small set of non-heme-iron oxygenases and oxidases to use "pre-ferryl" complexes to abstract hydrogen from their substrates allows them to obtain the electrons needed to cleave the O-O bond of O2, providing the thermodynamic driving force for their difficult oxidation reactions, while retaining all four oxidizing equivalents for conferral to their substrates. Their ability to target two adjacent or distal positions of the substrate with the pre-ferryl and subsequent ferryl complexes, often for fundamentally different types of oxidation, confers remarkable catalytic versatility that undoubtedly includes reaction types yet to be discovered. It appears that the key underlying chemical principle permitting this novel mode of O2 and C-H activation is heteroatom coordination and deprotonation to activate the substrate C-H bond and probably also tune the cofactor for facile reaction with O2 (Figure 6). In many cases, a second group on the substrate (e.g., hydroxyl or phosphonate oxygen) also coordinates, possibly to anchor the crucial heteroatom to the metal and ensure its deprotonation. Whether some enzymes are capable of using pre-ferryl complexes for H-abstraction without this means of substrate activation (e.g., HppE) remains to be determined. An important objective for the immediate future, to date achieved only for MIOX, is the detection and characterization of the proposed pre-ferryl complexes and direct demonstration that they are, in fact, the H-abstracting intermediates.
Figure 6.
Active sites of co-crystal structures of (A) IPNS, (B) MIOX, (C) HEPD, and (D) HppE with their substrates.
Acknowledgements
This work has been supported by the National Institutes of Health (GM58822 to WAV and GM69657 and DK074641 to JMB and CK), the National Science Foundation (MCB-0642058 and CHE-724084 to CK and JMB), the Petroleum Research Fund of the American Chemical Society (47214-AC3 to JMB and CK). The authors thank Megan Matthews (Penn State) and Satish Nair (UIUC) for assistance with preparation of Figure 6.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wilfred A. van der Donk, Department of Chemistry and Howard Hughes Medical Institute, University of Illinois at Urbana-Champaign, 600 S. Mathews Ave, Urbana, Illinois 61801, USA, vddonk@uiuc.edu
Carsten Krebs, Department of Chemistry and Department of Biochemistry and Molecular Biology, Penn State University, 332 Chemistry Building, University Park, PA, 16802, USA, ckrebs@psu.edu.
J. Martin Bollinger, Jr., Department of Chemistry and Department of Biochemistry and Molecular Biology, Penn State University, 336 Chemistry Building, University Park, PA, 16802, USA, jmb21@psu.edu
References and recommended reading
- 1.Krebs C, Galonic Fujimori D, Walsh CT, Bollinger JM., Jr Non-heme Fe(IV)-oxo intermediates. Acc Chem Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollinger JM, Jr, Krebs C. Enzymatic C-H activation by metal-superoxo intermediates. Curr Opin Chem Biol. 2007;11:151–158. doi: 10.1016/j.cbpa.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Que L, Jr, Ho RYN. Dioxygen activation by enzymes with mononuclear non-heme iron active sites. Chem Rev. 1996;96:2607–2624. doi: 10.1021/cr960039f. [DOI] [PubMed] [Google Scholar]
- 4.Kovaleva EG, Lipscomb JD. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nat Chem Biol. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krebs C, Galonić Fujimori D, Walsh CT, Bollinger JM., Jr Non-Heme Fe(IV)-Oxo Intermediates. Acc Chem Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Que L., Jr The oxo/peroxo debate: a nonheme iron perspective. J Biol Inorg Chem. 2004;9:684–690. doi: 10.1007/s00775-004-0574-8. [DOI] [PubMed] [Google Scholar]
- 7.Bollinger JM, Jr, Krebs C. Enzymatic C-H activation by metal-superoxo intermediates. Curr Opin Chem Biol. 2007;11:151–158. doi: 10.1016/j.cbpa.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin JE, Bradley M. Isopenicillin N synthase: mechanistic studies. Chem Rev. 1990;90:1079–1088. [Google Scholar]
- 9.Burzlaff NI, Rutledge PJ, Clifton IJ, Hensgens CM, Pickford M, Adlington RM, Roach PL, Baldwin JE. The reaction cycle of isopenicillin N synthase observed by X-ray diffraction. Nature. 1999;401:721–724. doi: 10.1038/44400. [DOI] [PubMed] [Google Scholar]
- 10.Enemark JH, Feltham RD. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coord Chem Rev. 1974;13:339–406. [Google Scholar]
- 11.Brown CD, Neidig ML, Neibergall MB, Lipscomb JD, Solomon EI. VTVH-MCD and DFT studies of thiolate bonding to [FeNO]7/[FeO2]8 complexes of isopenicillin N synthase: substrate determination of oxidase versus oxygenase activity in nonheme Fe enzymes. J Am Chem Soc. 2007;129:7427–7438. doi: 10.1021/ja071364v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye S, Price JC, Barr EW, Green MT, Bollinger JM, Jr, Krebs C, Neese F. Cryoreduction of the NO-adduct of taurine:alpha-ketoglutarate dioxygenase (TauD) yields an elusive {FeNO}8 species. J Am Chem Soc. 2010;132:4739–4751. doi: 10.1021/ja909715g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollinger JM, Jr, Diao Y, Matthews ML, Xing G, Krebs C. myo-Inositol oxygenase: a radical new pathway for O2 and C-H activation at a nonheme diiron cluster. Dalton Trans. 2009:905–914. doi: 10.1039/b811885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicchillo RM, Zhang H, Blodgett JAV, Whitteck JT, Li G, Nair SK, van der Donk WA, Metcalf WW. An unusual carbon-carbon bond cleavage reaction during phosphinothricin biosynthesis. Nature. 2009;459:871–874. doi: 10.1038/nature07972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pojer F, Kahlich R, Kammerer B, Li SM, Heide L. CloR, a bifunctional non-heme iron oxygenase involved in clorobiocin biosynthesis. J Biol Chem. 2003;278:30661–30668. doi: 10.1074/jbc.M303190200. [DOI] [PubMed] [Google Scholar]
- 16.Roach PL, Clifton IJ, Hensgens CMH, Shibta N, Schofield CJ, Hajdu J, Baldwin JE. Structure of isopenicillin N synthase complexed with substrate and the mechanism of penicillin formation. Nature. 1997;387:827–830. doi: 10.1038/42990. [DOI] [PubMed] [Google Scholar]
- 17.Steigerwald ML, Goddard WA, III, Evans DA. Theoretical studies of the oxy anionic substituent effect. J Am Chem Soc. 1979;101:1994–1997. [Google Scholar]
- 18.Ogle JM, Clifton IJ, Rutledge PJ, Elkins JM, Burzlaff NI, Adlington RM, Roach PL, Baldwin JE. Alternative oxidation by isopenicillin N synthase observed by X-ray diffraction. Chem Biol. 2001;8:1231–1237. doi: 10.1016/s1074-5521(01)00090-4. [DOI] [PubMed] [Google Scholar]
- 19.Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Dioxygen activation and methane hydroxylation by soluble methane monooxygenase: a tale of two irons and three proteins. Angew Chem Int Ed Engl. 2001;40:2782–2807. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Xing G, Diao Y, Hoffart LM, Barr EW, Prabhu KS, Arner RJ, Reddy CC, Krebs C, Bollinger JM., Jr Evidence for C-H cleavage by an iron-superoxide complex in the glycol cleavage reaction catalyzed by myo-inositol oxygenase. Proc Natl Acad Sci, USA. 2006;103:6130–6135. doi: 10.1073/pnas.0508473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreou A, Feussner I. Lipoxygenases - Structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Higgins LJ, Yan F, Liu P, Liu HW, Drennan CL. Structural insight into antibiotic fosfomycin biosynthesis by a mononuclear iron enzyme. Nature. 2005;437:838–844. doi: 10.1038/nature03924. [DOI] [PubMed] [Google Scholar]
- 23.Que L., Jr One motif - many different reactions. Nature Struct Biol. 2000;7:182–184. doi: 10.1038/73270. [DOI] [PubMed] [Google Scholar]
- 24.Koehntop KD, Emerson JP, Que L., Jr The 2-His-1-carboxylate facial triad: a versatile platform for dioxygen activation by mononuclear non-heme iron(II) enzymes. J Biol Inorg Chem. 2005;10:87–93. doi: 10.1007/s00775-005-0624-x. [DOI] [PubMed] [Google Scholar]
- 25.Xing G, Diao Y, Hoffart LM, Barr EW, Prabhu KS, Arner RJ, Reddy CC, Krebs C, Bollinger JM., Jr Evidence for C-H cleavage by an iron-superoxide complex in the glycol cleavage reaction catalyzed by myo-inositol oxygenase. Proc Natl Acad Sci USA. 2006;103:6130–6135. doi: 10.1073/pnas.0508473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitteck JT, Cicchillo RM, van der Donk WA. Hydroperoxylation by hydroxyethylphosphonate dioxygenase. J Am Chem Soc. 2009;131:16225–16232. doi: 10.1021/ja906238r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon NJ, Evans SA., Jr Acyl phosphates from acyl phosphonates - a novel Baeyer-Villiger rearrangement. J Org Chem. 1993;58:4516–4519. [Google Scholar]
- 28.Whitteck JT, Malova P, Peck S, Cicchillo RM, Hammerschmidt F, van der Donk WA. On the Stereochemistry of Hydroxyethylphosphonate Dioxygenase. 2010 doi: 10.1021/ja1113326. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koehntop KD, Marimanikkuppam S, Ryle MJ, Hausinger RP, Que L., Jr Self-hydroxylation of taurine/alpha-ketoglutarate dioxygenase: evidence for more than one oxygen activation mechanism. J Biol Inorg Chem. 2006;11:63–72. doi: 10.1007/s00775-005-0059-4. [DOI] [PubMed] [Google Scholar]
- 30.Paine TK, Paria S, Que L., Jr Oxidative decarboxylation of alpha-hydroxy acids by a functional model of the nonheme iron oxygenase, CloR. Chem Commun. 2010;46:1830–1832. doi: 10.1039/b925389k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge W, Clifton IJ, Howard-Jones AR, Stok JE, Adlington RM, Baldwin JE, Rutledge PJ. Structural studies on the reaction of isopenicillin N synthase with a sterically demanding depsipeptide substrate analogue. Chembiochem. 2009;10:2025–2031. doi: 10.1002/cbic.200900080. [DOI] [PubMed] [Google Scholar]
- 32.Brown-Marshall CD, Diebold AR, Solomon EI. Reaction coordinate of isopenicillin N synthase: oxidase versus oxygenase activity. Biochemistry. 2010;49:1176–1182. doi: 10.1021/bi901772w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hausinger RP. Fe(II)/{alpha}-Ketoglutarate-Dependent Hydroxylases and Related Enzymes. Crit Rev Biochem Mol Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 34.Hoffart LM, Barr EW, Guyer RB, Bollinger JM, Jr, Krebs C. Direct spectroscopic detection of a C-H-cleaving high-spin Fe(IV) complex in a prolyl-4-hydroxylase. Proc Natl Acad Sci U S A. 2006;103:14738–14743. doi: 10.1073/pnas.0604005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price JC, Barr EW, Tirupati B, Bollinger JM, Jr, Krebs C. The first direct characterization of a high-valent iron intermediate in the reaction of an alpha-ketoglutarate-dependent dioxygenase: a high-spin FeIV complex in taurine/alpha-ketoglutarate dioxygenase (TauD) from Escherichia coli. Biochemistry. 2003;42:7497–7508. doi: 10.1021/bi030011f. [DOI] [PubMed] [Google Scholar]
- 36.Price JC, Barr EW, Glass TE, Krebs C, Bollinger JM., Jr Evidence for hydrogen abstraction from C1 of taurine by the high-spin Fe(IV) intermediate detected during oxygen activation by taurine:alpha-ketoglutarate dioxygenase (TauD) J Am Chem Soc. 2003;125:13008–13009. doi: 10.1021/ja037400h. [DOI] [PubMed] [Google Scholar]
- 37.Hammerschmidt F. Biosynthesis of Natural Products with a P-C Bond. Part 8: On the Origin of the Oxirane Oxygen Atom of Fosfomycin in Streptomyces fradiae. J Chem Soc Perkin Trans 1. 1991:1993–1996. [Google Scholar]
- 38.Liu P, Murakami K, Seki T, He X, Yeung SM, Kuzuyama T, Seto H, Liu HW. Protein purification and function assignment of the epoxidase catalyzing the formation of fosfomycin. J Am Chem Soc. 2001;123:4619–4620. doi: 10.1021/ja004153y. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z, Liu P, Murakami K, Kuzuyama T, Seto H, Liu HW. Mechanistic Studies of HPP Epoxidase: Configuration of the Substrate Governs Its Enzymatic Fate. Angew Chem, Int Ed Engl. 2002;41:4529–4532. doi: 10.1002/1521-3773(20021202)41:23<4529::AID-ANIE4529>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 40.Liu P, Liu A, Yan F, Wolfe MD, Lipscomb JD, Liu HW. Biochemical and spectroscopic studies on (S)-2-hydroxypropylphosphonic acid epoxidase: a novel mononuclear non-heme iron enzyme. Biochemistry. 2003;42:11577–11586. doi: 10.1021/bi030140w. [DOI] [PubMed] [Google Scholar]
- 41.Yan F, Moon SJ, Liu P, Zhao Z, Lipscomb JD, Liu A, Liu HW. Determination of the substrate binding mode to the active site iron of (S)-2-hydroxypropylphosphonic acid epoxidase using 17O-enriched substrates and substrate analogues. Biochemistry. 2007;46:12628–12638. doi: 10.1021/bi701370e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valegard K, van Scheltinga AC, Lloyd MD, Hara T, Ramaswamy S, Perrakis A, Thompson A, Lee HJ, Baldwin JE, Schofield CJ, et al. Structure of a cephalosporin synthase. Nature. 1998;394:805–809. doi: 10.1038/29575. [DOI] [PubMed] [Google Scholar]
- 43.Pavel EG, Zhou J, Busby RW, Gunsior M, Townsend CA, Solomon EI. Circular Dichroism and Magnetic Circular Dichroism Spectroscopic Studies of the Non-Heme Ferrous Active Site in Clavaminate Synthase and Its Interaction with α-Ketoglutarate Cosubstrate. J Am Chem Soc. 1998;120:743–775. [Google Scholar]
- 44.Mirica LM, McCusker KP, Munos JW, Liu HW, Klinman JP. 18O kinetic isotope effects in non-heme iron enzymes: probing the nature of Fe/O2 intermediates. J Am Chem Soc. 2008;130:8122–8123. doi: 10.1021/ja800265s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moskala R, Reddy CC, Minard RD, Hamilton GA. An oxygen-18 tracer investigation of the mechanism of myo-inositol oxygenase. Biochem Biophys Res Commun. 1981;99:107–113. doi: 10.1016/0006-291x(81)91719-8. [DOI] [PubMed] [Google Scholar]